Introduction
Dry aging is an aging technique used to enhance the tenderness and flavor of meat (Kim et al., 2018). It exposes the primal/sub-primal cuts and/or whole carcasses of beef without packaging under controlled temperature, relative humidity (RH), and air flow conditions (Lee et al., 2017). For last decades, the application of dry aging had been limited due to its negative effects on salable yield followed by relatively high price (Lee et al., 2019a). Therefore, it had been in a small niche market and offered mostly in fine restaurants. However, in recent years, the consumption of dry-aged beef has been increasing worldwide, mainly due to the increase in consumer preference for its unique flavor (beefy and brown roasted) (Khan et al., 2016).
However, the direct exposure of meat during the dry aging process raises consumer concern over potential microbial contamination in dry-aged beef more than in wet-aged meat (Lee et al., 2017). According to previous studies, total aerobic bacterial count (TAB) was significantly higher in dry-aged beef when compared to that in wet-aged beef after 19 d of aging (Li et al., 2014). Degeer et al. (2009) also reported the increase in TAB of dry-aged beef from 6.6 to 9.4 Log CFU/g after 28 d of dry aging (p<0.05). Furthermore, significant growth of mold and yeast has been reported in dry-aged beef (Lee et al., 2018; Ryu et al., 2018). Changes in microbial growth are critical during the dry-aging process in that they can affect the initial numbers of microorganisms at the beginning of storage. Such changes may increase the risk of microbial contamination and meat spoilage, resulting in the deterioration of quality in dry-aged beef (Dashdorj et al., 2016). Therefore, the importance of microbial control during the storage of dry-aged beef has been reported by many researchers (Campbell et al., 2011; Choe et al., 2018; Dashdorj et al., 2016). The microbial quality of the outer surface of dry-aged beef is not directly incorporated into the expectation of quality or shelf-life of the products because the edible part of dry-aged beef is usually prepared after excising and trimming the crust completely. Therefore, the edible portion of internal dry-aged beef is less affected by outer surface microorganisms, contrary to consumer concerns. Instead it has been reported that mold and yeast produced in dry-aged beef were more impactful on flavor production of meat (Lee et al., 2019b). However, control of dry-aged beef remains important in the meat industry because microbial growth was higher in dry-aged beef than in wet/vacuum-aged beef.
Microbial control during storage can be attributed mainly to the packaging system (e.g., vacuum, wrap, and modified atmospheric packaging) (Lambert et al., 1991). Among them, vacuum packaging is likely the most effective in inhibiting microorganisms during storage as it eliminates air, which is an important factor in microbial growth. In addition, this system can retard lipid oxidation in meat during the storage period due to oxygen depletion (Mielnik et al., 2006). In consequence, vacuum packaging is widely used for improving the shelf-life of meat and meat products. Recently, consumption of dry-aged beef has been increasing worldwide due to its unique flavor. Despite this increase in consumption, information regarding the shelf-life of dry-aged beef is limited and guidelines and regulations are lacking. Therefore, the objectives of this study were to investigate the changes in microbial growth and physicochemical and sensory properties of vacuum-packaged dry-aged beef and to assess its storage stability at 4°C.
Materials and Methods
A total of nine strip sirloins were taken from nine beef carcasses (Holstein, quality grade 3; Jo et al., 2012) on three different slaughter days (three sirloins/trial) and dry aged in a dry aging cooler for 28 d (temperature, 4°C; RH, approximately 75%; air flow, 2.5 m/s). After the completion of dry aging, the crusts were trimmed off of the samples and the sirloins were cut (12.7×7.6×2.54 cm3, length×width×height) for packaging. Then the samples were vacuum packaged in polyethylene bags (O2 permeability, 2.3 mL/m2/d at 38°C) and stored at refrigeration temperature (4°C) for 21 d. During the 21 d of storage, vacuum-packaged dry-aged beef was obtained at 0, 7, 14, and 21 d for further analysis.
Five grams of dry-aged beef was blended with 45 mL of 0.85% saline solution for 2 min using a laboratory stomacher (BagMixer® 400, Interscience Ind., St. Nom, France). One-hundred microliters from each sample dilution was spread on the surface of agar plates. TAB, mold/yeast, and lactic acid bacteria (LAB) were enumerated using plate count agar (Difco Laboratories, Detroit, MI, USA), yeast mold agar (Difco Laboratories), and de Man Rogosa and Sharpe agar (MRS, Difco Laboratories), respectively. After spreading the dilution on the agar, the agar plates for TAB and LAB were incubated at 37°C for 48 h and yeast mold agar plates were incubated at 25°C for 120 h, respectively. The number of colonies was enumerated and expressed as Log CFU/g.
Each beef sample (1 g) was homogenized with 9 mL of distilled deionized water (DDW) for 30 s (T10 basic, Ika Works, Staufen, Germany). The homogenates were centrifuged (Continent 512R, Hanil Co., Ltd., Incheon, Korea) at 2,265×g for 10 min. After centrifugation, each supernatant was filtered through filter paper (No. 4, Whatman PLC., Kent, UK) and each filtrate was measured using a pH meter (SevenGo, Mettler-Toledo International Inc., Schwerzenbach, Switzerland) after calibration with standard buffers.
Three grams of each treatment sample was homogenized for 30 s (T25, Ika Works) followed by centrifugation (Continent 512R, Hanil Co., Ltd.) at 2,265×g for 10 min and filtration through filter paper (Whatman No. 1, Whatman PLC). One hundred microliters of each sample with 0.01 N boric acid and indicator solution [0.66% methyl red in ethanol:0.66% bromocresol green in ethanol=1:1 (v/v)] was placed individually in the inner section of a conway (Sibata Ltd., Sitama, Japan); then, 1 mL of sample and 50% potassium carbonate was added into the outer section of the conway, after which the lid was sealed immediately. Then, the conway was incubated at 37°C for 1 h and titrated with 0.01 N hydrogen chloride. The VBN value was calculated as follows:
Lipid oxidation was measured for the TBARS value using a spectrophotometer (X-ma 3100, Human Co. Ltd., Seoul, Korea). Five grams of each sample was homogenized with 15 mL of DDW and 7.2% butylated hydroxyl toluene in ethanol for 30 s (T25, Ika Works). After homogenization, 2 mL of the homogenates was transferred to 15 mL Falcon® tubes and 4 mL of 20 mM 2-thiobarbituric acid in 15% trichloroacetic acid was added. The tubes were heated in a laboratory water bath at 90°C for 30 min, cooled, and centrifuged at 2,265×g for 15 min (HM-150IV, Hanil Co., Ltd.). The absorbance of the supernatant was measured at 532 nm. The TBARS value was expressed as mg malondialdehyde (MDA)/kg of meat sample.
After cutting and opening the package and allowing the beef to bloom for 30 min, lightness, redness, and yellowness of the meat were measured and expressed as CIE L*, a*, b* values, respectively, using a spectrophotometer (CM-5, Konica Minolta Censing Inc., Osaka, Japan). The colorimeter was calibrated using a standard white and black plate before each measurement. Color difference (△E) was calculated as follows:
where L*ref, a*ref, and b*ref represents lightness, redness, and yellowness in vacuum-packaged dry-aged beef at day 0, respectively.
For Mb content and the composition of its related pigments, deoxymyoglobin (DeoxyMb), oxymyoglobin (OxyMb), and metmyoglobin (MetMb) were analyzed following the methods of Krzywicki (1979). Mb was extracted from 4 g beef samples with 20 mL of 0.4 M phosphate buffer (pH 6.8). Each sample was homogenized (T10 basic, Ika Works) for 30 s and the homogenates were stabilized for 1 h at refrigeration conditions (4°C) with foil. After allowing to stand, the samples were centrifuged (Combi 514R, Hanil Co., Ltd.) at 5,000×g for 30 min. The filtrates were filtered with filter paper (Whatman No. 1, Whatman PLC) and the absorbance of the supernatant was measured at 525, 572, and 700 nm using a spectrophotometer (X-ma 3100, Human Co. Ltd.).
Texture profile (hardness, adhesiveness, springiness, chewiness, and cohesiveness) was analyzed with a texture analyzer (TA1, Lloyd Instruments Ltd., Fareham, UK). Ten grams of ground sample was placed into a petri dish (35×10 mm2), cooked in a laboratory water bath at 85°C for 15 min, and cooled. The conditions of the texture analyzer were set as follows: pre-load speed 10 mm/min, post-load speed 2 mm/s, maximum cell load 50 kg, compression level 60%.
Sensory evaluation was conducted with nine consumer panelists to determine the sensory properties of vacuum-packaged dry-aged beef during 21 d of storage (IRB no. 1810/003-001). There were three independent sensory tests for each storage day. The samples were cut into pieces of the same size (4×2×2.54 cm3) and grilled until the core temperature reached 72°C. Sensory analysis was evaluated with a 9-point hedonic scale (1, extremely dislike; 9, extremely like) and scored for appearance, odor, taste, tenderness, juiciness, and overall acceptability of beef.
All experiments were conducted in triplicate and averaged (n=3). Vacuum-packaged dry-aged beef samples at different storage days (0, 7, 14, and 21 d) were analyzed in each trial. A generalized linear model was used to perform the analysis using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and results were reported as mean values with SEM. Significant differences among the mean values were determined on the basis of Tukey’s multiple comparison test at a significance level of p<0.05.
Results and Discussion
Microbial growth of meat depends on all environmental conditions during the slaughter and aging process, ultimately impacting meat spoilage and quality deterioration (Nychas et al., 2008). Therefore, the control of microbial growth (especially TAB) is important in meat during storage. In the Korean market, TAB count in meat and meat products is limited to <6 Log CFU/g at the point of consumption (MFDS, 2018). In the present study, the initial numbers of TAB, LAB, mold, and yeast in vacuum-packaged dry-aged beef were 4.4, 2.4, 3.6, and 5.9 Log CFU/g, respectively (Fig. 1). During 21 d of storage, TAB count steadily increased and exceeded the legal standard at day 14 (6.5 Log CFU/g). Therefore, the shelf-life of vacuum-packaged dry-aged beef may be limited to less than 14 d of storage based on TAB count. Furthermore, the regression equation (r2=0.99) for vacuum-packaged dry-aged beef based on the quality limit of TBA revealed that the shelf-life for dry-aged beef could possibly reach 11 d (data not shown) with vacuum packaging during refrigerated storage. Regarding important information for producers and sellers, vacuum-packaged dry-aged beef after completion of aging could be stored and sold within 11 d.
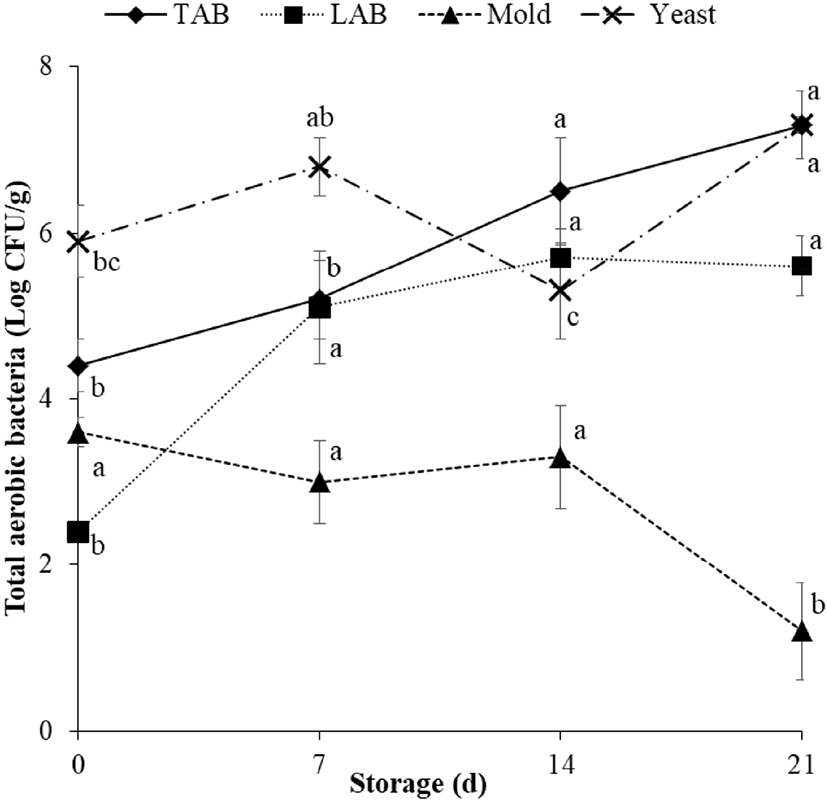
LAB count in vacuum-packaged dry-aged beef also increased over the first 7 d; these levels were maintained thereafter. However, given that the growth of LAB did not affect meat spoilage during storage in both vacuum- and wrap-packaged beef (Lee et al., 2018; Nychas et al., 2008), it was not considered a factor in the quality deterioration category. While mold count decreased significantly between days 14 and 21, possibly due to the depletion of oxygen—an element crucial for its growth (Kemp et al., 1983), yeast count fluctuated and reached its highest level at day 21 (Fig. 1). As the detection of mold and yeast is generally scarce in most meat and meat products, no recommendations for acceptable mold and yeast levels during storage are available. However, as the presence of mold and yeast has been consistently reported in dry-aged beef (Kim et al., 2018; Lee et al., 2018; Ryu et al., 2018), their impact on meat quality is currently being studied.
pH and VBN have been used to evaluate meat freshness/spoilage during storage because these indicators have been closely associated with microbial growth (Byun et al., 2003). During storage, the generation of protein-derived basic products (VBN including amine and/or ammonia) by the proteolysis of microorganisms can cause increases in pH as well as VBN content in meat (Byun et al., 2003; Lee et al., 2018). In contrast, decreases in pH can be caused mainly by the generation of lactic acid by LAB growth (Dave and Ghaly, 2011). According to Lee et al. (2018), the quality limits of pH and VBN in Korea are 6.2 and 20 mg/100 g, respectively, for fresh meat. In this study, the highest pH value was 5.69 occurring at day 14 and decreasing thereafter (Table 1). In contrast, the VBN content of vacuum-packaged dry-aged beef did not change significantly until 14 d of storage; however, thereafter, it increased and exceeded its recommended level for fresh meat (20 mg/100 g) at day 21. Consequently, the re-establishment of spoilage indicators for both dry- and wet-aged beef is necessary (Jang et al., 2014; Lee et al., 2018). Based on current recommendations, vacuum-packaged dry-aged beef may be considered fresh until day 14 at 4°C.
Lipid oxidation in meat is a very important factor, as it can cause quality deterioration (e.g., in color, flavor, texture, and nutritive value) in meat and meat products (Kim et al., 2018). It can be measured by the TBARS value and tends to increase during storage. However, in the present study, the TBARS value decreased significantly after 14 d of storage (Table 1), possibly as a result of excessive microbial growth in the vacuum-packaged dry-aged beef during that period (Fig. 1). According to Branen et al. (1978), the reaction of MDA and 2-thiobarbituric acid can be inhibited by protein-derived amines generated by microbial growth. Similarly, the TBARS value of raw pork decreased during storage (Kim et al., 2004). An et al. (2017) also reported a decrease in TBARS value in frozen pork over 7 d of storage with the degradation of MDA by microbial growth. Given that the initial TBARS value was high and did not increase significantly, it could not be considered to represent quality deterioration of vacuum-packaged dry-aged beef during storage. Therefore, the TBARS value is not a relevant metric impacting the shelf-life of vacuum-packaged dry-aged beef; this finding is consistent with Lee et al. (2018), who reported no correlation between the TBARS value and quality attributes of dry-aged beef. However, further investigation of pH, VBN, and lipid oxidation may be necessary to clarify their changes in dry-aged beef during storage.
Meat color can affect acceptability by consumers when they purchase meat and meat products at the market (Yong et al., 2018). CIE L*, a*, and b* values are used to measure meat color; among them, CIE a* may be important to consumers as it determines the redness of meat, which confers freshness at the market. Meat color is attributed to the chemical status of Mb (OxyMb, bright red color; MetMb, brown color; DeoxyMb, purple color) (Yong et al., 2018). Therefore, changes in Mb content is a main determinant for color stability during storage. Meat color and Mb content of vacuum-packaged dry-aged beef are shown in Tables 1 and 2, respectively. In the present study, significant increases in the composition of OxyMb was found between days 7 and 14, which was not expected, especially in the middle of vacuum packaging, as the generation of OxyMb is attributed exclusively to oxygen binding (Mancini and Hunt, 2005). However, Lee et al. (2018) also reported a sudden increase in OxyMb composition in wrap-packaged dry-aged beef at day 3. Hence, regardless of packaging methods, the composition of OxyMb may change during the storage of dry-aged beef based on unknown factors that require further investigation of the chemical changes of myoglobin in dry-aged beef. In contrast, OxyMb composition in vacuum-packaged dry-aged beef decreased (p<0.05) after day 14 of storage, possibly via its oxidation to MetMb with a decrease in pH. Lower pH at day 21 may promote the oxidation of OxyMb to increase the content of MetMb (Faustman et al., 2010).
During the storage of vacuum-packaged dry-aged beef, CIE L* increased significantly at day 7 and decreased thereafter, whereas CIE a* and b* increased significantly between days 7 and 14 and then decreased (Table 1). The change in CIE L* may be related to microbial growth, especially TAB (Robach and Costilow, 1961). Meanwhile, the change in CIE a* could be affected by OxyMb content (Table 2), which reached its highest value at day 14 and exhibited a similar tendency with CIE a*. Moreover, as CIE b* is positively correlated to CIE a*, the tendency of CIE b* in vacuum-packaged dry-aged beef during storage was similar to that of CIE a*. In addition, higher pH can contribute to the darker, redder, and more yellow color of meat by the increase in water holding capacity (Allen et al., 1997). In this study, the highest pH value of vacuum-packaged dry-aged beef at day 14 correlated with high CIE a* and CIE b* on day 14 of storage.
Total color difference (△E) of vacuum-packaged dry-aged beef did not change between day 7 and 14 but did change between day 7 and 21 (p<0.05). However, as the CIE a* of vacuum-packaged dry-aged beef reached its highest at day 14, the acceptability to the consumer may also be higher on this day when compared to the others.
Texture profile analysis (i.e., hardness, springiness, chewiness, and cohesiveness) is useful to predict the sensory texture of cooked meat and adhesiveness can reveal texture defects like slime (Perez-Santaescolastica et al., 2018). In this study, the adhesiveness of vacuum-packaged dry-aged beef did not change significantly during 21 d of storage (Table 2), signifying that deterioration in texture was not observed in vacuum-packaged dry-aged beef during storage. In contrast, the values of other parameters decreased significantly at day seven, possibly due to protein degradation by microbial growth during storage (Fig. 1). Texture was maintained thereafter, except for hardness and springiness (first and second bites of hardness; De Huidobro et al., 2005) (Table 2). Hardness decreased at day 7 (p<0.05), similar to the other parameters; however, it increased slightly but significantly at day 14, whereas springiness decreased only at day 21 (p<0.05). Considering all of the results from texture profile analysis, we assumed that the texture of vacuum-packaged dry-aged beef may not be substantially different after 7 d of storage.
In our previous study of wrap-packaged dry-aged beef, appearance and odor did not change (p<0.05), whereas taste and overall acceptability significantly decreased at day 7 (Lee et al., 2018). In the present study, sensory properties (appearance, odor, taste, tenderness, juiciness, and overall acceptability) of vacuum-packaged dry-aged beef were evaluated at 7-d interval over 21 d (Table 3). All parameters showed no significant changes throughout the entire storage period, except for juiciness and overall acceptability (p<0.05). Juiciness decreased significantly at day 14, while overall acceptability did not change until 14 d of storage, significantly decreased thereafter.
Taken the results together from the present study, vacuum-packaged dry-aged beef could be stored for 11 d at 4°C without any adverse effect on its microbial and sensory quality.













