Introduction
Recently, the issue of food contamination has triggered considerable interest in food safety, and the food industry is enforcing an increasing number of stringent regulations concerning acceptable foodborne pathogens. The Shiga toxin-producing Escherichia coli causes hemorrhagic diarrhea, hemolytic uremic syndrome, and kidney failure in patients and are among the most dangerous of the life-threatening foodborne pathogens (Ivnitski et al., 1999). Salmonella is one of the most common bacterial pathogens inducing foodborne illness, with 4,000 cases of salmonellosis reported annually in the United States (CDC, 2011). Staphylococcus aureus is notorious for its production of thermostable toxins in food (Soriano et al., 2002). Undercooked meat (Gould et al., 2013; Jackson et al., 2013), milk (Kozak et al., 2013; Oliver et al., 2009), dairy products (Gould et al., 2014; Tamarapu et al., 2001), and eggs (Gould et al., 2014) have been associated with instances of contamination with these bacteria. Therefore, taking into account the importance of these pathogens to food safety and public health, the development of sensitive and rapid detection methods to accurately identify their presence in complex food matrixes is highly desirable and should enable more effective monitoring of contaminated foods.
For the rapid detection of these pathogens in foods, polymerase chain reaction (PCR), real-time PCR, enzyme-linked immunosorbent assays (ELISAs), and biosensors have been developed to replace conventional culture-based methods (Park et al., 2014; Shukla et al., 2011). These approaches provide detection results within 12–48 h and are powerful tools, providing both high accuracy and low detection limits. However, due to the potential for very low levels of pathogenic bacteria in foods, the enrichment of food samples is still required to isolate pathogens from the food matrix and increase the number of target microorganisms to detectable levels. This requirement limits the ability of these approaches to provide same-day analysis by increasing the overall time needed to perform the assay. Therefore, a simple and efficient sample pretreatment technique is needed, not only to minimize effects from the sample matrix but also to enable the selective separation and enrichment of target pathogens from background microflora.
Immunomagnetic separation (IMS) technology is a strong candidate for food pretreatment systems, providing rapid analysis and exhibiting high sensitivity for pathogen detection in complex food samples (Benoit and Donahue, 2003). In recent years, applications of IMS coupled with PCR and flow cytometry assay have greatly increased the detection sensitivity for pathogens in food samples (Aydin et al., 2014; Chan et al., 2013; Yang et al., 2016); however, expensive instruments and experienced laboratory personnel are required, making these techniques unsuitable for routine field detection. As a result, conventional methods remain the preferred approach for the detection of foodborne pathogens in the food industry.
Studies using IMS have mostly focused on liquid foods such as milk and juice (Amaro et al., 2012; Herzig et al., 2016; Wei et al., 2016), despite the fact that animal-derived foods, such as beef, ham, eggs, and cheese, are potential growth media for bacteria. Thus, application-based studies are required in order to investigate more complex food matrix. Furthermore, because IMS-based assays are highly influenced by the composition and viscosity of the food matrix, as well as the specificity of the antibody attached to the magnetic beads, such approaches should be tested in a variety of animal-derived foods and using multiple pathogens. Therefore, in the present study, performance of IMS assay was evaluated by direct detection of E. coli, S. aureus, and Salmonella spp. in animal-derived foods, including beef, ham, eggs, and ricotta cheese.
Materials and Methods
E. coli serotypes O157:H7 (ATCC 43890), O104:H4 (NCCP 15656), O26 (NCCP 14539), O111 (NCCP 14540), and O103:H2 (NCCP 15957); Salmonella enterica serovars Typhimurium (ATCC 19585), Newport (ATCC 6962), Virginia (CCARM 8206), Enteritidis (CCARM 8200), and Choleraesuis (KCCM 40763); S. aureus strains ATCC 11778, KCCM 41291, KCCM 12214, CCARM 3A011, and ATCC 12113; Shigella sonnei (KCCM 11903); Listeria monocytogenes (CCARM 0019); and Clostridium perfringens (KCCM 12098) were cultured in tryptic soy broth (TSB; BD Biosciences, Franklin Lakes, NJ, USA) at 37°C for 18 h.
Anti-E. coli serotype O/K (rabbit/IgG), anti-Salmonella (rabbit/IgG), and anti-S. aureus (rabbit/IgG) polyclonal antibodies (Invitrogen, Carlsbad, CA, USA) were incubated with Dynabeads Protein G (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions to generate immunomagnetic beads (IMBs).
Ham, ground beef, eggs, and ricotta cheese were selected because of their different textures and compositions. The absence of microflora in samples was pre-evaluated with agar media selective for various microbes [Baird-Parker agar (BPA), eosin methylene blue (EMB), xylose lysine deoxycholate (XLD), Oxford, and tryptose sulfite cycloserine (TSC) media]. To collect egg contents, eggs were surface-sterilized with Lugol’s solution (iodine/potassium iodide solution) in 75% alcohol for 10 s and then air dried in a sterile chamber for 10 min. Egg contents, ham, ground beef, and ricotta cheese samples (10 g each) were inoculated with serially diluted concentrations (100 to 106 CFU/mL) of pathogens. Each inoculated food sample was mixed with 90 mL of phosphate-buffered saline (PBS; pH 7.6) and homogenized with a stomacher blender (Bag Mixer 400, Interscience, St. Nom la Bretèche, France) for 2 min.
Food samples (10 mL) were mixed with 1.5 mg (50 µL) of Dynabeads conjugated with a polyclonal antibody and incubated at room temperature for 10 min with rotation at 15 rpm. After separation, the supernatant was discarded, and cell-attached IMBs were suspended in 200 µL PBS. IMBs were then spread onto selective plates for enumeration after appropriate dilution and incubated overnight at 37°C. To determine the capture efficiency (CE), IMBs were measured by plating captured beads on tryptic soy agar. CE was calculated using the following equation: CE (%)=(B/A)×100, where A is the total number of cells present in the sample (CFU/mL) and B is the number of IMB-bound cells (CFU/mL).
Five strains each of E. coli, Salmonella, and S. aureus, along with three non-target bacteria (S. sonnei, L. monocytogenes, and C. perfringens) were cultured in TSB at 37°C for 18 h, followed by serial dilution to 103 CFU/mL. A mixed culture was spread onto agar media selective for IMB-bound target bacteria and non-target bacteria. CE was calculated as above.
The mixture of E. coli serotypes, mixture of S. aureus strains, and mixture of Salmonella serotypes were prepared to a final concentration of 103 CFU/mL. The mixed culture was used to inoculate ham, ground beef, eggs, and ricotta cheese samples (10 g each). A mixture of IMBs specific for each of the three target bacteria (a total of 4.5 mg) was added to the food samples containing the three bacteria and mixed. Bacteria-attached IMBs were plated onto each type of selective media, and CE was calculated as above.
This study was performed in triplicate. Data were expressed as means±SD. Statistical analysis was performed with SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). An independent samples t-test or analysis of variance (ANOVA)-Tukey test was used to determine statistical differences between two or more comparisons, respectively, after confirming a normal distribution of the data. Differences were considered statistically significant at p<0.05.
Results and Discussion
To determine the optimal amounts of IMBs and immunoreaction times for bacterial isolation, CE analysis against E. coli O157:H7 was performed with various concentrations of magnetic beads and incubation times. As shown in Fig. 1, the highest CE of 94.3% (5.6×104 CFU/mL) was obtained at 0.15 mg IMBs (corresponding to 1.0×107 magnetic particles) with and immunoreaction time of 10 min. Longer immunoreaction incubation times or IMB concentrations did not result in a significant increase in CE (all p>0.05). In fact, increasing the concentration of IMBs from 0.15 to 0.3 mg resulted in a slight decrease in CE (Fig. 1). Bacterial capture readily occurs in strong magnetic fields, but excessive IMBs can damage bacteria by inducing more antigenic sites on the bacterial surface that bind to the beads, thereby reducing CE (Zhang et al., 2016). Therefore, the optimal conditions of 0.15 mg IMBs and a 10 min immunoreaction time were used in subsequent experiments.
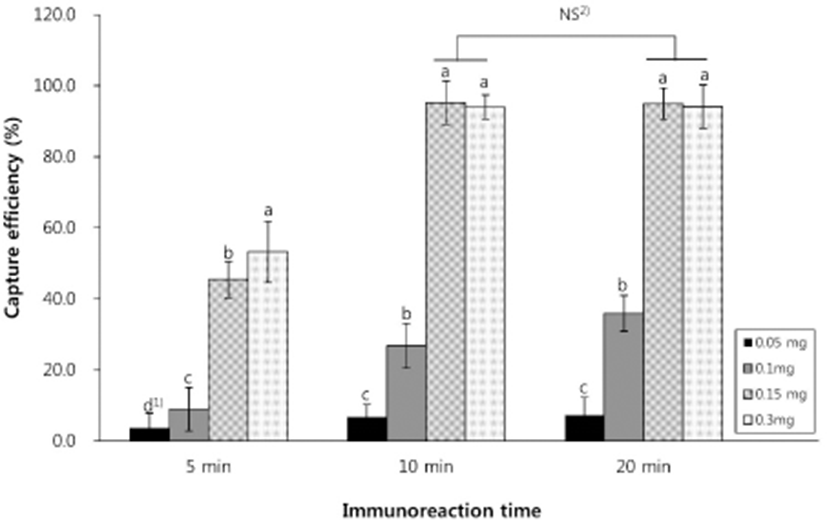
Differences in calculation methods can lead to the underestimation of CE. Varshney et al. (2005) compared two methods to calculate CE in an IMS assay and found that CEs of cells bound to magnetic beads (bound cell-based calculations) were lower than those with unbound cells (unbound cell-based calculations). This observation is supported by other studies, for example, using an unbound cell-based calculation, high CEs of 98.8% and 98% have been reported for E. coli O157:H7 (Xiong et al., 2014; Zhu et al., 2011). Generally, the use of the unbound cell-based calculation is more suitable for the determination of quantitative results in an IMS assay when the initial concentration of microorganisms is known. However, this method cannot be directly used in contaminated foods containing unknown microbial concentrations. Thus, we used the bound cell-based calculation method for subsequent experiments to recover bacteria from real food samples, in order to simulate the situation in the food industry.
The CE and specificity of target antibodies in the assay were examined in three mixed cultures (Fig. 2). The Salmonella polyclonal antibody showed a very high sensitivity for all Salmonella serotypes tested (CEs of 92.5%–100.4%), and the IMS assay also exhibited high CE values (87.0%–97.8%) in detecting the five strains of S. aureus. The conjugation of the IMBs with the anti-E. coli serotype O/K resulted in CEs of 81.1%–99.6% among the five serotypes of E. coli tested. The polyclonal antibodies for E. coli, S. aureus, and Salmonella spp. used in the assay showed high specificity for the target bacteria, with a less than 10% capture rate of non-target bacteria (Fig. 2). These results indicate that the IMS assay can effectively distinguish between the target organisms and other bacteria with minimal cross-reactivity.
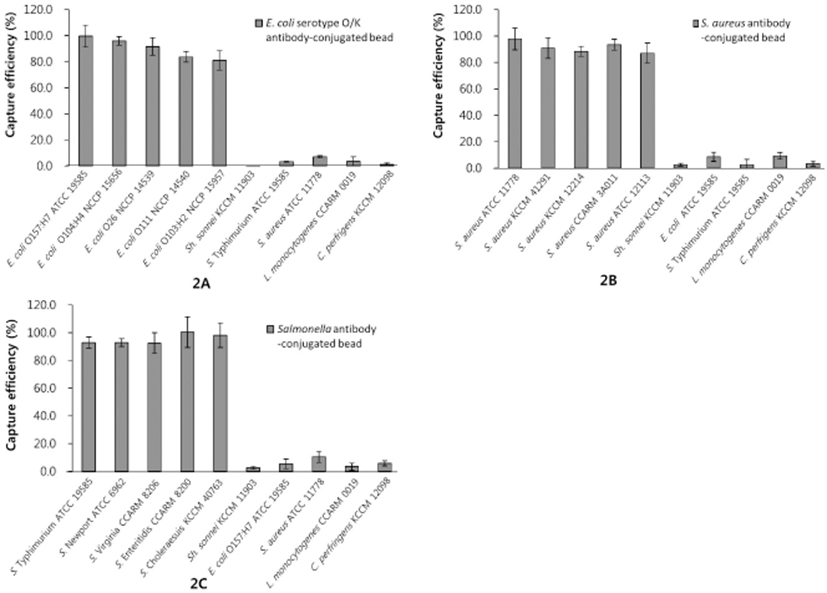
Even though the highly specific binding of polyclonal antibody-coated IMBs to E. coli O157:H7 has been reported by other researchers (Aydin et al., 2014; Herzig et al., 2016; Luciani et al., 2016), there are few studies involving the use of IMS assays to detect various antigenic serotypes of E. coli. In this study, the IMS assay returned a CE of 99.6% for E. coli O157:H7, as well as CEs of over 80% for the other E. coli serotypes tested. Moreover, consistent with our results, a Salmonella polyclonal antibody exhibited high specificity in an IMS assay for the detection of various strains of Salmonella spp. in a recent study (Herzig et al., 2016). Therefore, our results suggest that an IMB-based assay with high specificity has potential for rapid, immunological, presumptive identification.
Numbers of recovered cells of the three pathogenic microbes from artificially contaminated animal-derived foods were calculated using our IMB-based assay without any conventional enrichment steps and were compared to results using a conventional method. Using the IMB-based method, the average CEs of E. coli O157:H7, S. aureus, and S. Typhimurium are shown in Tables 1–3. The IMS assay detected E. coli O157:H7, S. aureus, and S. Typhimurium in all cases at concentrations as low as 10 CFU/mL, with average detection values among the four animal-derived food types of 7.4 CFU/mL for E. coli O157:H7, 12.4 CFU/mL for S. aureus, and 8.4 CFU/mL for S. Typhimurium. The highest CE values (8.5%–28.4%) for these three microbes were observed at inoculum concentrations of 102–104 CFU/mL, as the recovery rate decreased (3.6%–9.8%) at an inoculum concentration of 106 CFU/mL. This is likely because at high inoculum concentrations, fewer cells are bound by the IMBs due to the lower relative concentration of IMBs (Xiong et al., 2014). Simultaneous detection in a sample mixture showed similar CEs when compared to individual detection. The CEs of the mixture of E. coli serotypes, mixture of S. aureus strains, and mixture of Salmonella serotypes were 26.3% to 29.4% for ham, 28.7% to 30.8% for beef, 16.5% to 21.4% for egg, and 26.8% to 29.0% for ricotta cheese (Fig. 3), demonstrating that the mixing of various serotypes of bacteria did not lead to interference among serotypes.
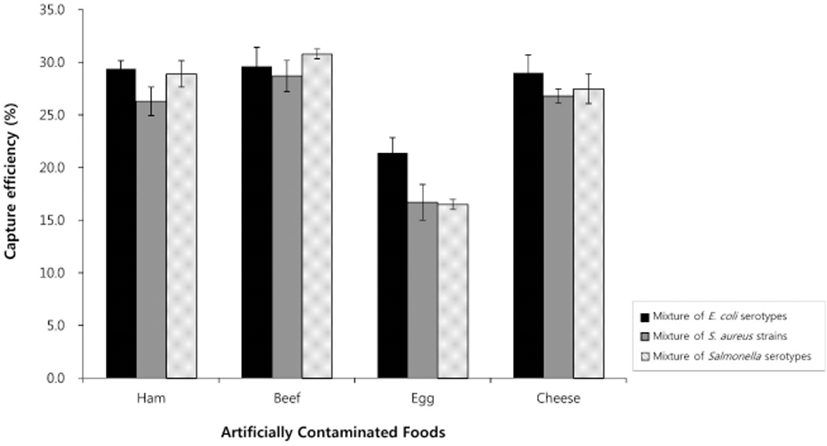
These results are consistent with previous findings that demonstrated a CE of 10%–15% in ground beef inoculated with 104 CFU/mL E. coli O157:H7 (Fu et al., 2005). Furthermore, Chu et al. (2009) used IMBs and immunoliposomal nanovesicles for the isolation and detection of Bacillus cereus in milk, showing a detection limit without pre-enrichment of 10 CFU/mL.
Thus, in comparison, our IMB-based assay without enrichment has similar detection limits, demonstrating the applicability of this approach for testing of animal-derived foods with complex matrixes. Several studies have reported that the detection limit of such assays can be further improved by either using a short enrichment procedure requiring less than 4 h or joining this with another method such as PCR. Xiong et al. (2014) reported that 1–10 CFU/mL E. coli O157:H7 in whole milk and ground beef could be detected by IMS after an enrichment step, whereas Ma et al. (2014) simultaneously detected Salmonella, S. aureus, and Shigella in fresh pork samples using an IMB-based multiplex real-time PCR assay, demonstrating detection limits of <10 CFU/g for all three pathogenic bacteria.
Many researchers have suggested that the composition of the food matrix has a considerable influence on IMS (Amaro et al., 2012; Fu et al., 2005). Previous studies using IMS-based approaches have shown that the percentage of captured cells in pure culture is significantly reduced in real foods (Fu et al., 2005; Laube et al., 2014; Wei et al., 2016), with researchers further pointing out that samples with high fat or protein contents or high acidity can interfere with antigen–antibody binding. This observation was consistent with our findings of a higher detection limit in beef (30%), which has a higher fat content, than in ham (17%) (Tables 1–3). Other factors affecting IMS efficiency in the capture of bacteria from food are the viscosity and density of the suspension containing bacteria and small food particles. A previous study found that the high viscosity of cheese and a concentrated bacterial suspension weakened the magnetic force and thus allowed for only the partial recovery of Dynabead-conjugated L. monocytogenes (Uyttendaele et al., 2000). In the present study, we found that the two solid matrixes (beef and ham) showed higher CEs in detecting the three tested pathogenic bacteria compared to those of the fluid (egg) and semi-fluid (ricotta cheese) matrixes. Because the state of the food suspension with contaminated bacteria can affect the CEs of IMB-based methods, we evaluated the food suspensions in this study and observed that large particles from the homogenization of ham and beef were completely removed by the stomacher bag filter during food sample pretreatment, while the very small particles from the ricotta suspension passed through the filter. These particles from the ricotta suspension and the viscosity of the egg suspension would both interfere with the IMS assay.
Conclusion
In this study, an IMS assay was developed for the simultaneous detection of E. coli, Salmonella spp., and S. aureus and was tested in four different types of animal-derived foods without the use of culture enrichment. The assay exhibited high specificity for five serotypes each of E. coli and Salmonella spp. and five strains of S. aureus, with a detection limit of 10 CFU/g. In addition, the method is simple to perform in a standard laboratory and requires no sophisticated techniques or equipment, making it suitable for ready adoption in the food processing industry. Future studies should focus on improving the CEs of the developed IMB-based method in real food samples, including validation in a wider variety of foods.













