Introduction
Researches have been conducted to develop functional raw materials to improve physiological activity. For example, enzymatic treatments have been proven to be effective for improving the quality of certain existing raw materials by modifying their structure or redistributing their composition (Lebesi and Tzia 2012; Liu et al., 2017; Ma and Mu 2016; Santala et al., 2014). It has been proven that reduction in raw material particle size not only alters structural characteristics, but also improves technological properties when using a raw material in food development (Chen et al., 2013; Raghavendra et al., 2006). Enzymatic hydrolysates possess many biological activities as potential physiological modulators. They usually contain various amino acid residues, and the activities are based on amino acid composition. Recently, studies have reported their possible utility as alternative antioxidants (Saiga et al., 2003).
Antioxidant is a broad term defined as any substance that significantly delays or inhibits oxidation of a substrate. Antioxidant agents protect living organisms from oxidative damage, resulting in the prevention of various diseases, such as cancer, cardiovascular diseases, and diabetes (Napolitano et al., 2006). Therefore, the existence of protective agents against free radicals and other oxidants in living cells is very important. It is becoming important to identify antioxidants from natural sources by modify technique.
RJ is a traditional product commonly used as a supplement to protect against diseases. Royal jelly is made by young worker bees and is the sole food for young larvae and queen bee. Royal jelly is composed of important compounds with biological activities, such as free amino acids, proteins, sugars, fatty acids, minerals, and vitamins. Also it contains physiologically active substances such as 10-hydroxy-2-decenoic acid (10-HAD) (Suguru Fujiwara et al., 1990). It has been reported to have functional effects, such as activation of the autonomic nervous system, antibacterial activity, prevention and alleviation of circulatory diseases, anti-inflammatory and anti-cancer effects (Guo et al., 2007; Sver et al., 1996). Above all, RJ’s antioxidant functions have been suggested to have an excellent effect (Nagaia et al., 2001). However, RJ contains two proteins, one is 47 kDa and the other is 55 kDa, which are considered to be major allergens (Rosmilah et al., 2008). The patients with sensitivity to RJ showed high serum IgE level. Therefore, many studies have been conducted to solve these problems and to make RJ easier to ingest (Kim et al., 2013; Yoon et al., 2014).
In this study, RJ was converted into an easy-to-absorb shorter chain monomer using three commercial protease enzymes. The objective of this study was to prepare ERJ hydrolysates using protease enzymes and to evaluate the antioxidant effect of ERJ on peritoneal macrophages obtained from mice.
Materials and Methods
Freeze-dried RJ powder was supplied from Zhejiang Jiangshan Bee Enterprise CO., LTD (Jiangshan, China). Proteolytic enzymes, including endo-protease obtained from cultures of Bacillus licheniformis, and endo- and exo-protease obtained from cultures of Aspergilus oryzae, were purchased from Vision Biochem Co., Ltd (Gyeongsan, Korea). RJ powder was mixed with purified water at a ratio of 1:10. The mixture was added endo- and exo-proteases, and mixed well in 380 mmHg for respective 3 h at 55-58℃, 45-50℃, 50-55℃ and for 18 h at 55℃ without measuring the pH value. Finally, it was heat-treated at 85-95℃ for 15 min to inactivate the enzymes, followed by drying to effect pulverization.
The test solution of 10-HDA, a standard component of RJ, was prepared as follows. 1 g of ERJ powder was added to 50 mL of distilled water, reacted at 50℃ for 1 h. And then the mixture was added 350 mL of methanol, mixed using ultrasonic waves for 30 min. The supernatant obtained by centrifugation (12,000 rpm, 10 min) with addition of 100 mL of methanol was filtered through a 0.2 μm syringe filter (Adventec, Tokyo, Japan) and used as a test solution. 10-HDA standards were diluted with methanol to 100, 10, or 1 μg/ml, respectively, and the same amount was injected to prepare a standard curve. HPLC (Dionex Ultimate 3000, Thermo Scientific, Milan, Italy) analysis was carried out on a Zorbax Extend-C18 Column.
For measuring total free amino acid content, 1 g of ERJ powder was diluted 10-fold in distilled water, treated in a water bath at 100℃ for 1 h, centrifuged to extract the supernatant and used as a test solution. Standards were prepared by diluting 17 different kinds of amino acid solutions (Pickering Laboratories, Inc., Mountain View, CA, USA) with 0.1 N HCl. The same amount of each standard solution was injected to prepare a standard curve. HPLC (Dionex Ultimate 3000, Thermo Scientific, Milan, Italy) analysis was carried out using a cation ion exchange column.
After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), coomassie blue staining (Wang et al., 2006) was carried out for observing changes in allergic proteins. Quantification of the total protein was performed with BCA protein assay reagents (Bio-Rad Laboratories, Hercules, CA, USA). After resolving the proteins with SDS-PAGE (10% acrylamide gels), the gel was stained with Coomassie brilliant blue G-250 (Sigma, St. Louis, MO, USA).
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay was performed as previously reported (Fan et al., 2014). Each sample was diluted to 1 mg/ml, and ascorbic acid was used as a positive control. The absorbance was measured at 517 nm with a microplate reader (BioTek, Winooski, VT, USA).
A cell viability assay was performed with peritoneal macrophages obtained from BALB/c mice to investigate the influence of ERJ on macrophages. The macrophages were plated in 96-well plates for 4 h at 37℃ in a 5% CO2 incubator (Sanyo Electric Co., Osaka, Japan). The macrophages was treated with various concentrations of ERJ (25, 50, or 100 μ g/mL) or RJ (100 μg/mL) for 24 h, without or with LPS (1 μg/mL) co-treatment. Cell viability was determined using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay kit from Sigma according to the manufacturer’s instructions.
To investigate the antioxidant activity of ERJ, macrophages was treated with ERJ or RJ, followed by LPS (1 μg/mL) for 24 h. The level of Reactive oxidative species (ROS) was quantified by fluorescence using 2′, 7′-dichlordihydrofluorescin diacetate (H2DCF-DA) (Invitrogen, CA, USA) according to manufacturer’s instructions. Superoxide dismutase (SOD) and Glutathione (GSH) levels were measured using a commercial SOD/GSH Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), and Nitric oxide (NO) level was measured using the Griess Reagent System (Promega, Madison, WI, USA). All experiments were conducted according to the manufacturer's instructions.
All values were expressed as mean ± SEM. Statistical differences among experimental groups were assessed with Student`s t-test for two groups. One-way analysis of variance (ANOVA) was used for comparison among more than two groups. Differences were considered statistically significant when p<0.05 (Andrikopoulos et al., 2008).
Results and Discussion
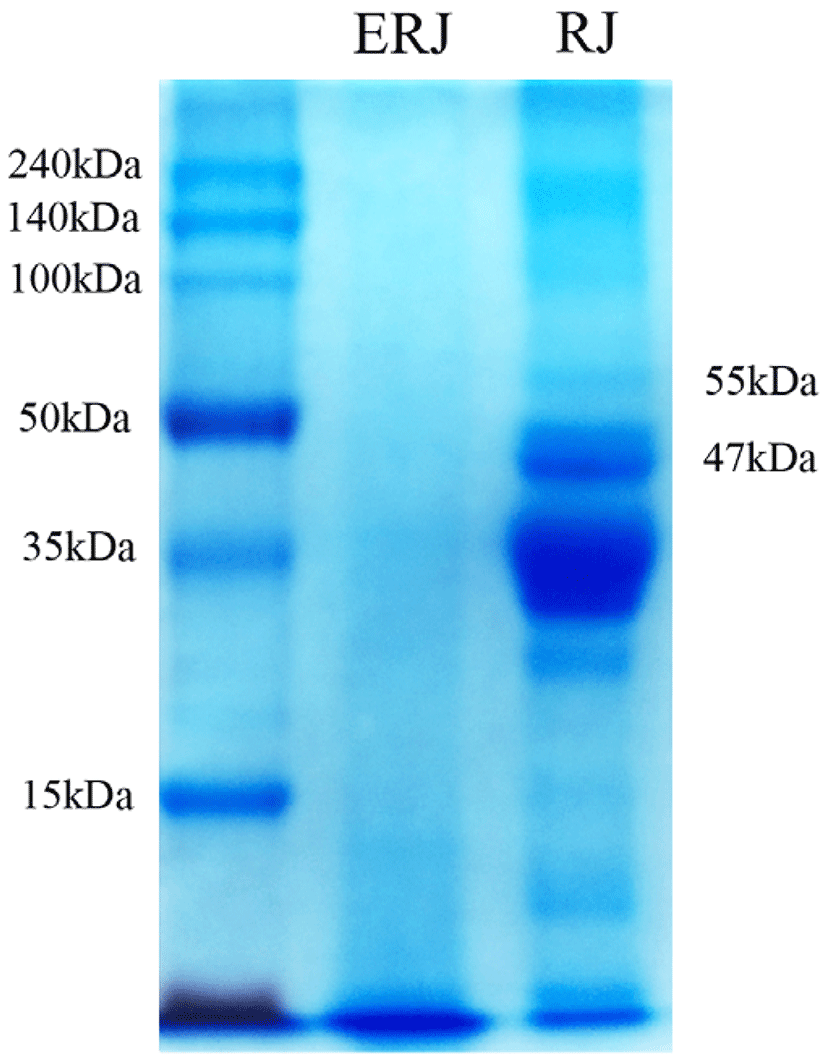
The water-soluble proteins of RJ have been studied in terms of their characteristics and physiological functions according to size in a total of nine fractions (Tamura et al., 2009). Among them, water-soluble protein 1 (molecular weight (MW): 55 kDa) and water-soluble protein 2 (MW: 47 kDa) have been reported as major proteins causing allergies (Yoko et al., 2011). Water-soluble proteins 1 and 2 have been reported to stimulate the secretion of TNF-α, a pro-inflammatory cytokine, in macrophages in mice (imúth et al., 2004) and to cause acute bronchial asthma, skin hypersensitivity, and colitis. Therefore, in this study, we examined whether these allergen-induced proteins were removed and the level of 10-HAD was changed by enzyme treatment.
The electrophoretic patterns of the RJ hydrolysates obtained from the enzyme treatments are shown in Fig. 1. Compared with RJ, ERJ did not show expression of either of the two proteins (MW: 47, 55kDa) causing allergic reaction. The enzyme treatment also resulted in an increase in the level of total free amino acids due to the decomposition of small molecules of polymer (Table 1). Nevertheless, the level of 10-HDA was not changed by the enzyme treatment (Table 1). Therefore, with ERJ, there is no need to worry about the risk of allergies when ingested, and the enzymatic processing of RJ is expected to improve the absorption rate.
| RJ | ERJ | |
|---|---|---|
| 10-HDA | 50.0 ± 0.0 | 51.0 ± 0.1 |
| Total free amino acid content | 5.72 g/mg | 83.89 ± 2.44 g/mg |
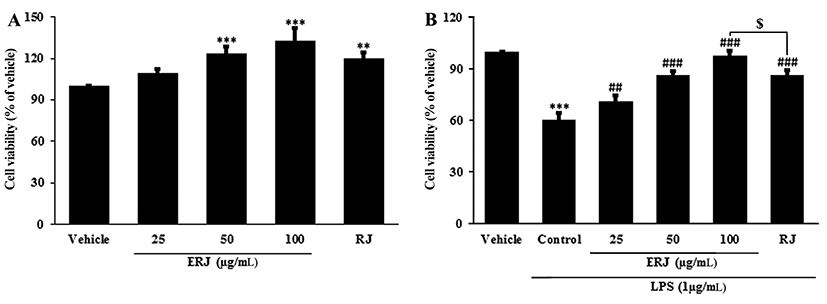
To assess the cytotoxicity of ERJ in peritoneal macrophages, MTT assays were conducted (Fig. 2A). The cell viability of macrophages treated with ERJ increased in a dose-dependent manner. To investigate the cell protective effect of ERJ, MTT assays were performed in LPS-treated peritoneal macrophages (Fig. 2B). The cell viability after 1 μg/mL LPS treatment for 24 hours was reduced to 60% of the normal group. Thus, 1 μg/mL LPS was used in all subsequent experiments. The viabilities of ERJ or RJ, co-treated with LPS for 24 h, were significantly increased compared to cells treated with LPS alone. ERJ resulted in a more effect on proliferation compared to treatment with the same concentration (100 μg/mL) of RJ. This result shows that ERJ had the protective effect against LPS-treatment-induced cell stress.
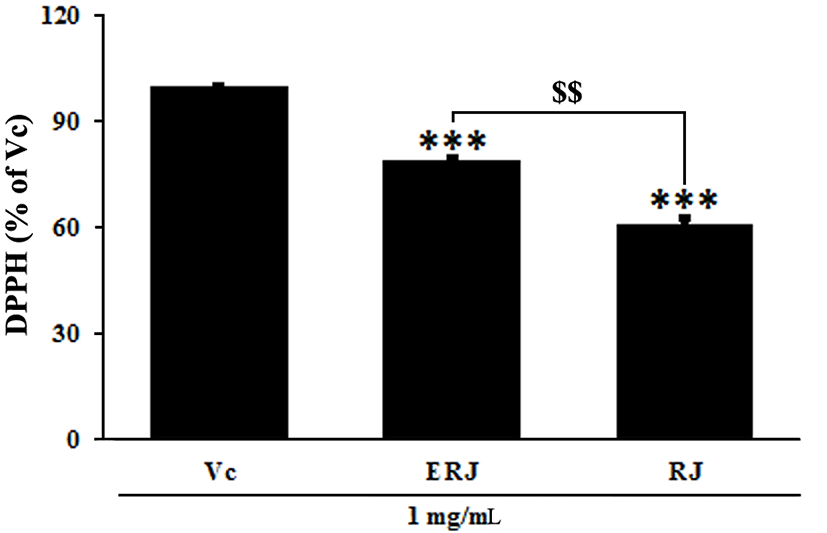
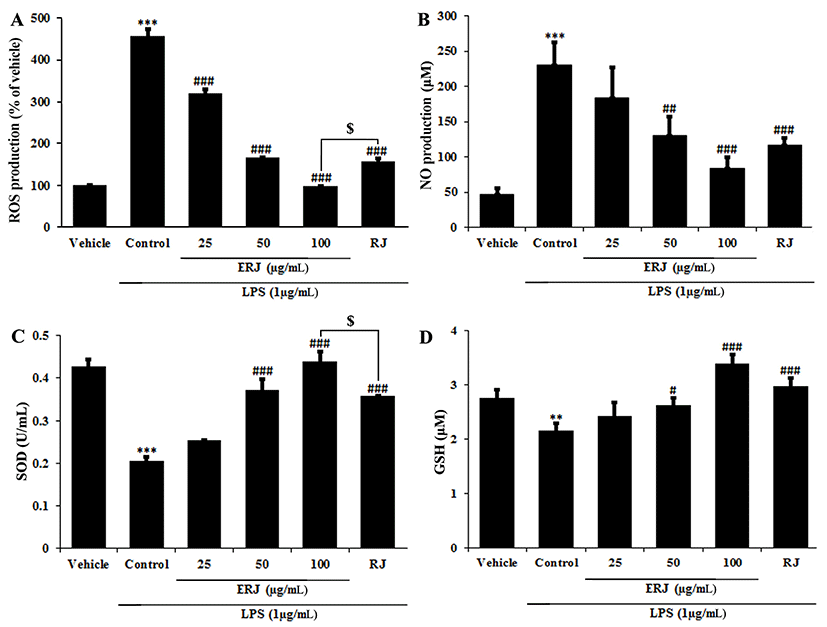
RJ exerts its positive effects on the human body due to its natural pharmacological activities, such as its antioxidative, immunomodulating, hypotensive and blood regulatory, and anti-inflammatory effects (Lebesi and Tzia 2012; Viuda-Martos et al., 2008). The antioxidant activity of ERJ was confirmed by DPPH assay, ROS and NO productions, and SOD and GSH densities. A DPPH assay was used to assess antioxidant activity by measuring the free radical scavenging ability of ERJ. At the same concentration of 1 mg/mL, ERJ showed 20% higher DPPH free radical scavenging activity than RJ (Fig. 3). ROS produced during the metabolic process causes oxidative stress in the body, resulting in an increase in NO production and an acceleration of cell damage caused by the inflammatory reaction. In addition, SOD and GSH are produced as part of a protective mechanism (Freeman et al., 1983) that occurs as a response to oxidative damage (Creppy et al., 1989). The experimental results showed that ROS and NO production after treatment with LPS alone significantly increased compared with non-treated cells. However, ROS and NO production in ERJ and LPS co-treated cells significantly decreased in a dose-dependent manner. Especially, compared to treatment with the same concentration (100 μg/mL) of RJ, treatment with ERJ resulted in a decrease in ROS production (Fig. 4A, B). Antioxidant enzymes that act as a defense mechanism against ROS include SOD, GSH, and catalase (CAT), all of which are present in various organelles, the cytoplasm, and nucleus (Lopes et al., 2001). SOD converts the superoxide anion to hydrogen peroxide. The resulting hydrogen peroxide is degraded by CAT and glutathione peroxidase (GSH-Px). The excess hydrogen peroxide, which has not been degraded, reacts with Fe2+ radicals and damages cellular proteins, cell membranes, and DNA, leading to cell aging and necrosis (Emerit et al., 2001; Kim et al., 2008). In this study, SOD and GSH production in cells treated with LPS alone were significantly decreased, whereas ERJ significantly increased their production in a dose-dependent manner. Compared to treatment with the same concentration (100 μg/mL) of RJ, treatment with ERJ resulted in higher SOD and GSH production (Fig. 4C, D).
Conclusions
The results obtained in this study demonstrate that ERJ is a bioactive compound based on its excellent antioxidant activities. In this study, when two allergen-inducing proteins were removed from ERJ, there was the increase in total amino acid content compared to non-treated RJ. In addition, no difference in the content of 10-HDA was observed in ERJ. Also, the evaluated antioxidant activities of ERJ were stronger than those of RJ. These results suggest that ERJ could be used as a new material in the development of medicinal foods with a potential antioxidant capacity.