Introduction
For last decades, abuse of antibiotics has increased the prevalence of antibioticresistant bacteria, resulting in significant problems to human health (Fani et al., 2007). Therefore, investigation into new antibacterial agents is required. Currently, there are negative perceptions regarding synthetic additives, and natural materials are attractive as alternative antimicrobial agents because of their limited effects on the environment and their low toxicity to human cells(Kaya et al., 2008). Various lifeforms, including microorganisms, plants, and animals, have been investigated to identify novel antimicrobial agents that possess these favored characteristics (Guliani et al., 2007; Yoon and Choi, 2010).
Plant-derived extracts, which are biodegradable and ready available, are known to possess antimicrobial activity against bacteria, yeast, and mold. Furthermore, they contain various antimicrobial components and phytochemicals, including alkaloids, terpenes, essential oils, and phenols. Therefore, plant-derived compounds are considered to be valuable sources of potential novel antimicrobial agents (Liu et al., 2007). For instance, a mixture of lemon and cherry powder was effective at inhibiting Clostridium perfringens growth in frankfurters and ham (Jackson et al., 2011), and an extract from the stem bark of Irvingia gabonensis, known as African mango, also showed antimicrobial activity on various bacteria such as Enterococcus faecalis, Staphylococcus aureus, and Bacillus cereus at concentrations of 75-160 μg/mL (Kuete et al., 2007).
Psoraleae semen is the seed of natural herb Psoralea corylifolia L. (Shim et al., 2009). It has been used in traditional Asian medicine, and it has been proved that they have many pharmacological effects including anti-angiogenesis, anti-cancer, and promoting bone formation (Hwang et al., 2013; Xiong et al., 2003). Extract of the plant contains many useful compounds such as bakuchiol, bavachinin, corylin, corylifolin, isopsorlen, psoracorylifols, psoralen, and psoralidin (Wong and Rabiem, 2010). In particular, bakuchiol was found to inhibit growth of Helicobacter pylori (Zaide et al., 2009), and Yoon and Choi (2012) reported that among various therapeutic herbal extracts, Psoraleae semen had a considerable antilisterial effect. Nevertheless, the antimicrobial effect of this extract on other important pathogenic bacteria remains to be determined.
In the present study, various therapeutic herbal plant extracts were screened for antimicrobial activity, and one of them, Psoraleae semen was evaluated as an antimicrobial agent in in vitro and in sausages for application in food, and its effect on bacterial cells was observed by measuring protein leakages and observing a scanning electron microscope (SEM).
Materials and Methods
Sixty-nine of herbal plant extract powder were acquired from New Natural Material Bank of Korea National Research Resources (Korea) (Table 1). The powder was produced by heating 70% ethanol extraction, followed by vacuum evaporation of the filtrate. A liquid stock was made by dissolving the extract powder in 100% dimethyl sulfoxide (DMSO; Sigma, USA) to give a final concentration of 50 mg/mL.
NNMBS: Standard sample from new natural materials bank.
2AB, Acinetobacter baumannii ATCC19606; BT, Burkholderia thailandensis E264; KP, Klebsiella pneumoniae ATCC25306; PA, Pseudomonas aeruginosa PAO1; SE, Salmonella enterica serovar Derby ATCC6960; EFS, Enterococcus faecalis ATCC35038; EFM, Enterococcus faecium ATCC19434; SA, Staphylococcus aureus ATCC25923; SM, Streptococcus mutans ATCC25175.
3-, not determined; *, no clear zone; †, <10 mm; ‡, 10 mm-12 mm; §, >12 mm.
The following bacterial strains were used in this study; Gram-negative bacteria (Acinetobacter baumannii ATCC 19606, Burkholderia thailandensis E264, Klebsiella pneumoniae ATCC25306, Pseudomonas aeruginosa PAO1, Salmonella enterica serovar Derby ATCC6960) and Grampositive bacteria (E. faecalis ATCC35038, Enterococcus faecium ATCC19434, S. aureus ATCC25923, Streptococcus mutans ATCC25175), and brain heart infusion (BHI, Becton, Dikinson and Company, USA) broth was used as the bacterial growth medium.
Each bacteria was activated and subcultured in BHI broth, and incubated at 37°C until they were grown to mid-logarithmic phase. The bacteria were mixed with 3 mL of BHI medium containing 0.8% agar to obtain an optical density of 0.1 at 600 nm (OD600), and the mixtures were overlaid on BHI agar plates. After solidifying the agar under room temperature, the paper discs (6 mm in diameter) containing 3 μL of the extract (50 mg/mL) were placed on the agar plates. After 24 h incubation at 37°C, the diameters of inhibition zones were measured.
The minimum inhibitory concentration (MIC) of Psoraleae semen extract on bacterial growth was determined using the two-fold broth micro-dilution method (NCCLS, 1997). Briefly, each bacterial strain was subcultured and was grown to mid-logarithmic growth phase in BHI broth. The bacterial cells were diluted in BHI broth to an optical density of 0.01 at 600 nm. An equal volume of the bacterial diluent was added to each well on the 96-well plate containing 100 μL of the serially-diluted Psoraleae semen extract (0-256 μg/mL). The 96-well plates were incubated at 37°C for 24 h, and the MICs were determined by measuring OD600 using a spectrophotometer (Sunrise, Tecan, Austria).
Biofilm formation experiment was performed by referring to the protocol provided by Loo et al. (2000). Bacterial cells were prepared and treated with Psoraleae semen extract following the method used to determine the MIC. Each bacterial culture was incubated in a 96-well plate containing Psoraleae semen extract (0-32 μg/mL) at 37°C for 24 h. The suspensions were carefully discarded, and the biofilm formed on the surface of the well plate was stained using 200 μL of crystal violet (1%; v/v). The stained cells were rinsed twice with 300 μL of PBS, followed by solubilizing the cells in 200 μL of 95% ethanol, and the degree of biofilm formation was determined by measuring the OD575 with a spectrophotometer.
Subcultured bacterial cells were suspended in phosphate buffered solution (PBS, pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4, 8.0 g of NaCl, and 0.2 g of KCl in 1 L of distilled water) for adjusting OD600 to 0.7. The bacterial cells were exposed to 2×MIC of Psoraleae semen extract or equal volume of DMSO for 1 h. After centrifugation, protein leakage was determined by measuring optical density of the cell supernatants using a spectrophotometer (Biophotometer, Eppendorf, Germany) at OD280. Values were calibrated with control group treated in PBS instead of cultured cells, and relative ratio (OD280 of bacterial cell exposed to the extract/OD280 of bacterial cell exposed to DMSO) was calculated.
Bacteria were incubated in BHI broth containing a glass slide to allow bacterial cells to adhere to the glass slide. Adherent cells were exposed to 2×MIC of Psoraleae semen extract in PBS, and the bacterial cells that had been treated with DMSO at the same volume as Psoraleae semen extract, were used as a negative control (Bereksi et al., 2002). After pre-fixing the treated cells in 1.8% glutaraldehyde solution (Sigma, USA), they were washed using distilled water. The bacterial cells were post-fixed with 2% osmium tetra oxide (Sigma, USA), followed by washing and dehydrating sequentially in 25, 50, 75, 90, and 100% ethanol. Bacterial cells were platinum-coated using a sputter coater 108 auto (Cressington Scientific Instruments Ltd., England) and then observed under field emission SEM (JEOL Ltd., JSM-7600F, Japan).
The experiments were replicated twice with six samples in each replication. The results were analyzed by the mixed model procedure of SAS® version 9.2 (SAS Institute, USA). Mean comparisons for the fixed effect were performed with the pairwise t-test, and significance was determined at alpha = 0.05.
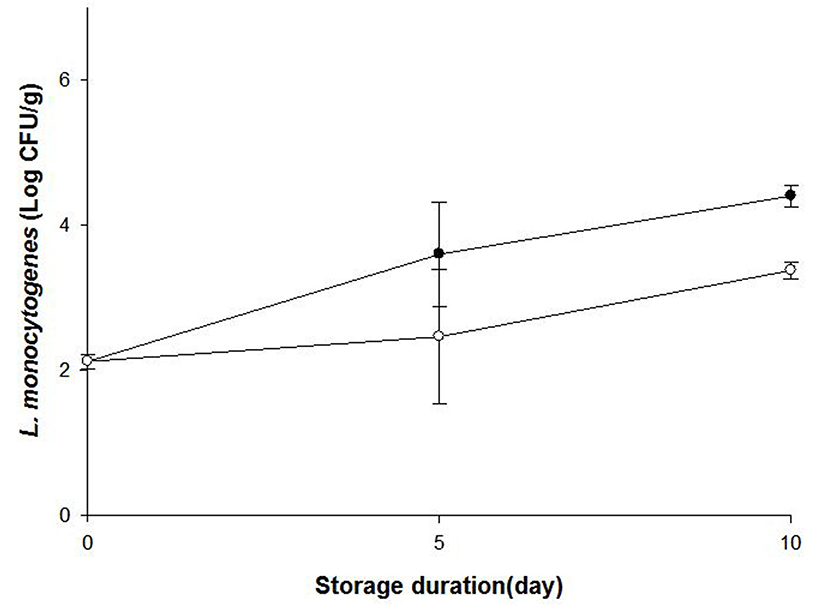
Inoculum for sausage contamination was prepared as follows. Two strains of Listeria monocytogenes (sausage isolate) cells identified and provided by Korea Consumer Agency (Korea) were incubated in BHI broth. The cultures were centrifuged at 1,912 g and 4°C for 15 min. The cell pellet was washed with PBS and resuspended in PBS. Sausage formulation was prepared by mixing lean pork (58.5%), pork fat (19.5%), NaCl (1.2%), sodium phosphate (0.3%), isolated soy protein (0.5%), spice (0.4%), and ice water (19.5%) using a blender (HR1372, Phillips, China). Ten grams of homogenized formulations were transferred into a 6-well plate, and were boiled at 80°C in a waterbath for 40 min, followed by cooling. Psoraleae semen extract (final concentration: 2×MIC) or same volume of DMSO was spread on the sausage samples, and the samples were dried. The sausages were inoculated with L. monocytogenes inoculum to obtain 2 Log CFU/g. During storage at 4°C, L. monocytogenes growth was determined by plating sausages samples on Palcam agar on day 0, 5, and 10.
Results and Discussion
In a previous study, Psoraleae semen exhibited antimicrobial activity against L. monocytogenes, and it was suggested that the herb has potential to be used as an antibacterial agent (Yoon and Choi, 2012). This preliminary evaluation was further progressed and the effect was assessed against an extended range of pathogens such as Enterococci, S. aureus and Salmonella Derby in the present study. In addition, antimicrobial activities of 69 herbal plant extracts on various pathogens including Gram-positive and Gram-negative bacteria were tested using a disc diffusion assay. Among them, only five extracts (Rubi fructus, Galla rhois, Moutan xortex Radicis, Sanguisorba officinalis Linne, and Psoraleae semen) were effective for inhibiting more than three different bacteria (Table 1). Rubi fructus inhibited growth of all bacterial strains tested in this study. Interestingly, there were no clear zones formed on the disc containing Psoraleae semen extract against any Gram-negative bacteria, but obvious clear zones (7-13 mm) were observed against Gram-positive bacteria such as S. mutans, E. faecium, E. faecalis, and S. aureus (Table 1). This contrary result observed against Gram-positive and Gram-negative bacteria in the presence of Psoraleae semen might be caused by the structural dissimilarity of the cell wall between them (Henie et al., 2009). Low permeability against lipophilic solutes and good adaptability of outer membrane is the key of resistance in Gram-negative bacteria (Plesiat and Nikaido, 1992). For example, Gram-negative bacteria have outer membranes, which may prevent the extract used in this study from exerting its antimicrobial activity. However, further study is necessary to elucidate the antimicrobial mechanisms of this extract against Gram-negative bacteria.
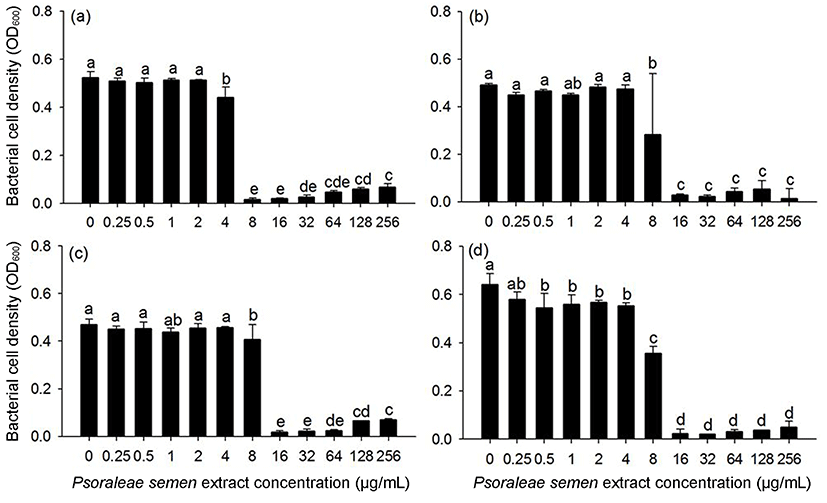
The antimicrobial effect of Psoraleae semen extract was further assessed by MIC determination for Gram-positive bacteria, which were susceptible to Psoraleae semen extract. As a result, S. mutans ATCC25175 exhibited no visible growth at 8 μg/mL, and E. faecalis ATCC35038, E. faecium ATCC19434, and S. aureus ATCC25923 exhibited no growth at 16 μg/mL (Fig. 1). Yim et al. (2013) also found that the MIC of Psoraleae semen extract was 625 μg/mL for E. faecalis and 156 μg/mL for S. mutans. This discrepancy may be due to use of different fractions of the extract for the experiment. Yim et al. (2013) used a water-soluble fraction of Psoraleae semen, whereas our group used total extract, which contains both water-soluble and water-insoluble fractions. Yoon and Choi (2012) suggested that the water-insoluble fraction of Psoraleae semen exhibits higher antimicrobial activity than the water-soluble fraction. Psoraleae semen extract contains bakuchiol in water-insoluble fraction, which has been known to have antimicrobial activity on various oral bacteria (Katsura et al., 2001), as Yoon and Choi (2012), and Yim et al. (2013) suggested that it may be a major compound responsible for the antimicrobial activity of Psoraleae semen against the bacteria.
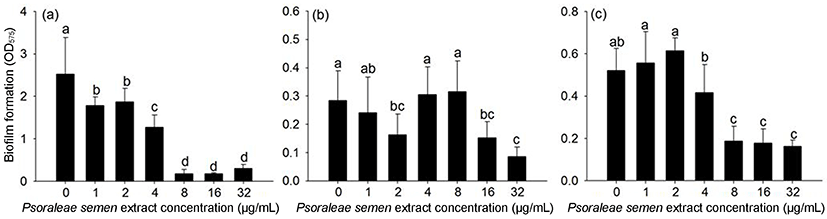
Biofilm is a collection of bacterial cells that form on surfaces in reaction to stressful environmental conditions, and biofilm formation is of concern in various areas including food safety, dental health, and medicine (Loesche, 1986). In this study, the effect of Psoraleae semen extract on biofilm formation was investigated. Biofilm formation of S. mutans ATCC25175 and S. aureus ATCC25923 was inhibited when the extract was present at concentrations greater than 8 μg/mL, and it was significantly decreased when concentrations exceeded 16 μg/mL in E. faecalis ATCC35038 (Fig. 2). S. mutans biofilms, namely plaque, are responsible for dental diseases like caries (Loesche, 1986), and S. aureus and E. faecalis biofilms are a problem in the food industry and medical devices (Hood and Zottola, 1995). Thus, our results indicate that Psoraleae semen extract may be of potential use for the inhibition of biofilm formation by these bacterial strains.
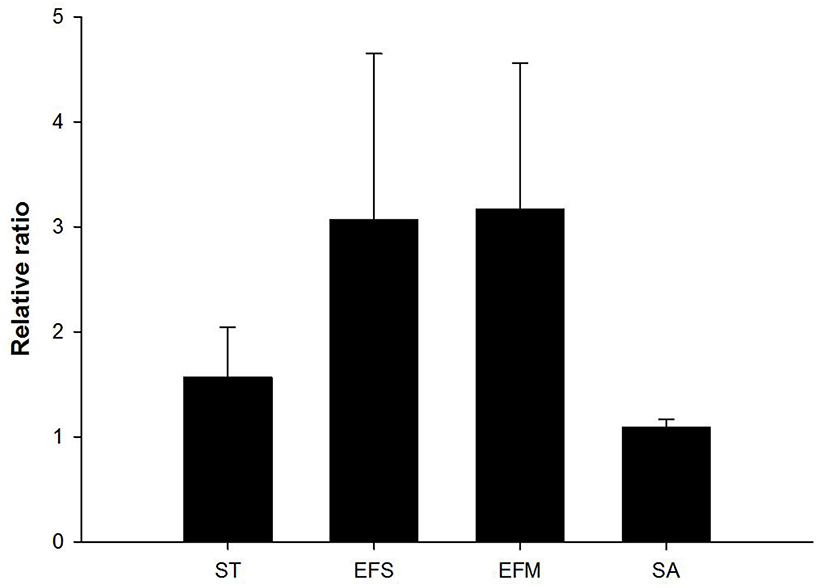
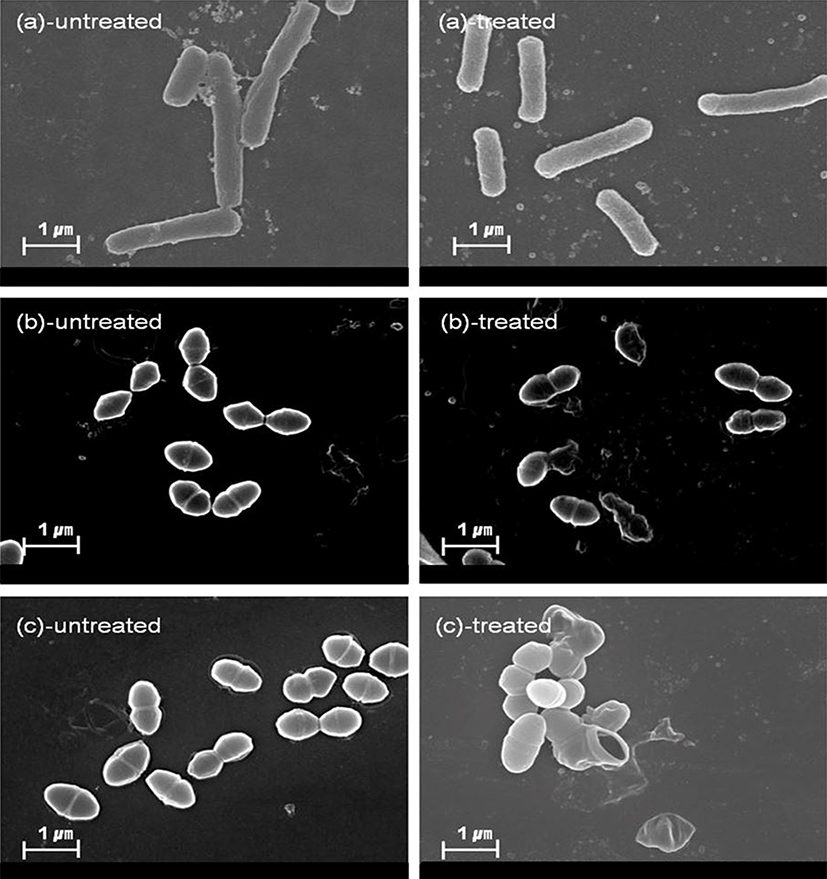
The effects of antimicrobial agents on bacterial cell structures and membranes have been widely used to investigate the mode of action of natural antimicrobial agent (Leela and Satirapipathkul, 2011). In this study, protein leakage induced by cell membrane damage was measured, and the membranes were observed by SEM analysis following exposure to Psoraleae semen. In case of Gram-positive bacteria (E. faecalis and E. faecium), values of OD280 were increased in extract-treated cells, compared to non-treated cells (relative ratios = 3.17 and 3.07 in E. faecalis and E. faecium, respectively). This result suggests that exposure of the extract to the bacterial cells induce membrane damage, resulting in leakage of intracellular proteins. Whereas, relative ratio of OD280 (= 1.57) was relatively low in Salmonella Typhimurium, a Gram-negative bacteria (Fig. 3). Further, SEM images showed that the membranes of Gram-positive bacteria, such as E. faecalis, E. faecium, and S. aureus cells have been damaged by the treatment of Psoraleae semen extract, whereas no damage was observed in Sal. Typhimurium (Fig. 4). Taken together, antibacterial activity of Psoraleae semen extract on Gram-positive bacteria seems to be attributed to this membrane damage-mediated action, but Sal. Typhimurium possessing outer membrane, which contributes to homeostasis and adaptability, avoids membrane injury by the extract. Although S. aureus is gram-positive bacteria, protein leakage was not observed in this species. This can be explained by two possible reasons as follows. First, longer incubation time may have been required for inhibition of the S. aureus growth. Briefly, MIC against S. aureus was determined after 24 h, whereas protein leakage by the extract was observed only after 1 h treatment, which can be too short to induce the effects on S. aureus compared to the other bacteria. Second, structural difference of the bacteria probably causes discrepant outcome. S. aureus cell wall is solubilized by lysostaphin, unlike the ones of other Gram-positive bacteria can be solubilized by lysozyme (Navarre and Schneewind, 1999).
A Gram-positive bacterial pathogen L. monocytogenes usually contaminates livestock products such as ham and sausages, and can grow at low storage temperature. Our research group previously demonstrated that Psoraleae semen extract (named bogolgi) had antibacterial effects on this pathogen in in vitro environment (Yoon and Choi, 2012). Subsequently, current study investigated this antimicrobiacl activity of Psoraleae semen extract on livestock products-originated L. monocytogenes in sausages at refrigeration temperature. As a result, the growth of L. monocytogenes was impeded in the sausages treated with the extract, compared to the ones treated with DMSO as a negative control on day 10 at 4°C (Fig. 5). This result may suggest that Psoraleae semen extract could be applied to control L. monocytogenes growth in processed meat products. Although Psoraleae semen extract exhibits these antimicrobial activities, it was also reported that high dosage of psoralens, the component of Psoraleae semen could induce phototoxicity (Wu et al., 2005). Thus, further study is necessary to find appropriate conditions and procedure to use Psoralea semen extract as food antimicrobial.
In conclusions, Rubi fructus, Galla rhois, Moutan xortex Radicis, Sanguisorba officinalis Linne, and Psoraleae semen have antimicrobial activity against various pathogenic bacteria. Among them, Psoraleae semen extract could be used as a natural antimicrobial to control the growth of Gram-positive bacteria including Enterococci, S. aureus, and L. monocytogenes.













