Introduction
Yogurt is a probiotic dairy product generated by lactic acid bacteria through fermentation of milk. Yogurt can promote the reduction of blood cholesterol, absorption of vitamins and minerals, improvement of digestive tract, and absorption of digestion and lactose (Gilliland and Seck, 1977; MacGregor et al., 2002). In addition, yogurt can inhibit colorectal cancer and diabetes mellitus. Therefore, yogurt has been expanded to prevent and cure diseases such as hyperlipidemia (Cho et al., 2006; Goldin and Gorbach, 1984).
Recently, interest in natural food additives and integration of substance for enhancement of health has increased (Varga, 2006). Natural food materials have been added to yogurt to increase its functionality, including the addition of mulberry, green tea, white tea, and black tea (Muniandy et al., 2016) wine grape pomace (Tseng and Zhao, 2013) and grape (Karaaslan et al., 2011). Studies that manufacture yogurt with more intensified biological activity are progressing.
Olive oil and table olive are important components of Mediterranean diet. They are consumed all over the world (Pereira et al., 2006) Olive is a fruit containing abundant antioxidant substance, including phenolic compounds such as verbascoside, ligstroside, and oleuropein (Ryan et al., 1999) of these antioxidants, oleuropein has been found in Olive (Olea europea) leaves and raw olive (Bianco and Uccella, 2000; Soler-rivas et al., 2000). In many in vivo and in vitro studies, oleuropein has shown various biological activities, including antioxidant and antimicrobial effect (Bisignano et al., 1999; Speroni et al., 1998). According to scientific view of European Food Safety Authority (2011), oleuropein can lower LDL cholesterol (LDL-C) level. However, no study has reported the antioxidant and quality characteristics of yogurt added with green olive powder. Therefore, the objective of this study was to determine the antioxidant and quality characteristics of yogurt added with green olive powder in shelf-life.
Materials and Methods
Green olives were used as salted green olives imported from Spain. First, to remove saltness of green olive, green olives were took out after soaking in water for 6 h. They were then freeze-dried using a freeze dryer (Ilshin Lab, MCFD 8510, Korea). Dried green olives were ground for 2-3 min with a grinder. Green olive powder was kept at −80°С in a deep freezer until use.
Lactic acid bacteria including Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and Bifidobacterium animalis ssp. lactis were used as yogurt fermentation starter (Lyofast YAB 450 AB, Sacco srl., Italy).
Skim milk powder, pectin, white sugar, and green olive powder at 0%, 1%, 3%, and 5% were homogenized for 5 min using a homogenizer (Table 1). The mixture was sterilized at 85°С for 30 min followed by cooling to 42°С in a water bath. Yogurt culture was inoculated to the mixture and fermented at 37°С for 8 h until pH reached 4.5. After fermentation, all yogurt samples were stored at 4°С in a fridge. Samples were collected at interval of 5 d for 15 d for analysis (1, 5, 10 and 15 d).
GY0: control; GY1: yogurt added with 1% green olive; GY3: yogurt added with 3% green olive; GY5: yogurt added with 3% green olive.
pH was determined using a pH meter (pH 900, Precisa Co., Switzerland). Titratable acidity was determined for all groups through neutralization titration until pH reached 8.3 using distilled water (3 g of stored yogurt sample in 27 mL of water). Then 0.1 N NaOH was used to calculated the amount of lactic acid (%) using the following equation:
Viscosity of yogurt sample stored at 4°С was measured using a viscometer (Model LVDV-E, Brookfield Engineering Lab. Inc., USA). Samples were stirred for 5 min before measurement. Then, all viscosity values were measured at 50 rpm with spindle No.63.
Yogurt syneresis (release of whey) was determined using the centrifugation method described by Keogh and O’Kennedy with some modifications (1998). Briefly, yogurt (30 g) was centrifuged (640×g, 20 min, 4°С). The clear supernatant was harvested and weighed. Syneresis was calculated using the following equation (Keogh and O’Kennedy, 1998):
Syneresis = weight of supernatant (g)/ weight of yogurt sample (g) × 100%
The cell viability of lactic acid bacteria (LAB) was measured using the spread plate method with MRS agar (Oxoid Ltd.) at 37°С for 24 h. Samples (100 μL) were added to 900 μL of 0.85% sterile saline and sequentially diluted 10 times with 0.85% saline, then 100 μL of each dilution was spread on plates, followed by incubation at 37°С for 24 h. Total viable cell numbers were expressed as log values (Park and Oh, 2005).
Color was checked using a colorimeter (NR-300, Nippon Denshoku, Japan). The instrument was calibrated before measurements using standard white plate supplied with the instrument. Lightness (CIE L*), redness (CIE a*), and yellowness (CIE b*) values were measured. Measurements were repeated three times for each treatment group.
Total phenolic content was determined using the method of Wei (2011) with slight modification. Briefly, after mixing 100 μL of test solution and 100 μL of Folin-Caltheous phenol reagent 1 N solution, the mixture was allowed to react at room temperature for 3 min. Then 300 μL of 1 N Na2CO3 solution was added to the mixture followed by incubation at room temperature for 90 min. Then 1 mL of distilled water was added to the mixture to complete the reaction. After that, the absorbance of the mixture was measured at wavelength of 725 nm using a spectrophotometer (OPTIZEN 2120UV, Mecasys Co., Ltd., Korea). Results were expressed as mg of gallic acid equivalents (GAEs) per 10 g of sample using gallic acid as the standard.
The reducing power of tannic acid was determined using the method of Oyaizu (1986) with slight modification (Gülçin, 2006). Briefly, tannic acid in 1 mL of distilled water at different concentrations (15-45 μg/ mL) was mixed with sodium phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°С for 20 min. Aliquot (2.5 mL) of trichloroacetic acid (10%) was added to the mixture. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%). After that, the absorbance of the mixture was measured at wavelength of 700 nm using a spectrophotometer (OPTIZEN 2120UV, Mecasys Co., Ltd., Korea).
DPPH radical scavenging activity was determined using the method of Blois (1958). To determine the antioxidant activity of sample, DPPH radical scavenging activity (α, α-Diphenyl-β-picrylhydrazyl, sigma) was measured. Briefly, 1 mL of 1.5×10-4 M DPPH radical scavenging activity solution was added to 4 mL of test solution and stirred. The mixture was then incubated at room temperature for 30 min. The absorbance of the mixture was then measured at wavelength of 517 nm using a spectrophotometer (OPTIZEN 2120UV, Mecasys Co., Ltd., Korea).
Sensory evaluation of GY0, GY1, GY3 and GY5 (stored at 4°С) was estimated by 30 trained panelists, 3 d after production. These panelists were members of staff and students at Konkuk University, Seoul, Korea. Each item evaluated was given a score of 7 points hedonic scale; like extremely = 7, like very much = 6, like moderately = 5, neither like nor dislike = 4, dislike moderately = 3, dislike very much = 2, dislike extremely = 1. The following items were evaluated: color, flavor, sweet, sour, texture, oily, and overall sensory characteristic. Descriptions about these items were given to panelists prior to evaluation.
All data from three replicates were analyzed by one-way analysis of variance using SPSS/PC Statistics 18.0 software (SPSS Inc., USA). All data are presented as means with standard deviation. Duncan’s multiple range tests were used to determine significance among means. Statistical significance was considered when p-value was less than 0.05 (p<0.05).
Results and Discussion
Results of pH and titratable acidity value of yogurt added green olive powder stored at 4°С for 15 d are shown in Table 2. The pH value of yogurt after finishing fermentation ranged from 4.38 to 4.41. After that, incipient pH of storage ranged from 4.44 to 4.55. The decrease of pH during the storage might be due to accumulation of lactic acid by metabolic activity of bacteria (Tseng and Zhao, 2013). The pH was decreased in all treatment groups except the GY5 group during the storage. It might be resulted from the number of lactic acid bacteria, and that of GY5 was fewer than other groups (Table 4) in every measured day. The conversion of lactic acid from lactose by lactic acid bacteria would be relatively decreased in GY5 than other groups due to its lower amount of lactic acid bacteria during storage and it result in constant pH in GY5.
1)GY0: control; GY1: yogurt added with 1% green olive; GY3: yogurt added with 3% green olive; GY5: yogurt added with 3% green olive.
2)Means with different superscripts (A-D in the same column and a-d in the same row) differ significantly (p<0.05).
All values are means ± standard deviation for three replicates.
According to Lee and Hwang (2006), the optimum pH of thick fermented milk coming into the market is from 3.27 to 4.59. In this study, after storing yogurt for 15 d at 4°С, the pH also fell into this optimum range. This mean that the quality of yogurt added green olive yogurt is not different from the quality of fermented milk in the market.
The initial titratable acidity values of yogurt ranged from 0.92 to 0.94%. After storage, titratable acidity values were increased in all treatment groups. After 15 d of storage, titratable acidity values ranged from 1.07 to 1.14%. Davis (1970) has reported that titratable acidity of normal product is 0.72 to 1.20%. Titratable acidity of yogurt samples in this study fell into this range.
Results of viscosity and syneresis of yogurt samples after storing at 4°С for 15 d are shown in Table 3. Experimental groups showed no significant changes in viscosity up to 5 d except for GY5 which showed significant reduction in viscosity. After 10 d of storage, viscosity of GY1 was not significantly changed compared to its initial value. After storage of five days, viscosity of GY1, GY3, and GY5 were higher than that of GY0 (p>0.05). The more time went by, the more viscosity of GY5 decreased (p<0.05), and GY3 decreased after 10 d significantly. GY1 didn't show significant difference through all storage duration, and GY0 showed a decreasing tendency after 5 d when comparing with 1 d.
1)GY0: control; GY1: yogurt added with 1% green olive; GY3: yogurt added with 3% green olive; GY5: yogurt added with 3% green olive.
2)Means with different superscripts (A-D in the same column and a-d in the same row) differ significantly (p<0.05).
All values are means ± standard deviation for three replicates.
This result was in agreement with the result of a previous study showing that yogurt added with yuza extract has higher viscosity than that of the control (Lee and Kim, 2008). Because green olive powder was added to yogurt during manufacturing of green olive yogurt, solid content was increased which might have increased the viscosity (Lee and Kim, 2008). Ramirez-Santiago et al. (2010) have reported that viscosity is increased when fiber is added to yogurt. Therefore, dietary fiber from table olive might have affected the viscosity of yogurt containing green olive powder as suggested by Jiménez et al. (2000).
Syneresis is directly affected by acidity and is inversely proportional to pH (Fox et al., 2000) Results of syneresis of yogurt in this study revealed that the longer the storage duration, the higher the syneresis. When pH is lower (especially below 5.0), casein will approach to isoelectric point. Therefore, electrostatic repulsion is minimized because protein-to-protein interaction is promoted (Visser et al., 1986; Marchesseau et al., 1997). The ability of protein interacting with water and water-holding capacity of protein matrix will be decreased below pH 5.0 (Pastorino et al., 2003).
Results of the number of lactic acid bacteria in yogurt stored at 4°С for 15 d are shown in Table 4. The number of initial lactic acid bacteria in all groups was over 9.0 Log CFU/g. The number of lactic acid bacteria in yogurt after storage for 15 d was decreased to 7.7-8.7 Log CFU/g. Such number of lactic acid bacteria in all groups after 15 d of storage was high enough compared to standard yogurt. In intestinal canal, microbial count of probiotics for useful action is estimated to be at least 6 Log CFU/g (Akalin et al., 2004) The number of total lactic acid bacteria of fermented drinks in Codex is regulated to be more than 7 Log CFU/g. However, when green olive powder is added to yogurt, lactic acid bacteria tend to be decreased more than control yogurt after 10 d of storage. Therefore, additional studies are needed to determine the antimicrobial action and the proper amount of green olive powder to be added to yogurt.
1)GY0: control; GY1: yogurt added with 1% green olive; GY3: yogurt added with 3% green olive; GY5: yogurt added with 3% green olive.
2)Means with different superscripts (A,B in the same column and a,b in the same row) differ significantly (p<0.05).
All values are means ± standard deviation for three replicates.
Results of total polyphenol contents and antioxidant activity in yogurt after storage at 4°С for 15 d are shown in Table 5. TPC values in GY0, GY1, GY3, and GY5 were 4.30, 4.51, 5.85, and 6.96 mg GAE/kg, respectively in the 1st day. The longer the storage duration, the lower the TPC (p>0.05). This result was consistent with results of a previous study showing that TPC values of yogurt added with grape and callus extracts were decreased when storage period was longer (Karaaslan et al., 2011) Temporary decrease of TPC in yogurt could be decomposition of polymeric phenolics in the presence of lactic acid bacteria during refrigerated storage (Dalling, 1986).
1)GY0: control; GY1: yogurt added witah 1% green olive; GY3: yogurt added with 3% green olive; GY5: yogurt added with 3% green olive.
2)Means with different superscripts (A-C in the same column and a-d in the same row) differ significantly (p<0.05).
All values are means ± standard deviation for three replicates.
Reducing power values of all groups were decreased throughout the overall storage period compared to their initial values. This result was in agreement with results of Trigueros et al. (2014) showing that longer storage period decreased the reducing power of pomegranate yogurt. However, on day 15 of storage, reducing power values of GY0, GY1, GY3, and GY5 were not significantly different.
DPPH radical scavenging activity of GY5 and GY3 were at 47% and 44% each, which was the highest among all groups. After 15 d of refrigerated storage, DPPH radical scavenging activities of GY0, GY1, GY3, and GY5 groups were decreased to 21%, 26%, 27%, and 29%, respectively. This is consistent with results of a previous study showing that DPPH radical scavenging activity of yogurt added with grape and callus extracts yogurt after storage of 14 days is decreased 1.16-3.78 times (Karaaslan et al., 2011).
It is generally known that antioxidant activities of plants are highly correlated with their phenolic compounds (Pérez-fons et al., 2010), consistent with the results of this study. It has been reported that green olive has plenty of polyphenol compounds such as oleuropein (Amiot et al., 1986). Hydrolysis of milk protein or organic acid production might have also contributed to the antioxidant activity of olive yogurt due to microbial metabolic activity during fermentation and refrigerated storage.
Results of color L*, a*, and b* values of yogurt after storage at 4°С for 15 d are summarized in Table 6. The higher the concentration of green olive added to yogurt and the longer the storage duration, the lower the value of L*. When green olive was added at higher concentration, the value of a* was decreased more. Because the color of green olive is dark green, if large amount of green olive is added to yogurt, values of L* and a* would be lower naturally. Furthermore, this is because green olive contains chlorophylls, pheophytins, and β-carotene that can decrease the value of a* (Rahmani and Saari, 1991). Longer storage duration increased the value of a* more. The more concentration of green olive powder increased, the more value of b* increased (p<0.05), and the longer period, the more value decreased generally (p>0.05).
1)GY0: control; GY1: yogurt added with 1% green olive; GY3: yogurt added with 3% green olive; GY5: yogurt added with 3% green olive.
2)Means with different superscripts (A-D in the same column and a-d in the same row) differ significantly (p<0.05).
All values are means ± standard deviation for three replicates.
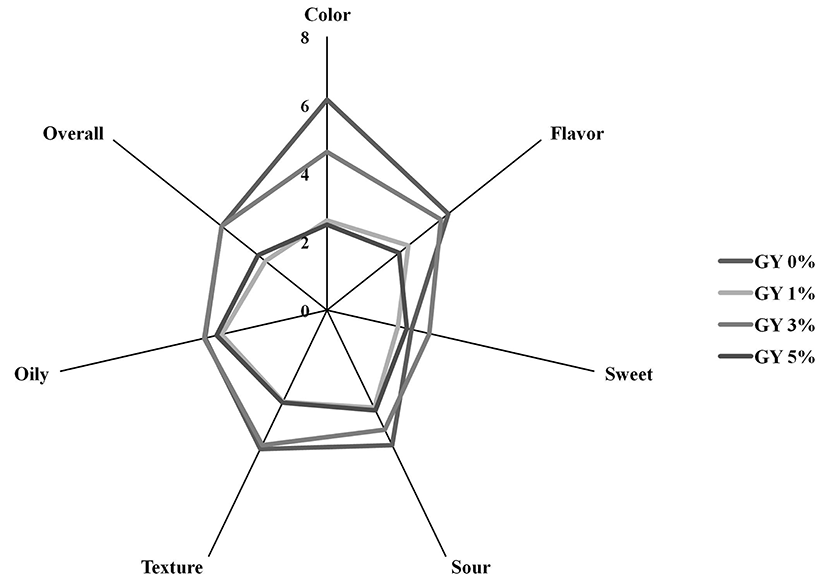
Sensory test of the manufactured yogurt was implemented through acceptability test after different amounts of green olive powder was added to yogurt. As shown in Fig. 1, GY3 yogurt color value was 4.63, which was the highest among all experimental groups. GY0 had the highest sour score. Sour scores of GY3 and GY5 groups were 3.88 and 3.25, respectively, without significant difference. Sour score of GY1 was the lowest. Texture scores of GY0 and GY3 were 4.5 and 4.38, respectively, without significant difference between the two. These scores were higher than those of the other two groups. Oily scores of all groups were not significantly different from each other. Flavor scores of GY0 and GY3 were 4.53 and 4.25, respectively, without significant difference between the two. Sweetness score of GY3 was 3.06 which was the highest among all groups. Overall scores of GY0 and GY3 were both 3.94, which were higher than those of GY1 and GY3 (at 2.31 and 2.59, respectively, p>0.05). Because sweetness can influence sensory characteristics, the overall value of GY3 is found to be the highest.
Conclusions
Adding green olive powder to yogurt for intensifying fiber improved the antioxidant function. GY3 and GY5 were indicated higher viscosity than that in GY0 and GY1 until 5 d stored. Total count of lactic acid bacteria was no significant among experimental group except GY5 until 10 d. Antioxidant activity in green olive powder added yogurt was higher than that in GY0 during 15 d. Overall scores of GY0 and GY3 were both 3.94 in sensory evaluation. Yogurt added 3% of the green olive powder has better antioxidant effect than GY0. In sensory evaluation, it shows similar score with GY0. This study showed that yogurt added 3% of green olive produced the acceptable product that influenced to substantial helpful health. Therefore, potential functionality of yogurt added green olive can be confirmed.