Introduction
Egg white ovotransferrin (OTF) is a well-characterized transferrin protein with strong iron-binding properties and is the second major protein in egg white and it account for 12%-13% of protein content (Wu and Acero-Lopez, 2012). OTF is consisted of 686 amino acids and its molecular weight is known as 78 kDa. OTF has two lobes containing each a single iron-binding site (N-lobe: 1-329 amino acids; Clobe: 330-686 amino acids). This is the reason that OTF have a strong iron-binding activity. And this activity gave an antimicrobial activity to OTF by removing iron ion from microbes (Abdallah and Chahine, 1999). Furthermore, OTF has been reported to have antifungal, antioxidant, antiviral, and anticancer activity (Giansanti et al., 2002; Ibrahim et al., 2007; Moon et al., 2013; Valenti et al., 1985). Although recent studies have revealed diverse functional activities of OTF, little is known about its immune-enhancing activity.
The immunomodulators used in enhancing the host defense system have being highlighted in recent years (Zhao et al., 2014). The modulation of immune response is especially important for people who have impaired immune responses such as the elderly and cancer patients, because it plays a crucial role in diseases prevention (Salvioli et al., 2006). Accordingly, immunomodulation induced by active substances have been the focus of many studies (Castro et al., 2008; Hong et al., 2017; Shen et al., 2017). They found new active substances from natural materials which are herb, egg, and grape etc. In these natural materials, polysaccharides, proteins, and resveratrol are separated, and they are reported to have immunomodulating activity.
Macrophages play an important role in both the innate and the adaptive immune system. They exist in the body widely. When the body is attacked by pathological stimuli or is injured, macrophages defend the body through the production of pro-inflammatory chemokines and cytokines which are nitric oxide (NO), interleukin family (IL-1β, IL-6, and IL-12), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) (Wang et al., 2017; Yu et al., 2017). Macrophage activation is an essential process for defending the body against pathogens. When macrophages are activated, they initiate and propagate defensive reactions such as phagocytosis and production of pro-inflammatory mediators (Yu et al., 2017). Macrophage activation is associated with the major signaling pathways such as mitogen activated protein kinase (MAPK) which include p38 MAPK, extracellular signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK) (Han et al., 2009). Therefore, many researchers have been studying new substances with immunomodulatory and immunostimulatory activities that activate macrophages via MAPK pathway (Hong et al., 2017; Li et al., 2017; Seo et al., 2015).
In the current study, the immune-enhancing activity of OTF obtained from hen egg white was investigated by measuring the pro-inflammatory chemokine and cytokine levels in RAW 264.7 macrophages. Furthermore, we assessed whether activation of macrophages induced by OTF is associated with MAPK pathways.
Materials and Methods
The iron-free OTF was prepared using the method of Abeyrathne et al. (2013). Dulbeco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin were purchased from HyClone Laboratories, Inc. (Logan, MI, USA). N-(1-naphthyl)-ethylenediamine, sulfanilamide, thiazolyl blue tetrazolium bromide (MTT), lipopolysaccharides (LPS), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against phospho-p38 (p-p38), total p38 (t-p38), phospho-ERK (p-ERK), total ERK (t-ERK), phospho-JNK (p-JNK), and total JNK (t-JNK) were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Goat anti-mouse IgG-HRP and materials for western blot were obtained from Bio-Rad (Hercules, CA, USA).
RAW 264.7 macrophages were obrained from Korean Cell Line Bank (Seoul, Korea). Macrophages were cultured in DMEM medium supplemented with 10% FBS, penicillin-streptomycin (100 U/mL and 100 μg/mL) at 37℃ in an incubator containing 5% CO2 (MCO-18AIC, SANYO, Osaka, Japan). The culture medium was replaced every 2-3 days.
Cell viability of macrophages against OTF was measured using the MTT assay with slight modifications (Lee et al., 2017). RAW 264.7 macrophages (2×105 cells/well) were added to a 96-well plate and cultured at 37℃ for 4 h. Various concentration of OTF (0–2 mg/mL) were then added to each cell suspension and cultured for 24 h. After then, MTT (2.5 mg/mL) solution dissolved in PBS was added to each well and the plate was incubated at 37℃ for an additional 4 h. The supernatant was then removed from each well and DMSO was added to dissolve the formazan crystals. The absorbance of each solution was then measured using a microplate reader (Model 680, Bio-Rad, Hercules, CA, USA) at 570 nm.
The effects of OTF on NO levels of macrophages were measured using the Griess assay (Chen et al., 2014). RAW 264.7 macrophages (2×105 cells/well) were added to a 96-well plate and cultured at 37℃ for 4 h. Various concentration of OTF (0–2 mg/mL) and LPS (10 ng/mL) were then added to each cell suspension and cultured for 24 h. Lipopolysaccharides was used as a positive control. Griess reagent (mixed with 1% sulfanilamide dissolved in 5% phosphoric acid and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride dissolved in distilled water) were mixed with each well supernatant. Under aerobic conditions, NO is rapidly converted into nitrite, which then produces the azo dye when mixed with Griess reagent. The absorbance was measured using a microplate reader at 540 nm and NO concentration was calculated using standard solutions of sodium nitrate dissolved in DMEM medium.
The effects of OTF on expression levels of cytokine were measured using quantitative real-time PCR (qRT-PCR). RAW 264.7 macrophages (1×106 cells/well) were added to a 6-well plate and cultured at 37℃ for 24 h. Various concentration of OTF (0–2 mg/mL) and LPS (10 ng/mL) were then added to each cell suspension and cultured for another 24 h. RNeasy Mini Kit (Qiagen, Milan, Italy) was used for isolating total RNA from macrophages according to the manufacturer’s instructions and the RevertAid First strand cDNA synthesis Kit (Thermo Fisher Scientific, Carlsbad, CA, USA) was used for synthesis cDNA. The cytokine primer sequences are shown in Table 1 and the SYBR green reagent (PhileKorea, Daejeon, Korea) was used. The cDNA and primer mixture were pre-heated at 95℃ for 2 min, followed by PCR for 40 cycles, using a denaturing temperature 95℃ for 5 s, and annealing/extension temperature 65℃ for 30 s. The ΔΔ Ct method was used for analyzing qRT-PCR results and melting curve analysis was used for assessing the purity of PCR products.
The effects of OTF on phagocytic activity of RAW 264.7 macrophages were measured using neutral red uptake assay (Wang et al., 2016). RAW 264.7 macrophages (2×105 cells/well) were added to a 96-well plate and cultured at 37℃ for 4 h. Various concentration of OTF (0–2 mg/mL) and LPS (10 ng/mL) were then added to each cell suspension and cultured for 24 h. After incubation, the supernatant was removed and 100 μL of 0.075% neutral red solution was added to the each well and incubated for additional 30 min. After washing three times, lysis buffer [100 μL, 0.01% glacial acetic acid: ethanol = 1:1, (v/v)] was added. The absorbance was measured using a microplate reader at 540 nm.
For western blot analysis, RAW 264.7 macrophages (2.5×106 cells/well) were added to a 6-well plate and cultured at 37℃ for 24 h and then treated with either OTF (0–2 mg/mL) or LPS (10 ng/mL) for 30 min. Total protein was extracted using RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with protease and phosphatase inhibitor. Protein concentrations were determined according to the Lowry method. Thirty microgram of total protein was separated on a 12% SDS-PAGE gel and subsequently transferred to PVDF membranes. The membranes were blocked with 5% skim milk dissolved in TBST (Tris-buffered saline with 1% Tween-20) for 1 h and then incubated with primary antibody (p-p38, t-p38, p-ERK, t-ERK, p-JNK, and t-JNK antibodies) overnight at 4℃. After washing in TBST for 45 min (3 times×15 min), the membranes were incubated with secondary antibodies at room temperature for 2 h. After washing in TBST for 45 min (3 times×15 min), using the chemiluminescence western blot detection system the protein bands were detected.
All the data are present as the mean with standard deviation. The experiments were performed in triplicates and the assays were repeated three times. Statistical significance for the difference between groups was using Student’s t-test. SPSS software version 18 (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analysis.
Results and Discussion
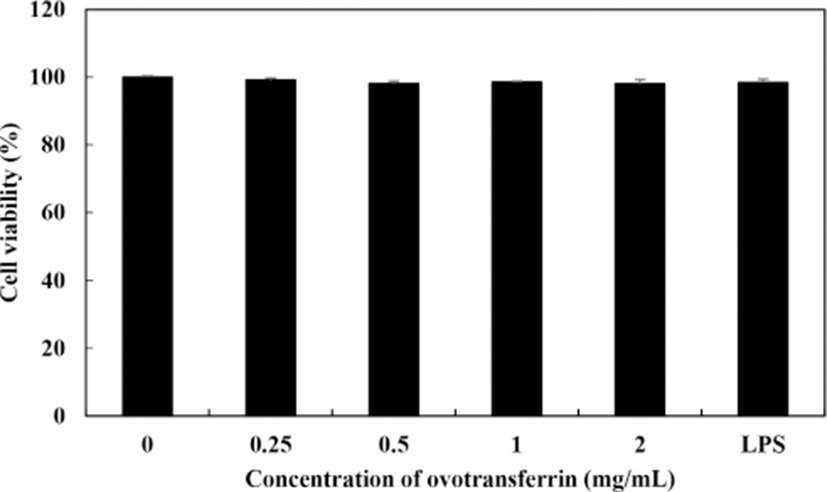
Cell viability of RAW 264.7 macrophages with OTF treatment was measured using the MTT assay. All concentrations of OTF (0–2 mg/mL) did not cause any cytotoxicity to RAW 264.7 macrophages (Fig. 1). To assess whether OTF affected the production of NO and iNOS expression, we performed Griess assay and qRT-PCR analysis. Fig. 2A shows the amount of NO production in RAW 264.7 macrophages produced by OTF. Nitric oxide concentration increased dose-dependently increased with OTF treatment (p<0.001 for 0.5–2 mg/mL, or p<0.01 for 0.25 mg/mL). At 2 mg/mL concentration, OTF increased NO concentration to 31.9±3.5 μM, whereas LPS only increased NO concentration to 27.5±3.3 μM. Nitric oxide is widely involved in physiological processes such as modulation of vasodilation, neurogenesis and wound healing (Mayer and Hemmens, 1997; Rizk et al., 2004). Furthermore, NO synthesis is an important part of the inflammatory response. Because NO can inactivate mitochondrial respiratory chain enzymes, resulting in cell death in the pathogens (e.g., virus, bacteria, and tumor cells) (MacMicking et al., 1997). In living organisms, NO is synthesized by nitric oxide synthases (NOS) through the oxidative deamination of L-arginine. Nitric oxide synthases have three isoforms which are neuronal NOS, endothelial NOS, and inducible NOS (iNOS). Among the three isoforms, iNOS expression is induced by inflammatory conditions after cell stimulation by microbial or immunological stimuli (Lind et al., 2017). Therefore, we confirmed whether OTF stimulates expression of iNOS.
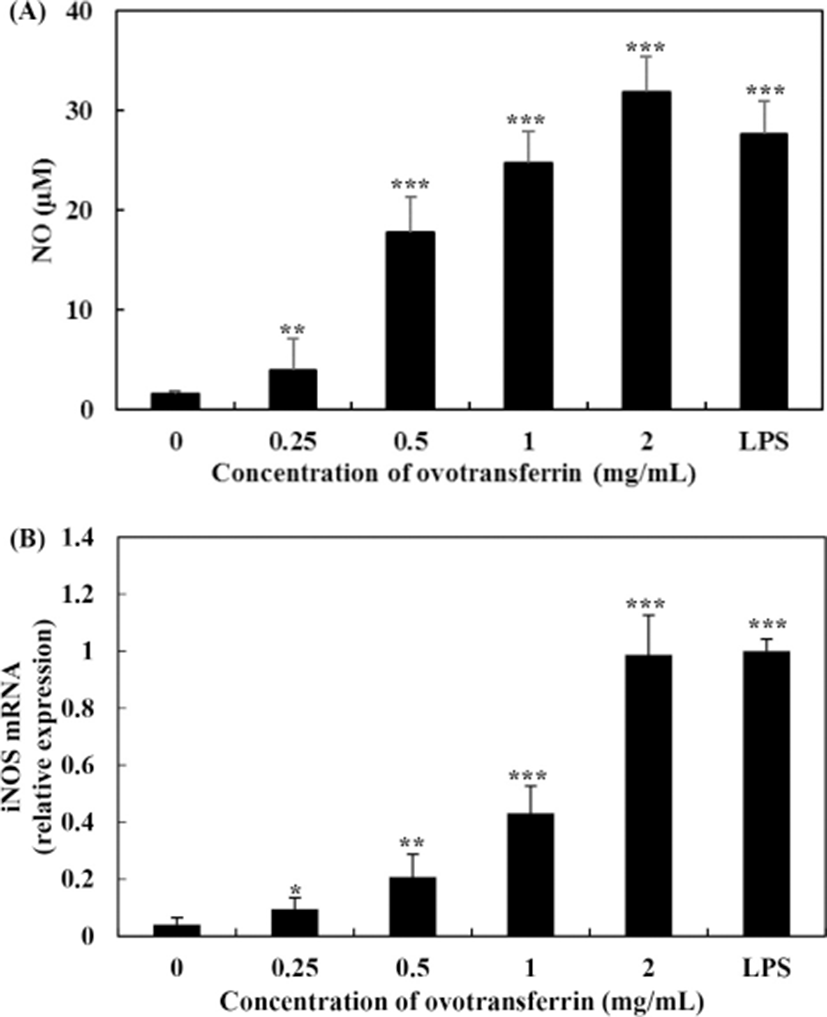
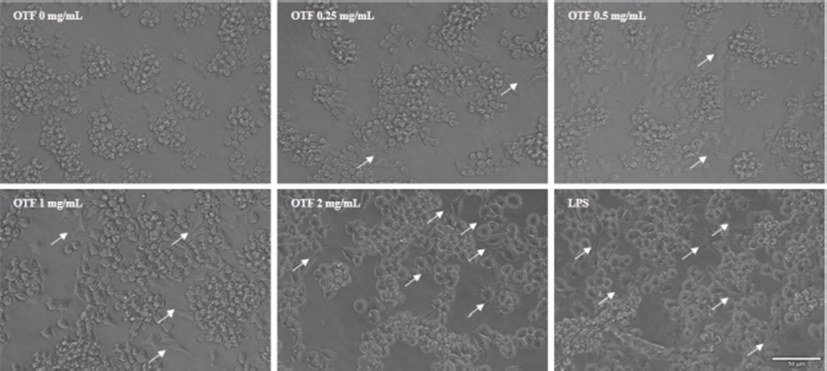
The effects of OTF on the expression of iNOS are shown in Fig. 2B. OTF (2 mg/mL) increased expression levels of iNOS by 24.0-fold (p<0.001) compared with negative control (0 mg/mL OTF treatment). OTF significantly increased expression levels (p<0.001 for 1 mg/mL, or p<0.01 for 0.25, 0.5 mg/mL) in a dose-dependent manner, suggesting that the increase of NO production was induced by stimulating the expression of iNOS by OTF treatment. Additionally, representative micrographs of RAW 264.7 macrophages showed some morphological changes between negative control and OTF treated groups (Fig. 3). After treated with OTF and LPS, RAW 264.7 macrophages were activated, and the single cell size became bigger and some branches were added in cells (white arrows in Fig. 3). The concentration of OTF was increased, the changes were more dramatically.
Many studies reported that increased NO via enhanced iNOS expression activates macrophages and improves immune-enhancing activity (Ha et al., 2013; Hong et al., 2017; Li et al., 2017). Li et al. (2017) and Hong et al. (2017) reported that extracted materials from Radix astragali and Cervus nippon mantchuricus have immune-enhancing activity. These extracted materials increased the iNOS mRNA expression and NO production in RAW 264.7 macrophages without causing cytotoxicity. Ha et al. (2013) found an immunomodulatory activity of Maillard-type lysozyme (conjugated with galactomannan). Specifically, it significantly increased NO expression and affected production of inflammatory cytokines.
Quantitative real-time PCR analysis was used for assessing the effects of OTF on the expression of pro-inflammatory cytokines (Fig. 4). Compared with the negative control (0 mg/mL OTF treatment), 2 mg/mL OTF increased levels of TNF-α expression by 22.2-fold (p<0.001) (Fig. 4A), similar to LPS (10 ng/mL, positive control), which increased TNF-α expression by 22.1-fold. Ovotransferrin increased TNF-α expression in a dose-dependent manner (1.6-, 4.8-, and 12.1-fold increase with 0.25, 0.5, and 1 mg/mL OTF treatment, respectively). Interleukin-1β and IL-6 expression levels also increased with OTF treatment (Fig. 4B, 4C). OTF (from 1 mg/mL) significantly increased IL-1β expression level (p<0.01). Interleukin-6 expression required a higher dose of 2 mg/mL OTF for a significant increase (p<0.001).
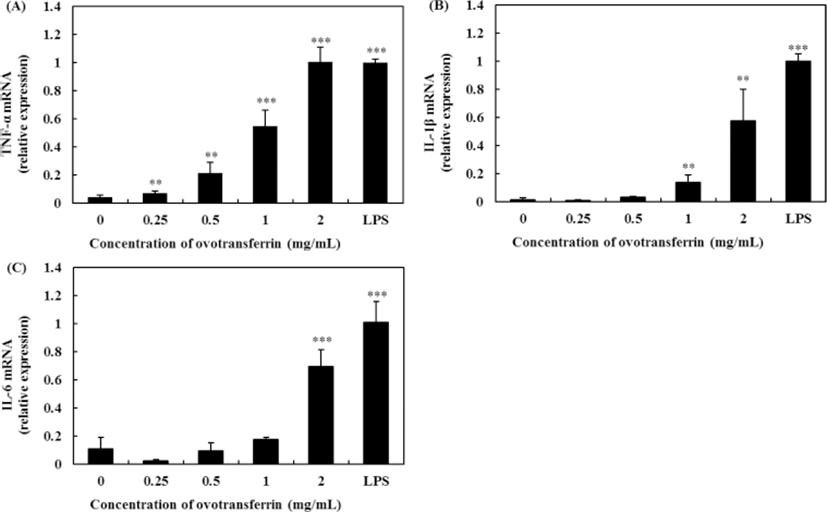
Several bioactive compounds have been reported to be involved in stimulating immune activity, resulting in an increase in secretion of pro-inflammatory cytokines (Hong et al., 2017; Li et al., 2016; Li et al., 2017; Ren et al., 2014; Wang et al., 2013). Tumor necrosis factor-α is the first cytokine which is appeared in the circulation after stimulation of macrophages and it plays an important role in the immune system and induces tumor cell apoptosis (Schooltink and Rose-John, 2002). Furthermore, TNF-α can stimulate the expression of IL-1β and IL-6 (Ahmad et al., 2018; Thieringer et al., 2000). Wang et al. (2013) found that Gelidium amansii gel extracts have immune-enhancing activity by causing an increase in NO and pro-inflammatory cytokines expression. And these pro-inflammatory cytokines (IL-1β and IL-6) are known to play key roles in inflammatory process. Hong et al. (2017) reported that Cervus nippon mantchuricus extract upregulated the IL-6 and TNF-α mRNA levels in a dose-dependent manner.
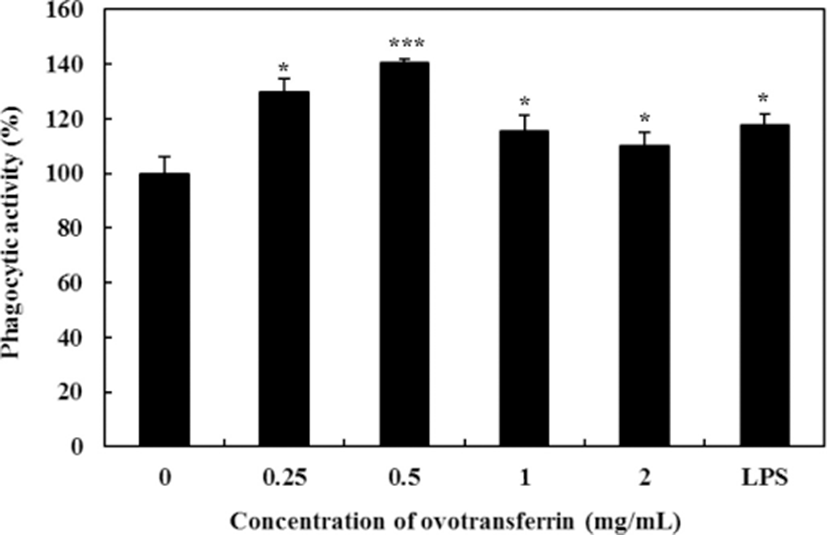
Phagocytic activity of macrophages is the characteristic feature. It is the important part in the immune system, and it is the first step of macrophages in the response to pathogens (Cheng et al., 2008). Using the Neutral Red uptake assay, the effects of OTF on phagocytic activity was determined. Upon treating with OTF, phagocytic activity of macrophages was increased (Fig. 5). Compared with negative control (0 mg/mL OTF treatment), all OTF treatment group significantly increased phagocytic activity (p<0.05 for 2, 1 mg/mL, or p<0.001 for 0.5 mg/mL, or p<0.01 for 0.25 mg/mL). Phagocytic activity of macrophages is closely related to the elimination of specific pathogens, tumor cells, and damaged cells (Cameron and Churchill, 1980; Yamamura and Azuma, 1983). Therefore, enhanced phagocytic activity of macrophages by OTF will help boosting the immune function.
To determine the mechanism by which OTF activates pro-inflammatory cytokines and upregulates NO production, we assessed signaling pathway activation associated with MAPK (p38, ERK, and JNK). Dauphinee and Karsan (2006) reported that LPS treatment in endothelial cells induced the activation of all three MAPK pathways. Upon treating RAW 264.7 macrophages with OTF, we found that all three MAPK (p38, ERK, JNK pathway) were phosphorylated (Fig. 6). From a concentration of 0.25 mg/mL, OTF induced p38 phosphorylation. Furthermore, phosphorylation of ERK and JNK were induced from a concentration of 0.5 mg/mL. Ovotransferrin induced phosphorylation of all three MAPK in a dose-dependent manner.
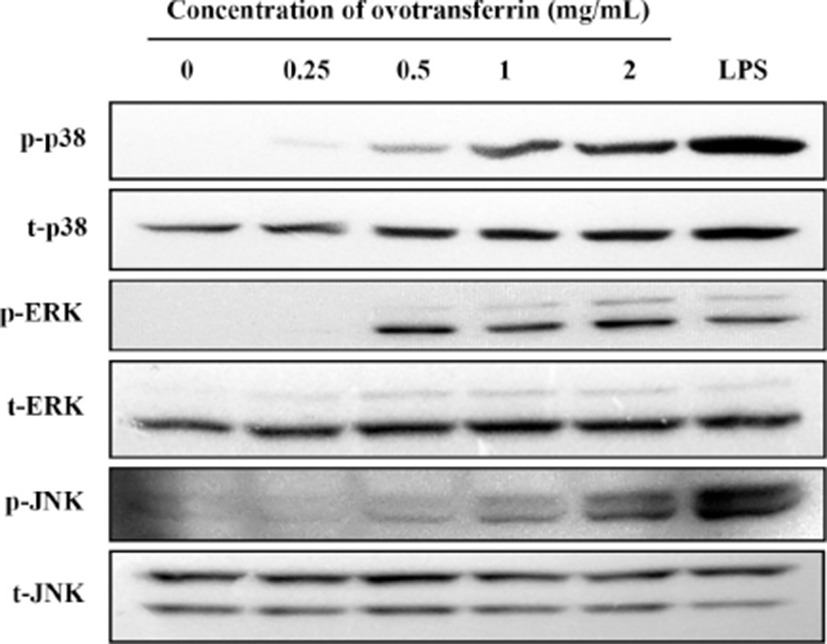
Mitogen activated protein kinase pathways are well known to have a crucial role in the innate immune response and adaptive immunity (Dong et al., 2002; Rincon et al., 2000). When MAPK pathways are activated, genes involved in the immune response are expressed by activating several transcription factors, such as NF-κB (Ninomiya-Tsuji et al., 1999). Active NF-κB then regulates the expression of iNOS and pro-inflammatory cytokines. Many studies about immune-modulating materials reported that they activate macrophages through MAPK pathways (Ha et al., 2013; Hong et al., 2017; Jeong et al., 2015; Seo et al., 2015). These materials induced phosphorylation of all three MAPK in a dose-dependent manner. Ha et al. (2013) found that lysozyme conjugated with galactomannan had immune-enhancing effects by inducing the phosphorylation of JNK. Hong et al. (2017) showed that NGE (extracts from Cervus nippon mantchuricus) activated RAW 264.7 macrophages, and that MAPK were phosphorylated upon treatment with NGE. They concluded that phosphorylation of MAPK elevates NO production and stimulates expression of iNOS and COX-2.
Conclusion
In the present study, we confirmed the immune-enhancing activity of OTF from egg white protein in vitro. OTF stimulated the production of NO in RAW 264.7 macrophages and expression of iNOS and pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. We also found that all three MAPK pathways were associated with macrophage activation. These results indicate that OTF has immune-enhancing activity by activating macrophages. Therefore, OTF should be considered as a potential immune-enhancing agent that can be used in the food or pharmaceutical industry. However, further in vivo study is needed to ensure their benefits before being used for human.













