Introduction
Sous-vide (SV) cooking generally uses a vacuum packaging machine and precisely thermo-controlled water bath to provide efficient and uniform heat penetration into food products (Park et al., 2020). It is considered one of the suitable methods for home-meal replacements, since SV cooking has several benefits, including extending shelf life, enhancing product yield, and preventing the nutrient loss (Baldwin, 2012). However, due to the lower cooking temperature, meat cooked with SV method exhibited a less developed brown color and flavor compared to meat cooked with conventional method (Park et al., 2020). These results were associated with lack of Maillard reaction (MR) development, since the MR products related to desired flavor increase with increasing cooking temperatures (Cho et al., 2021). Therefore, additional treatments, such as searing and marination, are necessary to compensate for the drawbacks of SV cooking.
Recently, since consumers strongly believe that foods can directly contribute to their health, they are becoming more interested in healthier food products that use natural additives (Choi et al., 2012). Thus, the food industry is employing plant-based products, which contain various antioxidant compounds, such as carotenoid and flavonoid, as natural ingredients (Kim et al., 2009). The medicinal plant extracts with a higher amount of antioxidant not only can improve the functionality of meat products, but also inhibit the deterioration of food quality by preventing lipid oxidation during processing and storage (Pompella et al., 2014). Additionally, herbal medicinal extracts can be used as a flavoring agent for the development of meat products due to their specific flavors (Aminzare et al., 2019).
Astragalus membranaceus (AM; called as Hwanggi), Adenophora triphylla (AT; called as Jandae), and Ulmus pumila (UP; called as Ugeunpi), which are widely distributed throughout the world, and are used as herbal medicines in Asian countries for liver cirrhosis, chronic bronchitis, and inflammation, respectively (Kim et al., 2009; Sun et al., 2007; Zhou et al., 2017). Also, these herbal medicines commonly contain greater polyphenolic compounds as like blueberry and rosemary, which have antioxidant properties (Kim et al., 2009; Li et al., 2014; Zhou et al., 2017). Thus, AM, AT, and UP extracts can be used an ingredient for improving the quality and shelf life of meat products. Therefore, to improve usability by enhancing the quality of SV chicken breast, this study investigated the effects of AM, AT, and UP extracts on the quality traits, palatability, and storage stability of SV cooked chicken meat.
Materials and Methods
The roots of AM, AT, and UP were purchased from a local medicinal plant market (Geumsan, Chungcheongnam-do, Korea). At 400 g in 10 L of water, each herbal medicine was used and boiled at 100°C for 2 h to obtain the extract. Extracts from the three herbs were poured into plastic containers and stored at 4°C until the marinades were prepared.
A total of 123 fresh boneless and skinless fresh chicken breasts were purchased from a local retail market. All the chicken breasts belong to the normal quality condition according to the chicken quality classification (Park et al., 2020). The samples were randomly assigned into 1 of 4 groups, the control and 3 herbal medicinal extract (AM, AT, and UP) groups. The control group was immersed in water at the meat-to-fresh water ratio of 1:2 without any addition of plant extracts, and the three experiment groups were marinated in AM, AT, or UP extracts at a ratio of 1:2 (meat:extract) for 1 h.
All the samples were weighed, put into a polyethylene pouch, and vacuumed using a vacuum packaging machine (Leepack, Hanguk Electronic, Incheon, Korea). Samples were then cooked in a circulating thermostatic water bath at 60°C for 2 h, the optimal condition for chicken breast cooked SV (Park et al., 2020). All the SV samples were cooled in an ice-slurry until equilibration; then, the quality traits were immediately examined using 24 samples. A total of 24 samples (6 samples per treatment) were stored at −20°C for the assessments of visual attributes and sensory quality traits. The contents of polyphenols and flavonoids were analyzed using 27 chicken breasts (9 samples per treatment without control group). The remaining 48 samples were stored at 4°C to measure storage stability during a cold storage (period from 0 to 14 d).
The pH of SV cooked samples was determined using a Testo 206-pH2 (Testo AG, Lenzkirch, Germany) with a penetration probe. Color parameters, including lightness (L*), redness (a*), and yellowness (b*), were measured using a colorimeter (CR-400, Minolta Camera, Osaka, Japan) at the surface and inner regions of SV samples according to the recommendations of the Commission Internationale de l’Eclairage (1978). Hue angle [tan–1 (b* / a*)] and saturation index [(b*2 + a*2)0.5] at the same regions were calculated. Cooking loss and Warner-Bratzler shear force (WBS) of each sample were measured based on a previous publication (Honikel, 1998). Samples were weighed before and after SV cooking to calculate the percentage of cooking loss. After measuring cooking loss, more than six core samples (1.27 cm diameter) were obtained parallel to the muscle fiber orientation for WBS measurement. The WBS value was collected using an Intron Universal Testing Machine (Model 1011, Instron, Canton, MA, USA) with the Warner-Bratzler blade (crosshead speed, 200 mm/min).
For analyses of visual attributes and eating quality characteristics, a total of 24 samples were randomly coded with a 3-digit number and used during four sessions (6 samples per session). Before each session, the frozen SV samples were thawed at 4°C overnight, then heated to and maintained at a core temperature of 54°C in a water bath until further analyses. All the panelists (6 women and 5 men aged 23 to 48) were trained according to the previous procedures (American Meat Science Association, 1995; Meilgaard et al., 1991), and evaluated the visual attributes and sensory quality characteristics of SV breasts to use a hedonic scale (1 to 9). Training of the panelists and sensory evaluations were conducted at the Kyungpook National University. Visual attributes, including color, moisture, appearance, and overall acceptability, were evaluated. A total of 11 eating quality attributes, including initial tenderness, rate of breakdown, amount of perceptible residue, juiciness, flavor intensity, off-flavor intensity, treatment flavor acceptability, sweetness, sourness, bitterness, and overall acceptability were assessed.
A total of 27 SV cooked samples (3 treatments×3 samples×3 repetitions) were used to measure the polyphenol and flavonoid contents. One gram of SV sample was homogenized, and extracted using 10 mL of 70% ethanol (v/v) and methanol (v/v) solutions for the assessment of polyphenol and flavonoid contents, respectively. For the determination of total polyphenol content by the Folin-Ciocalteu procedure, the method described by Singleton and Rossi (1965). The flavonoid content for each sample was evaluated according to the method described by Song et al. (2014), with some modifications. The results of both polyphenol and flavonoid contents were expressed as mg/100 g of experiment sample.
The levels of lipid oxidation in the SV cooked breasts during storage at 4°C was assessed by measuring the thiobarbituric acid-reactive substances (TBARS) according to the method described by Buege and Aust (1978) and Cho et al. (2020). A total of 48 samples (16 samples per each repetition) were used on 0, 3, 7, and 14 d as three repetitions. The TBARS values were expressed as milligrams of malondialdehyde (MDA) per kg of SV sample.
The general linear model in SAS software (SAS Institute, Cary, NC, USA) was performed to compare the quality traits, visual attributes, palatability characteristics, and storage stability, including levels of polyphenol, flavonoid, and TBARS, among the SV cooked chicken breasts with different herbal medicinal extracts. A linear mixed model was used to identify the factors influencing the quality traits, visual attributes, palatability, and polyphenol and flavonoid contents. In the model, the fixed effect commonly included the herbal extracts, and the random effects included the number of experimental repetitions and panelists. A linear mixed model was also used to compare the TBARS values of the SV cooked meats among the groups, with the extracts and storage periods as the fixed effects and repetitions as the random effects. Significant differences among the groups were determined by the probability difference at 5%. All the data were presented as the least-squares means with standard errors.
Results
Meat quality characteristics among the SV cooked chicken breasts marinated with different herbal medicinal extracts were compared (Table 1). No difference was observed in pH between the control and herbal extract groups (p>0.05). While all the groups displayed a similar redness value on the surface region (p>0.05), the SV samples with UP extract showed the lowest lightness and highest yellowness values compared to the SV samples with other extracts and samples without extracts (p<0.05). A similar hue angle was observed among the groups (p>0.05), and the UP group exhibited a higher saturation index compared to the other groups (p<0.05). In the inner region of SV cooked breasts, there were no differences in any of the color parameters among the groups (p>0.05). On the other hand, the control group exhibited a lower cooking loss compared to the AT group (17.4% vs. 18.5%, p<0.01), and showed a similar loss compared to the AM and UP groups (p>0.05). No difference was detected in WBS value among the groups (p>0.05).
Comparison of the visual attributes and palatability characteristics among the SV cooked meats with different extracts is shown in Table 2. The AM group exhibited lower color acceptability compared to the other groups (p<0.01), except for the UP group (p>0.05). There was no difference in the moisture intensity and appearance acceptability among the groups (p>0.05). The control group showed similar overall acceptability compared to the other groups (p>0.05), except for the AM group (p<0.01).
A similar score of initial tenderness was observed in the SV cooked meat with AM, AT, and UP extracts (p>0.05), and the AM group had a lower value compared to the control group (7.63 vs. 8.22, p<0.05). No differences were detected in rate of breakdown, amount of perceptible residue, and juiciness between the control and herbal extract groups (p>0.05). SV breast added UP extract showed a higher flavor intensity compared to SV breast added AM extract (6.87 vs. 6.37, p<0.01), and the other herbal treatments had similar scores compared to the control group (p>0.05). In contrast, a marked difference was observed in off-flavor intensity among the groups. The herbal plant extract groups were scored higher than the control group (p<0.001). Similar to the pattern in flavor intensity, the level of treatment flavor acceptability did not differ among the control and herbal extract groups (p>0.05), except for the UP group (p<0.05). While all the groups had similar values of sweetness and sourness (p>0.05), the control group scored higher on bitterness than the herbal extract groups (p<0.001), except for the AT group (p>0.05). The herbal plant treatments, except for the AM group, showed a higher score of overall acceptability compared to the control group (p<0.01).
Contents of total polyphenols and flavonoids among the AM, AT, or UP groups were compared (Fig. 1A). Although there was no significant difference in the total polyphenol content among the groups (p>0.05), a considerable difference was observed in the flavonoids content among the groups (p<0.001). Samples from the UP group had the highest flavonoid content among the groups, and a lower content was observed in the AM group compared to the AT group (1.13 vs. 1.54 mg/100 g, p<0.001).
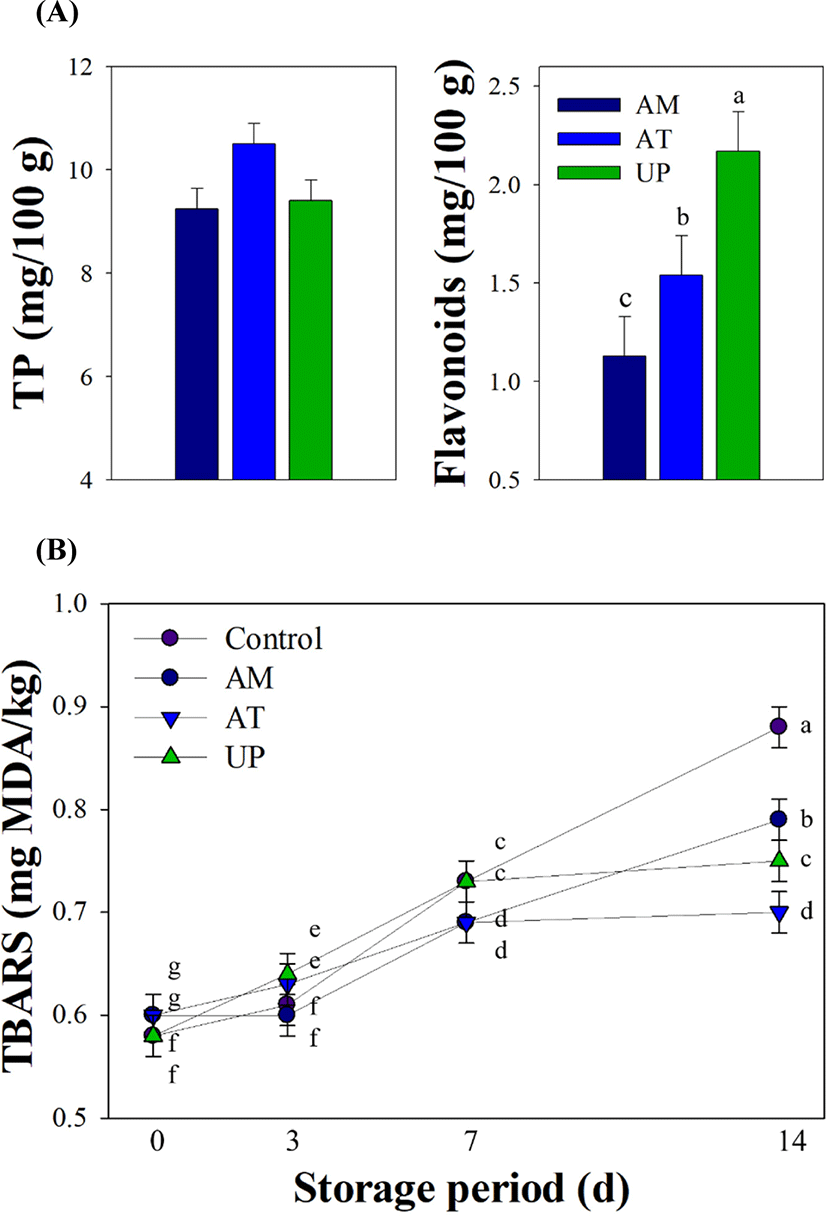
Changes in the TBARS values among the groups during the storage period are shown in Fig. 1B. The TBARS values of all the groups tended to increase during 0 to 14 d of the cold storage (p<0.05). After 7 d of storage, all the groups showed a higher value than that on 0 d (p<0.05). A difference in the increase of TBARS values was observed in the control (0.73 vs. 0.88 mg MDA/kg) or AM (0.69 vs. 0.79 mg MDA/kg) groups between 7 and 14 d of cold storage (p<0.05). However, no differences were observed in the AT (0.69 vs. 0.70 mg MDA/kg) or UP (0.73 vs. 0.75 mg MDA/kg) groups during 7 to 14 d of storage (p>0.05).
Discussion
The herbal plants are widely available and have been considered as a potential source for enhancing food functionality (Radha Krishnan et al., 2014). The extracts from herbal plants have various colors and flavors, which can influence the appearance characteristics of meat products (Jin et al., 2015). For instance, turkey breasts with rosemary extract showed a lower lightness value compared to untreated breasts due to yellowish color of rosemary extract (Yu et al., 2002). However, the raw meat characteristics and cooking methods also influence the color of final meat products. For examples, no differences were observed in the lightness value and appearance acceptability between the untreated beef meatballs and those with rosemary extract due to a darker color of raw beef (Fernández-López et al., 2005). Additionally, Akebia quinata extract did not influence the lightness value and sensory color score of emulsion-type pork sausage (Jin et al., 2015). In this study, the SV chicken breasts with UP extract exhibited darker and yellower color on the surface region compared to the other SV chicken breasts (p<0.05) due to a brown color of UP extract (Kim et al., 2016) and the lighter yellowish color of the other extracts (Li et al., 2015; Ny et al., 2021). However, the addition of UP extract influenced the color acceptability, and the control group was preferred to the UP group (p<0.001). In comparison, there were no differences in moisture, appearance, and overall acceptability between the control and UP groups (p>0.05). Additionally, all the groups did not differ in the cooking loss and WBS value (p>0.05) except for the AT group in cooking loss. Thus, herbal medicinal extracts in this study did not negatively affect the meat quality and visual attributes of SV cooked chicken breasts.
Poultry meat is more susceptible to quality deterioration mainly due to lipid oxidation with resulting off-flavors compared to red meat during storage and processing (Jayasena et al., 2013). Marination using various herbal plant extracts that act as flavoring agents to compensate for the disadvantage by masking the off-flavor can be applied to diverse meat types, especially chicken meat (Embuscado, 2015). In a previous study, the pork patties with cassia bark extract had a lower rancid flavor compared to the patties without extract, although the overall acceptability was similar between the groups (Kong et al., 2010). Addition of 0.02% rosemary extract to ground beef reduced the extent of warmed-over flavor compared to ground beef with distilled water (Ahn et al., 2002). However, herbal plant extracts generally have a limited effect on the other sensory traits, such as tenderness and juiciness, of processed meat products (Hayes et al., 2011; Jin et al., 2015). A similar result was found in this study; the AM, AT, and UP groups did not differ in tenderness attributes and juiciness compared to the control group (p>0.05). UP extract demonstrated specific flavor, sweetness, and bitter taste as assessed by trained panelists (Cho et al., 2016). Consistent with the previous findings, this study found the SV breasts with UP extracts had better flavor and lower off-flavor intensities compared to SV breasts without herbal plant extract (p<0.05). As these results, the trained panelists preferred the SV cooked meat with UP extract compared to the untreated meat (p<0.01), although samples from the UP group tasted more bitter than samples from the other groups (p<0.001) except for the AM group.
Lipid oxidation is well associated with protein oxidation, as the oxidation products of one substance can accelerate the oxidation of another substance (Cai et al., 2021). Thus, to assess storage stability, extents of these two or each oxidation are mainly measured. There is a need to suppress or delay the onset of lipid and protein oxidation in chicken products to increase shelf life. On the other hand, phytochemicals, especially flavonoids and phenolic acids derived from the herbal plant origins, are essential antioxidants due to their ability to scavenge free radicals (Embuscado, 2015). AM, AT, and UP as medicinal plants have also higher amounts of phenolic compounds (Kim et al., 2009; Li et al., 2014; Zhou et al., 2017); thus, these plant extracts can be used as food additives to improve the storage stability of meat products. In this study, the flavonoid contents of SV cooked chicken breasts were highest in the UP group, followed by the AT and AM groups (p<0.001), although the total polyphenol contents were similar among the groups (p>0.05). This finding may explain the previous observation that UP extract had better antioxidant (Im et al., 2017) and immunomodulatory properties (Chang and Woo, 2003) compared to AM and AT extracts, respectively. Due to its high flavonoid content, the UP group also exhibited lower TBARS concentrations compared to those the AM group after 14 d of cold storage (p<0.05). Moreover, adding herbal plant extracts to the SV cooked breasts significantly inhibited the formation of TBARS compared to the control breasts at 14 d of storage (p<0.05).
Conclusion
Taken together, the addition of AM, AT, and UP extracts before SV cooking enhanced the storage stability of chicken breasts during refrigeration without impairing the meat quality traits. In particular, the UP extract improved palatability of the chicken breasts by reducing the off-flavor and increasing the flavor intensities compared to the chicken breasts without plant extract. Therefore, herbal plant extracts, especially the UP extract, can be a good food additive for enhancing the overall quality of SV cooked chicken breasts and improving the utilization of plant extracts.













