Introduction
Camels are found in different parts of the world, particularly in the deserts of Africa and Asia. In the late 19th and early 20th centuries, several breeds of camels were introduced to Australia; consequently, at present, there are more than 500,000 wild camels in Australia. Worldwide, two camel species are common: dromedary (Arabian) camels with a single hump (Camelus dromedaries; camel of the plains) and Bactrian camels with a double hump (Camelus bactrianus) (Fukuda, 2013). Presently, it is estimated that there are over 20 million camels worldwide with the numbers continuously increasing (Al Haj and Al Kanhal, 2010). Usually, camels are reared for their milk, meat, fiber (wool and hair), and for the purpose of transportation of goods and people (El-Agamy, 2006).
In the arid regions of Africa and Gulf Cooperation Council (GCC) regions, camels are good and nutritious sources of meat (Kadim et al., 2013). There is significant interest in camel meat due to its health benefits. For example, it has lower fat and cholesterol content as well as higher polyunsaturated fatty acid content compared to other animals (Mejri and Hassouna, 2016). Consequently, camel meat seems to have a great potential to be used in preparation of functional food products providing various health benefits (Gul et al., 2016). The trade of functional food products is predicted to grow due to the magnitude of the human population. Majority of current reports about functional fermented sausages are on the bioactivities of fermented beef or pork sausages (Lafarga and Hayes, 2017; Liu et al., 2016). However, a few studies have recently employed camel meat for fermented sausage formulation. Commercial starter cultures were mainly used to ferment camel sausages with a focus on the non-bioactive properties such as texture, taste, and flavor (Kargozari et al., 2014; Maqsood et al., 2016; Mejri et al., 2017b). Mejri et al. (2017a) has explored the antioxidant and antihypertensive properties of fermented sausages; however, no systematic comparison was made with fermented pork or beef sausages. Recently, lactic acid bacteria exhibiting novel probiotic characteristics have been isolated from camel milk in our food microbiology lab at United Arab Emirates University (UAEU) (Abushelaibi et al., 2017). Among these novel probiotics, Lactococcus lactis KX881782 showed a very promising fermentation profile; therefore, we hypothesized that use of this strain in the fermentation of camel sausages could provide health-promoting benefits of camel meat.
This study aimed to understand the bioactivities of semi-dry fermented camel sausages using L. lactis KX881782, comprehensively. The antioxidant capacity, cytotoxicity against Caco-2 and MCF-7 cell lines, antidiabetic activity by α-amylase and α-glucosidase inhibition, antihypertensive activity by angiotensin-converting enzyme (ACE)-inhibition were the main indicators. The technical parameters such the degree of hydrolysis (DH) to assess proteolysis rate, lipid peroxidation activity, and texture profile of the fermented semi-dry camel sausages were assessed. The same parameters were assessed in fermented beef sausages for comparison.
Materials and Methods
Traditional commercial cultures (TCC) of Pediococcus pentosaceus and Staphylococcus carnosus (Chr-Hansen Holding A/S, Horsholm, Denmark) are usually used in the fermentation of sausages. L. lactis KX881782 was stored at –80℃ in 50% glycerol. For preparation of fresh stationary phase cultures, frozen strains were thawed at room temperature and a 0.1 mL aliquot of each strain was individually transferred into 9.9 mL of fresh De Man, Rogosa and Sharpe (MRS) broth (LAB-M, Lancashire, UK) and incubated at 37℃ for 24 h. The cultures were activated twice successively in MRS broth before the experimental day.
Fresh boneless chuck meat cuts and back-fats were purchased from a local market. The camel and beef sausages were formulated according to Mejri et al. (2017b) with minor modifications. The batters of camel or beef sausages were prepared with 80% lean meat and 20% fat (camel sausage includes 80% camel meat+20% camel fat, and beef sausage includes 80% beef meat+20% beef fat). All components (meats and fats) from each source (camel or beef) were minced individually using a commercial mincer (MK-G20NR-W, Panasonic, Osaka, Japan). Subsequently, seasoning was added as follows: NaCl, 25 g/kg; garlic, 10 g/kg; sucrose, 4 g/kg; mixed spices, 30 g/kg; NaNO2, 0.12 g/kg; and NaNO3, 0.12 g/kg.
All ingredients (meat+seasoning) were mixed at 4℃ and the mixture was portioned into 3 equal parts to be inoculated with 107–108 CFU/kg of TCC (control portion), L. lactis, or TCC+L. lactis. The final mixture was prepared by mincing the inoculated minced meat with cold fat in a 3-mm plate. A semi-manual stuffer was used to stuff the final mixture in presoaked (in water at 40℃ for 30 min) fibrous casings (55 mm diameter, Kalle GmbH & Co. Wiesbaden, Germany). The semi-dried fermentation and storage procedures were carried out according to Hughes et al. (2002). For fermentation, the camel or beef sausages were incubated for approximately 48 h at 30℃ with relative humidity of 90% until their pH values reached ≤5.3. Afterwards, the semi-dried fermented batters were vacuum-packaged and stored at 15℃ for 21 d. Sausages were sampled after fermentation at 0, 7, 14, and 21 d of storage. The sausage making was repeated three times at three different occasions.
Moisture content (oven-drying method at 105℃), protein content (Kjeldahl method), fat content (Soxhlet method), and ash content (muffle furnace method) of the sausage samples were determined according to AOAC (2005). The changes in pH values were monitored directly using a digital pH meter (Starter-3100, Ohaus, NJ, USA). HygroLab-C1 (Rotronic, NY, USA) was used to measure the water activity (Aw) of the sausage samples.
The population of LAB was enumerated on MRS agar (LAB-M) according to the method used by Mejri et al. (2017b). The inoculated plates were anaerobically incubated at 37℃ for 48 h using an anaerobic jar system (Don Whitley Scientific Limited, West Yorkshire, UK). An aliquot of 0.1 mL was spread on tryptone soy agar (TSA, LAB-M) plates which were aerobically incubated at 37℃ for 24 h to determine the total bacterial count.
The WSE was prepared by homogenizing 15 g of the sausage sample with 15 mL dd-water at 20,000×g for 30 s. A Whatman® filter No.1 was used to filter the mixture at room temperature. The filtrate was stored at –20℃ for further analysis (Van Ba et al., 2017). Centrifugation at 10,000×g at 4℃ for 5 min was carried out for each WSE before subsequent assays.
The DH% was determined according to the method detailed in (Ayyash et al., 2019). The following formulae were employed to assess the DH%:
where, the total number of peptide bonds per protein equivalent (htot) was 7.6 mEq/g protein in meat products.
where α=1.0 and β=0.40 mEq/g protein for meat products
The Serine-NH2 value
All the above values (htot, α, and β) were reported by Nielsen et al. (2001).
Lipid peroxidation activity assessed by the TBARS test expressed as mg malonaldehyde/kg (mg MDA/kg) was carried out according to Berardo et al. (2016).
Sausage samples were tested for hardness, adhesiveness, cohesiveness, springiness, gumminess, chewiness using Texture Analyzer CT3 (Brookfield AMETEK, Middleboro, Massachusetts, USA) according to Kargozari et al. (2014). Samples were tested immediately in duplicates.
The ability of the prepared WSEs to inhibit α-amylase and α-glucosidase activities was determined as described by Ayyash et al. (2018a). The following equations were employed to calculate the α-amylase and α-glucosidase inhibition:
where control was a solution without the WSE sample and blank was a solution without the substrate.
The scavenging capacity of the WSE was assayed by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2’-azino-bis (3- ethylbenzo-thiazoline-6-sulphonic acid) (ABTS·+) according to Ayyash et al. (2018b). The following equations were employed to calculate the scavenging percentages:
The capability of WSEs to inhibit the ACE was assayed according to the method detailed in (Al-Dhaheri et al., 2017). ACE-inhibition percentage was calculated using the following equation:
where control was without WSE addition.
The cytotoxicity of the WSEs were assayed against Caco-2 and MCF-7 cell lines as described by Ayyash et al. (2018b). The following equation was employed to calculate the cytotoxicity percentage:
where Rsample is the absorbance in presence of the WSE. Rctrl is absorbance in absence of the WSE (positive control). Ro is absorbance of the non-cell background (negative control).
Sausage making was repeated in triplicates at three different occasions. To examine the significant impact of the meat variety, starter culture type, and storage period, two-way ANOVA was performed (p<0.05). To compare means, Tukey’s test was performed at a significance level of p<0.05. The correlations between parameters were tested by Pearson’s correlation (Table S1). The AOVA analysis showing the significant impact of all factors on measured parameters are presented in Table S2. Statistical analysis software SAS v9.2 (SAS, NC, USA) was used to perform all the statistical analyses.
Results and Discussion
The initial (at d 0 of storage) and the final (at d 21 of storage) chemical compositions of camel and beef sausages fermented with TCC, L. lactis, or TCC+L. lactis are presented in Table 1. The initial chemical composition between the fermented sausages was not significantly different. The moisture content of the fermented camel or beef sausages significantly decreased (p<0.05) from approximately 15%–19% by d 21 of storage to 28.7%–29.7% and 30.8%–36.5%, respectively. Consequently, the protein, fat, and ash content of the sausages significantly increased (p<0.05) by 21 d. It is worth mentioning that the fermented camel sausages had lower moisture content than that of the beef sausages at 21 d of storage; however, there were no significant differences between the sausages (fermented with TCC, L. lactis, or TCC+L. lactis) from the same source except that beef sausages fermented with L. lactis had higher moisture content than other types of sausages. Kargozari et al. (2014) reported that Turkish fermented camel and beef sausages had moisture contents of 26.8% and 31.8%, respectively, at 16 d of storage. It is likely that fermented camel sausages had lower moisture content than beef sausages due to the lower emulsifying ability of camel fat, thus releasing more water during storage (Kargozari et al., 2014). Furthermore, camel meat had lower water-holding capacity than that of beef meat. In contrast, Soltanizadeh et al. (2010) found that non-fermented camel sausages had higher moisture content compared to beef sausages. The variation in moisture content between the present study and those reported by Soltanizadeh et al. (2010) could be attributed to the breed variation in camel meat and sausage preparation method. The drop in moisture content in fermented camel sausages might be the main cause for the increase in protein, fat, and ash content (Kargozari et al., 2014). It is evident that the prepared fermented sausages are comparable to the fermented sausages produced by the food industry.
a–e Mean values in the same column with different lowercase superscripts indicate significant difference at p<0.05.
Table 2 displays the populations of LAB and TBC, pH values and water activities (Aw) of camel and beef sausages. The populations of LAB and TBC were maintained at >8.0 Log CFU/g at the different time intervals tested. However, the populations of LAB at 7 and 14 d of storage were higher (p<0.05) by ≤1.5 Log CFU/g in fermented camel sausages than in beef sausages. At the end of storage, LAB numbers in camel sausages fermented by TCC+L. Lactis were lower than those in beef sausages fermented with the same bacteria. The LAB numbers in camel sausages fermented by L. lactis were slightly higher than those in beef sausages and this might be attributable to the ability of L. lactis, isolated from camel milk, to grow in camel meat better than in beef. We assume that L. lactis may have higher metabolomic activities which reflected into slightly greater cell count in camel sausage compared with beef sausage.
a–e Mean values in the same column with different lowercase superscripts indicate significant difference at p<0.05.
It should be noted that LAB numbers in camel sausages fermented with various starter cultures rose significantly (p<0.05) until d 14 of storage and a slight drop occurred on d 21. Similar results were obtained from TBC when TBC numbers were higher (~8.0 CFU/g) during the storage period in comparison to the initial numbers (Table 2). These results concur with the FAO/WHO definition of probiotics (FAO/WHO, 2002).
pH values declined significantly from ~6.20 to ~5.20–5.30 during the first 48 h of fermentation. pH values of camel sausages significantly declined (p<0.05) during the storage period, when camel sausages fermented with TCC+L. lactis had the lowest pH value (4.06) compared to the other fermented camel sausages at the end of the storage period. The pH values of beef sausages fermented with L. lactis had the lowest pH value (4.01) compared to the other fermented beef sausages. The drop in the pH values of camel or beef sausages fermented with L. lactis implies that this probiotic had good homofermentative properties and the ability to produce primary organic acids (Leroy et al., 2006). It is evident that this new probiotic possesses comparable technical properties to commercial cultures used in the meat industry. The water activities decreased significantly (p<0.05) from ~0.98 to 0.90 in camel sausages and from ~0.97 to 0.92 in beef sausages on d 21. ANOVA showed that the different fermented camel sausages had lesser (p<0.05) aw than beef sausages (Table 2). Similar outcomes were reported by Kargozari et al. (2014) who found that the Turkish camel sausages had lower pH and aw values than beef sausages at the end of the storage period. The reduction in Aw of sausages may be attributed to moisture loss during the storage period. Further, the low Aw value of camel sausages fermented by L. lactis alone or mixed with the TCC may be due to exopolysaccharides produced by this strain (Abushelaibi et al., 2017).
TBAR values of fermented camel and beef sausages were lower than 0.7 mg MDA/kg and 0.8 mg MDA/kg, respectively (Fig. 1). Generally, TBAR values were insignificantly changed during storage (Fig. 1). By the end of storage, the lowest TBAR values were in camel sausages fermented by only TCC and beef sausages fermented by TCC+L. Lactis. The highest value was in beef sausages fermented by L. lactis. Generally, camel sausages had lower TBAR values than beef sausages and this could be ascribed to the higher intramuscular fat content in beef which may facilitate lipid oxidation (Soltanizadeh et al., 2010). Similar findings were reported by (Kargozari et al., 2014) where the beef sausages were more susceptible to lipid oxidation than camel sausages, although the values of TBAR reported in the current study are slightly higher than those reported in their results.
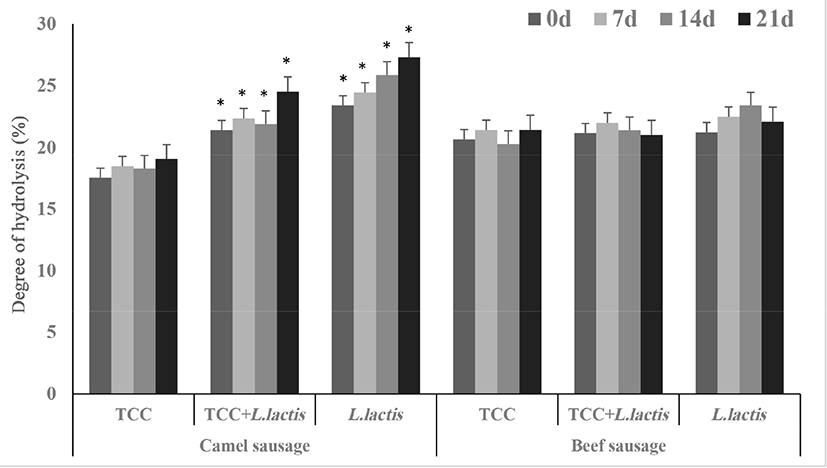
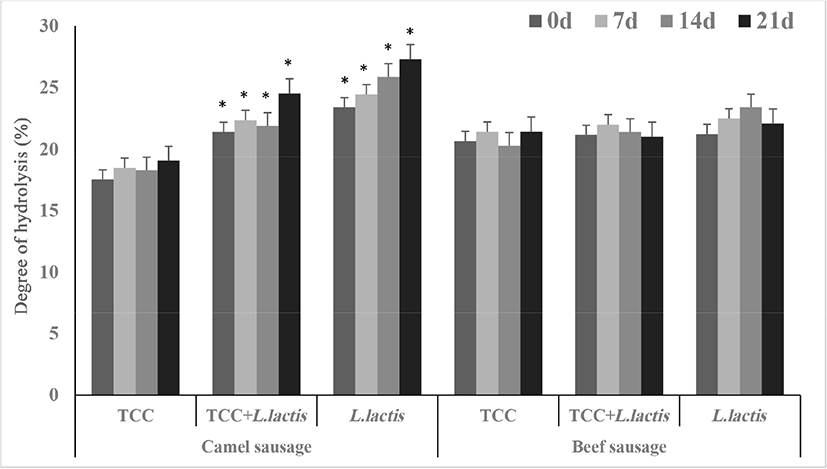
The DH (%) in fermented camel sausages gradually increased (p<0.05) during storage and the values were in the following order: camel sausage fermented by TCC<TCC+L. lactis<only L. lactis. Furthermore, these values were significantly higher (p<0.05) than DH% in fermented beef sausages, which remained stable during the storage period (Fig. 2). It has been reported that myofibril hydrolysis in camel semitendinosus at cold temperatures occurs in greater magnitude compared to that in beef during storage (Soltanizadeh et al., 2008). The DH (%) values were higher than those (16%) for fermented Petrovac sausages after 90 d (Vaštag et al., 2010). Enzyme activities of the microbial cultures used in sausage fermentation as well as the endogenous proteinases in meat play a critical role in the proteolysis process and liberation of free amino acids, which has a strong impact on the health-promoting benefits and physicochemical characteristics of the fermented sausages (Khan et al., 2011). In the current study, it is likely that the increase in DH% of camel sausages may be partly due to the camel meat endogenous proteinases since there was no significant effect of the bacterial culture on beef sausages (Leroy et al., 2006). Moreover, camel meat proteins could be more susceptible to hydrolytic enzymes produced by current probiotics compared to beef proteins.
The texture profile analysis (TPA) analysis of fermented camel or beef sausages is presented in Table 3. Camel or beef sausages became more (p<0.05) hard, gummy, and chewy during storage and this could be as a result of moisture reduction during storage. Hardness of camel sausages were lower (p<0.05) than beef sausages (Table 3) and this may be attributed to the high proteolysis that occurred in camel sausages. Benito et al. (2005) found that a fungal protease enzyme stimulated proteolysis, and consequently reduced the hardness of dry fermented sausages. The chewiness of beef sausages was higher by 2–4 folds than camel sausages. Springiness and adhesiveness values significantly increased in beef sausages during storage, while these values remained constant or reduced for camel sausages; however, the adhesiveness of camel sausages fermented with L. lactis increased by 3.5 folds and this could be attributed to the exopolysaccharides produced by the probiotic strain. In contrast, cohesiveness significantly decreased during storage of beef sausages. Similar results were obtained by Kargozari et al. (2014).
The DPPH (A) and ABTS (B) results are presented in Fig. 3. In general, the ABTS and DPPH scavenging activities in fermented camel sausages were greater (p<0.05) than in beef sausages. Overall, DPPH and ABTS scavenging activities in camel sausages increased (p<0.05) during storage except in camel sausages fermented with TCC+L. lactis which remained constant. Considering the effect of starter culture on antioxidant capacity, it is worth mentioning that the novel probiotic used in the current study showed comparable antioxidant activities to the commercial culture (Fig. 3).
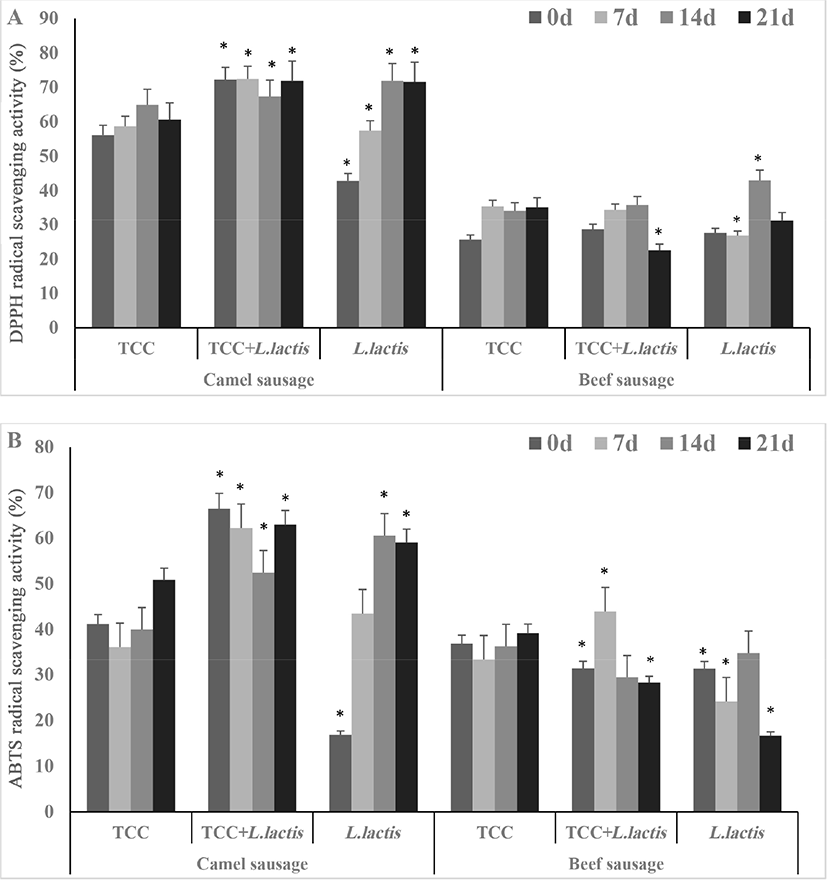
The reactive oxygen species (ROS) including superoxide (•O2-, •OOH), hydroxyl (•OH), and peroxyl (ROO•) radicals are produced by living organisms during normal cellular metabolism and their production is influenced by environmental stresses (Birben et al., 2012). It has been well established that bioactive compounds such as protein-derived peptides have a major role in reducing the effect of these compounds in food products by neutralizing the free radicals (Leroy et al., 2006). The higher antioxidant capacity of camel sausages fermented with different starter cultures may be accredited to the magnitude of their proteolytic rate (DH, Fig. 2) and/or to the characteristics of the released peptides. Similarly, Mejri et al. (2017a) found that camel sausages had high antioxidant capacity due to peptides with molecular weight below 3 kDa released by proteolysis. In contrast, Sun et al. (2009) found that the DPPH radical scavenging activity reached 92% in fermented pork sausages which is slightly higher than that reported in the current study with camel sausages (70%). Furthermore, DPPH and ABTS inhibitions positively correlated with DH% in fermented camel sausages (Table S1). This correlation was also reported by Vaštag et al. (2010).
α-Amylase (A) and α-glucosidase (B) inhibition activity is presented in Fig. 4. α-Amylase and α-glucosidase inhibition is efficient in managing diabetes by reducing carbohydrate hydrolysis (Ayyash et al., 2018b). The fermented camel sausages showed lower α-glucosidase and similar α-amylase inhibition activity than the beef sausages. The difference in the nature of the released peptides in beef and camel meat explains why camel sausages showed lower α-glucosidase and similar α-amylase inhibition activity as beef sausages. α-Amylase and α-glucosidase inhibition activity correlated positively with DH% in camel sausages (Table S1). Remarkably, sausages fermented by either L. lactis or TCC+L. lactis showed higher (p<0.05) α-glucosidase and α-amylase inhibition compared to those fermented with the commercial culture (TCC; Fig. 4). This could be ascribed to the bioactive peptides, especially the small ones (da Cruz et al., 2009), released as a result of hydrolytic enzymes secreted by L. lactis. In general, the inhibition activities of α-amylase and α-glucosidase increased (p<0.05) by the end of storage with the exception of α-amylase inhibition in camel sausages fermented with the commercial culture where the values remained constant at d 21. It is evident that the probiotic L. lactis added value, in terms of antidiabetic potential, to the fermented sausages.
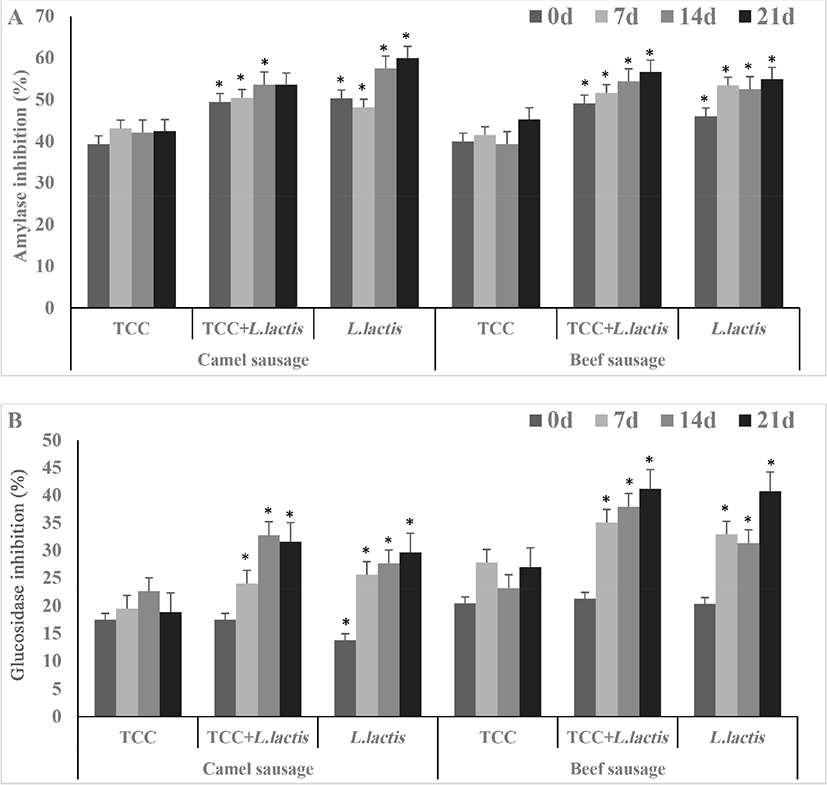
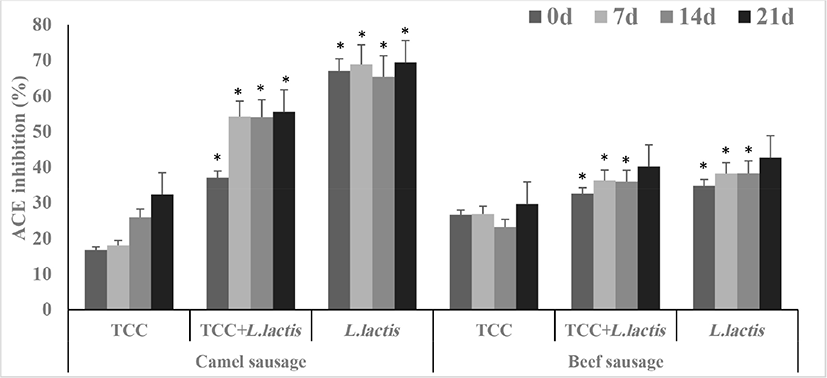
Fig. 5 displays the ACE-inhibitory activity in fermented camel or beef sausages. ACE-inhibitory activity could be used as a barometer for antihypertensive feature of functional products (Gobbetti et al., 2004). In general, ACE-inhibition in fermented camel or beef sausages increased significantly during the storage period. Further, the ACE-inhibitory activity in fermented camel sausages were greater by up to 2-folds (p<0.05) than in beef sausages. It is worth mentioning that camel sausages fermented by L. lactis had the highest value of ACE-inhibition. Nonetheless, ACE-inhibitory activity in camel sausages fermented by either L. lactis or TCC+L. lactis were higher than those fermented by only TCC. It is apparent that meat type and bacterial culture affect the ACE-inhibitory activity. The proteolytic rate and the characteristics of the released peptides in the camel sausages could cause higher ACE-inhibitory activity. Flores and Toldra (2011) reported that the microorganisms, including LAB, generate oligopeptides and free amino acids that inhibit ACE. Fernández et al. (2016) also found that ACE-inhibitory activity in dry fermented sausages “salchichón” is influenced by the starter culture strain. Mejri et al. (2017a) have reported high ACE-inhibitory activity of small peptides of <3 kDa molecular weight released during camel sausage fermentation. The ACE-inhibitory activity of fermented camel sausages by the probiotic L. lactis reported in the current study were higher than ACE-inhibition results previously reported, which suggests that this probiotic enhanced the functional properties of fermented camel sausages. This phenomenon was also observed in fermented beef sausages, which confirms the importance of the starter culture used. ACE-inhibitory activity had positive correlation with DH% in fermented camel (r=0.372) and beef (r=0.430) sausages. Furthermore, α-amylase inhibition had a strong positive correlation with ACE-inhibitory activity in fermented camel (r=0.726) and beef (r=0.650) sausages (Table S1).
The cytotoxicity against Caco-2 (A) and MCF-7 (B) cell lines are presented in Fig. 6. The cytotoxic activity against Caco-2 cell line increased (p<0.05) up to 55%–70% in camel sausages and up to 40-50% in beef sausages, fermented with either L. lactis or TCC+L. Lactis, after 14 to 21 d of storage. Further, camel sausage fermented with only TCC increased up to 32% (Fig. 6A). In general, the cytotoxic activity against colon-cancer cell (Caco-2) of fermented camel sausages were greater (p<0.05) than beef sausages. By d 21, the cytotoxic activity against breast-cancer cell line (MCF-7) in camel sausages fermented by L. lactis (Fig. 6B) was higher (p<0.05) compared to other camel or beef sausages. Moreover, beef sausages fermented by L. lactis+TCC exhibited lower cytotoxic activity against breast-cancer cell line (MCF-7) than its counterpart camel sausages. Overall, cytotoxic activity rose (p<0.05) along with storage time.
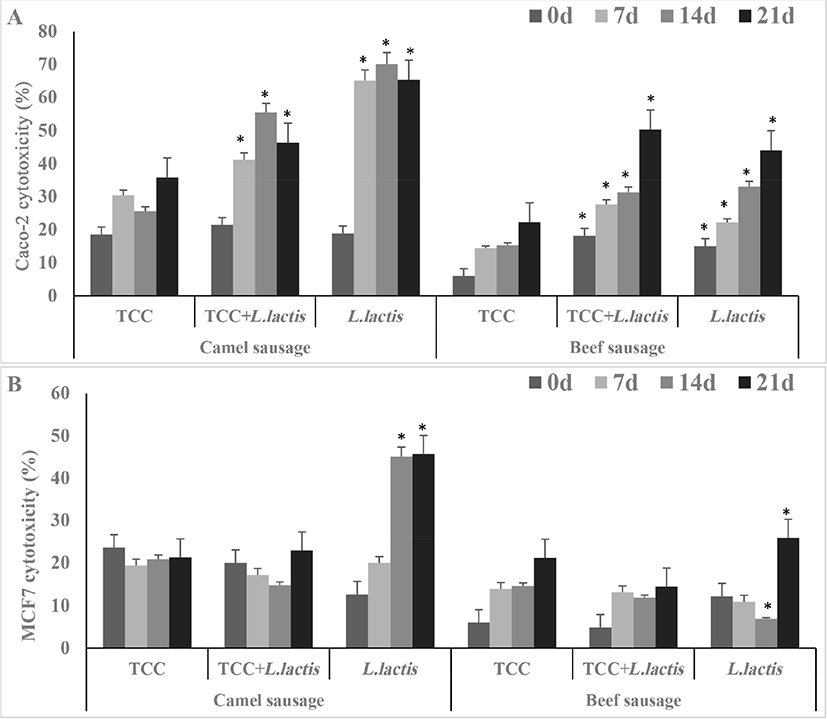
Bioactive peptides with anticancer properties might be used as cytotoxic agents (directly) or as carriers for cytotoxic agents (indirectly) (Cicero et al., 2017). It has been reported that the anticancer activity of these peptides is due to their competing ability with cancer growth factors on the receptors of cancer cell-membrane, their ability to induce apoptosis or necrosis in cancer cell; or to their capability to inhibit gene transcription/cell proliferation (Picot et al., 2006).
It is likely that higher cytotoxic activity displayed by camel sausages fermented with L. lactis may be accredited to the released peptides during proteolytic activity of this strain. Quality, quantity and structure of peptides affect their bioactivities and thus their ability to act as anticancer agents (Cicero et al., 2017; Sohaib et al., 2017). Liu et al. (2016) reported that oligopeptides containing 4–16 amino acids (<3 kDa) exhibited bioactivities including antihypertensive, antioxidant, antimicrobial, anticancer, and opioid activity. Pearson’s test revealed positive correlation between the cytotoxic activity against Caco-2 and MCF-7, DH%, inhibition of α-amylase and α-glucosidase and antioxidant activities (Table S1).
Conclusions
Several health-promoting benefits of fermented camel sausages such as ACE-inhibition, cytotoxicity, and α-amylase and α-glucosidase inhibition activity were greater than beef sausages. The new novel probiotic L. lactis provided an additional-value to the fermented sausages. In general, the health-promoting values of the fermented camel sausages were superior compared with the beef sausages. The present study indicated that camel meat might provide better health quality than that of beef meat. The new probiotic L. lactis had comparable industrial characteristics compared to the commercial culture.













