Introduction
Nitrite is a typical curing agent widely used in meat products because it promotes formation of the red color of cured meats, provides the cured flavor, has a bacteriostatic impact, and acts as an antioxidant for suppressing lipid oxidation (Pegg and Shahidi, 2000).
In the interest of health-conscious consumers in obtaining natural and organic foods, the demand for chemical-free foods using natural substances has increased in recent years (Choi and Chin, 2003). Despite the high price of organic and natural foods, they are preferred by health-conscious consumers instead of regular foods (Winter and Davis, 2006). For these reasons, the addition of natural materials to meat products instead of chemical compounds is being actively pursued in the meat processing area (Jin et al., 2014). Due to these trends, natural nitrite replacements have been developed as alternatives to synthetic nitrite in the meat processing industry (Alahakoo et al., 2015).
Vegetables such as Swiss chard, celery, spinach, and lettuce are known to contain a considerable amount of nitrates that can be converted to nitrite using nitrate-reducing starter cultures such as Staphylococcus carnosus and Staph. xylosus (Santamaria, 2006; Simonová et al., 2006). Krause et al. (2011) reported the commercial use of pre-converted vegetable juice and powders as alternatives to synthetic nitrite for production of meat products. Among the pre-converted vegetables, celery powder has been used as a natural nitrite source because addition of celery does not generate any off-flavors (less than 0.2-0.4%) in meat products (Sebranek and Bacus, 2007; Sindelar et al., 2007b). However, because celery is a frequent cause of food allergy, celery-allergic patients have to completely avoid it owing to the absence of alternatives (Ballmer-Weber et al., 2002). Therefore, meat-processing industries have been searching for novel sources of natural nitrite as substitutes for synthetic nitrite.
Swiss chard (Beta vulgaris var. cicla), a leafy vegetable, is well known for containing high levels of nitrate (2754.0 ppm) and has antioxidant components such as phenolic acids and flavonoids (Bosch Bosch et al., 1985; Pyo et al., 2004). Notably, Sebranek et al. (2012) reported that the main advantage of using Swiss chard was the absence of allergens, unlike the case with celery. Previous studies reported that Swiss chard has high antioxidant and antimicrobial capacities (De la Hoz et al., 1991; Ponce et al., 2003). However, scientific literature on nitrite replacement in meat products treated with pre-converted nitrite is still insufficient. Thus, the objective of this study was to determine the effect of Swiss chard as source of preconverted nitrite on the color stability and shelf-life of cooked pork patties over the course of 28 d at 4°C.
Materials and Methods
Commercial Swiss chard powder (Hangaram GF Co., Ltd., Korea) and pre-converted celery powder (Sias Co., Ltd., Korea) were purchased from the local market. Ten grams of Swiss chard powder was mixed with 100 mL of distilled water for 30 min. Subsequently, 0.025% of a nitrate reductase-active starter culture containing Staphylococcus carnosus (S-B-61, BactofermTM, Chr. Hansen Inc., USA) was added to the mixture, followed by incubation at 37°C for 24 h in a shaking incubator. The samples were filtered through a Whatman No.1 filter paper (Whatman International, UK) and concentrated at <50°C using a rotary evaporator (EYELA N-1000, Rikakikai, Japan). The concentrated extract was spray-dried using a spray dryer (SD Pilot 2010, Ain system, Korea). The physicochemical properties of the powders are summarized in Table 1.
All values are mean ± standard deviation of three replicates.
Fresh pork ham (M. semimembranosus) and pork back fat were purchased from a local processor at 48 h postmortem. All subcutaneous and intramuscular fat and visible connective tissue were removed from the fresh ham muscle and the pork ham and back fat were ground through a 3 mm plate using a meat grinder (PM-70, Mainca, Spain). The ground meat and back fat were packaged in polyethylene bags, vacuum-sealed, and used on the same day. Table 2 shows the formulation of the ground pork patties used in this study. The nitrite contents in the pork patties from pre-converted nitrite in Swiss chard powder (PS) and pre-converted nitrite in celery powder (PC) were adjusted to 120 ppm. The addition of 1% PS or 1% PC was approximately equal to 60 ppm nitrite (see Table 1). The nitrite levels in PS and PC were measured by applying the diazo coupling method (KFDA, 2013). Each batch was mixed for 12 min using a mixer (RM-90, Mainca, Spain). Each mixed sample was divided into 5 smaller portions (about 100 g each) and subjected to storage for the five periods (0, 7, 14, 21, and 28 d). The samples were produced as 100±1 g patties with a diameter of 10 cm and thickness of 1.5 cm using patty presses (small ground press, Spikomat Ltd., UK). The patties were then vacuumpackaged in polyethylene bags for cooking. The pork patties were considered fully cooked when the core temperature reached 75°C and was maintained for 30 min in a water bath; the sample was then cooled to room temperature for 2 h and vacuum packed in polyethylene bags and stored at 4±1°C for 28 d. Five treatments were formulated as follows: Control (120 ppm nitrite), T1 (2% PS), T2 (2% pre-converted nitrite from celery powder; PC), T3 (1% PS + 60 ppm nitrite), and NC (nitrite-free).
1)PS: pre-converted nitrite from Swiss chard powder.
2)PC: pre-converted nitrite from celery powder.
The pH of the cooked pork patties was determined using a pH meter (Model 340, Mettler-Toledo GmbH, Switzerland). Each 5 g sample of cooked pork patty was homogenized in a homogenizer (Ultra-Turrax T25, Janke and Kunkel, Germany) with 20 mL distilled water. All determinations were performed in triplicate.
The color was measured at the surface of the cooked pork patties using a colorimeter (Minolta Chroma Meter CR-210, Japan; Illuminate C, calibrated with a white plate, L*=+97.83, a*=−0.43, b*=+1.98) during the storage periods. The color is expressed in terms of the L* (lightness), a* (redness), b* (yellowness), ∆E (color difference), H˚ (hue angle), and C* (chroma) value. The ∆E, H˚, and C* values were calculated using the following equations: ∆E = [(L*−L0*)2 + (a*−a0*)2 + (b*−b0*)2]1/2, H˚ = tan(b*/a*) and C* = [(a*)2+(b*)2]1/2, respectively (Artes et al., 2002; Backhaus et al., 1998; Clydesdale, 1998).
The nitrosoheme and total pigment of the cooked pork patties were measured after extraction in 80% acetone and acidified acetone by using the method reported by Honsey (1956). The nitrosoheme and total pigment contents were calculated using the following equation: nitrosoheme pigment content (ppm) = A540 nm × 290; total pigment content (ppm) = A640 nm × 680; cure efficiency (%) = (ppm of nitrosoheme pigment ÷ ppm of total pigment) × 100.
The residual nitrite content in the pork patties was determined according to the diazo coupling method (KFDA, 2013) and is expressed as ‘ppm of pork patties’. Briefly, a 10 g sample of the pork patty was placed in a 200 mL volumetric flask, to which 150 mL of preheated distilled water (80°C) was added. The samples were combined with 10 mL of 0.5 N NaOH and 10 mL of 12% (NH4)2SO4 solution and then heated in a water bath at 80°C for 30 min. After cooling, the samples were made up to 200 mL by addition of 20 mL CH3COONH4 buffer (pH 9.0) and 10 mL of distilled water. The samples were held for 10 min at room temperature and filtered through a No. 1 filter paper. After filtration, 1 mL of sulfanilamide solution and 1 mL of N-(1-naphthyl)ethylenediamine dihydrochloride reagent were added to the tube containing 20 mL of filtrate and kept at room temperature for 20 min. The absorbance at 540 nm was read using a UV/VIS spectrophotometer (Optizen 2120 UV Plus, Mecasys Co., Ltd., Korea). The residual nitrite content was calculated from the standard curve constructed using nitrite solutions.
The lipid oxidation was assessed in triplicate for each sample using the thiobarbituric acid (TBA) method presented by Tarladgis et al. (1960) with minor modifications. Approximately, 10 g of sample was blended with 50 mL of distilled water for 2 min using a homogenizer (Model AM-7, Nihonseiki Kaisha Ltd., Japan) and then transferred to a distillation tube. The cup used for blending was washed with an additional 47.5 mL of distilled water, which was added to the same distillation flask along with 2.5 mL of 4 N HCl and a few drops of an antifoam agent (KMK-73, Shin-Etsu Silicone Co., Ltd., Korea). The mixture was distilled and 50 mL of the distillate was collected. Five milliliters of 0.02 M 2-thiobarbituric acid in 90% acetic acid (TBA reagent) was added to test tube containing 5 mL of distilled water and mixed thoroughly. The tubes were capped and heated in a boiling water bath for 30 min to develop chromogen and cooled to room temperature for 10 min. The absorbance was measured at 538 nm against a blank prepared with 5 mL of distilled water and 5 mL of TBA-reagent using a UV/VIS spectrophotometer (Optizen 2120 UV Plus, Mecasys Co., Ltd., Korea). The TBA values were calculated as mg MDA/kg meat using the formula: TBA (MDA mg/meat kg) = (optical density of sample-optical density of blank) × 7.8.
Each sample was evaluated in terms of the color, flavor, off-flavor, salty taste, tenderness, juiciness, and overall acceptability. The trained sensory panel consisted of ten researchers from the Department of Food Sciences and Biotechnology of Animal Resources at Konkuk University in Korea (Choi et al., 2008). The samples were cut into quarters with a length of 2 cm and served randomly to the panelists. Sensory evaluations were performed by the panelists under fluorescent lighting. The panelists were instructed to cleanse their palates between samples using water. The color (1 = extremely undesirable, 9 = extremely desirable), flavor (1 = extremely undesirable, 9 = extremely desirable), off-flavor (1 = extremely undesirable, 9 = extremely desirable), tenderness (1 = extremely tough, 9 = extremely tender), juiciness (1 = extremely dry, 9 = extremely juicy), and overall acceptability (1 = extremely undesirable, 9 = extremely desirable) of the cooked pork patties were evaluated using a 9-point descriptive scale, using the hedonic test (Bergara-Almeida and Da Silva, 2002).
To determine the total viable count (TVC) of E. coli and coliform bacteria for each sample during storage, 25 g samples were aseptically transferred to a sterile stomacher bag containing 225 mL of 0.1% peptone water, followed by pummeling the samples in the stomacher (Masticater-Paddle-Blender, IUL Instrument, Spain) for 2 min. The homogenates were serially diluted with 0.1% peptone water. The diluents (1 mL) were then placed in Petri dishes. A total of 20 mL of plate count agar (PCA; Difco, USA) was poured over the diluents. After the medium solidified, the plates were incubated at 37°C for 48 h. The colonies developed on these plates were manually counted. Petrifilm (3M, Korea) was used to cultivate the diluted sample. Colonies with blue bubbles were counted as E. coli and those with purple bubbles and blue bubbles were counted as coliform bacteria.
The 5 (treatment) × 5 (storage period) factorial design including two factors, treatment and storage period, carried out in triplicate, was analyzed by the two-way ANOVA (two-way analysis of variance) test. The ANOVA was performed on all the variables using the General Linear Model (GLM) procedure of SPSS Ver. 24.0 (SPSS Inc., USA). Mean separations were performed using Duncan’s multiple range tests and were used to determine significant differences among the treatments (p<0.05).
Results and Discussion
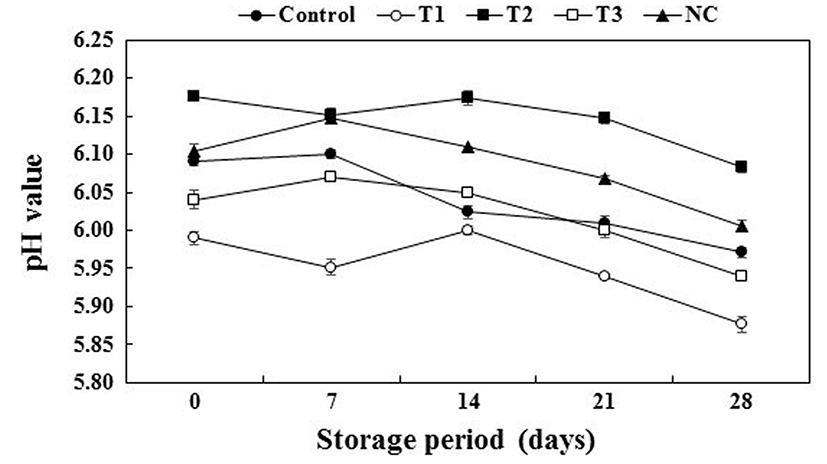
Fig. 2 shows the change in the pH values of the cooked pork patties during refrigerated storage for 28 d. The pH of the pork patties may be affected by the additives in the patties. The pH of the cooked pork patties decreased with increasing levels of PS (pH 5.39±0.01) (p<0.05). As a similar result, Kim et al. (2017) reported that the addition of different concentrations of pre-converted spinach extract affected the pH of meat products. In addition, the pH of the T2 sample was the highest due to the relatively high pH (8.83±0.01) of PC (p<0.05). Sindelar et al. (2007a) noted that the pH of cured meat increased after adding vegetable juice powder. As the storage period progressed, the pH values of all samples decreased. Choi et al. (2007) reported similar results, wherein the pH of meat products generally decreased during storage. Moreover, the major factor influencing the decrease in the pH was the storage time, and refrigerated storage also caused a decrease in the pH values due to the activity of lactic acid bacteria and dissolution of CO2 into the pork patties (Rubio et al., 2007).
The nitrosoheme and total pigment contents of the cooked pork patties are shown in Table 3. The samples to which PS was added had higher nitrosoheme pigment contents that those subjected to other treatments (p<0.05). T1 and T3 gave rise to higher nitrosoheme pigment contents than the other treatments (p<0.05). These results are in accordance with those of Kim et al. (2015), who reported that the use of various phenolic acids and flavonoids from natural sources as a natural reducing agent could promote the formation of nitrosoheme pigments. Pyo et al. (2004) noted that syringic acid and kaempferol were the main phenolic acid and flavonoids in Swiss chard. Moreover, the nitrosoheme pigment content of NC was about 5.66 ppm, despite the lack of addition of nitrite. According to the World Health Organization (2003), nitrate and nitrite are naturally generated ions that are commonplace in drinking water. Thus, the formation of the nitrosoheme pigment may occur with the use of tap water in this study. The total content of heme pigment in the cooked pork patties ranged from 60.52-68.34 ppm. T1 gave rise to the highest total pigment content relative to the other treatments (p<0.05). Deniz and Serdaroğlu (2003) reported that part of the nitrosoheme pigment was converted to total heme pigment, which is bound to nitric oxide by nitrogen dioxide. The cure efficiencies of T1 and T3 were higher than 80% (p<0.05). According to Pearson and Tauber (1984), well-cured meats have a nitrosoheme pigment content comprising above 80% of the total heme pigment content. Thus, the addition of PS to the pork patties might account for the formation of nitrosoheme pigment.
All values are mean ± standard deviation of three replicates.
a-dMeans within a row with different letters are significantly different (p<0.05).
1)Treatments: Control-pork patties with 120 ppm sodium nitrite; T1-pork patties with 2% PS; T2-pork patties with 2% PC; T3-pork patties with 60 ppm sodium nitrite and 1% PS; NC- nitrite-free.
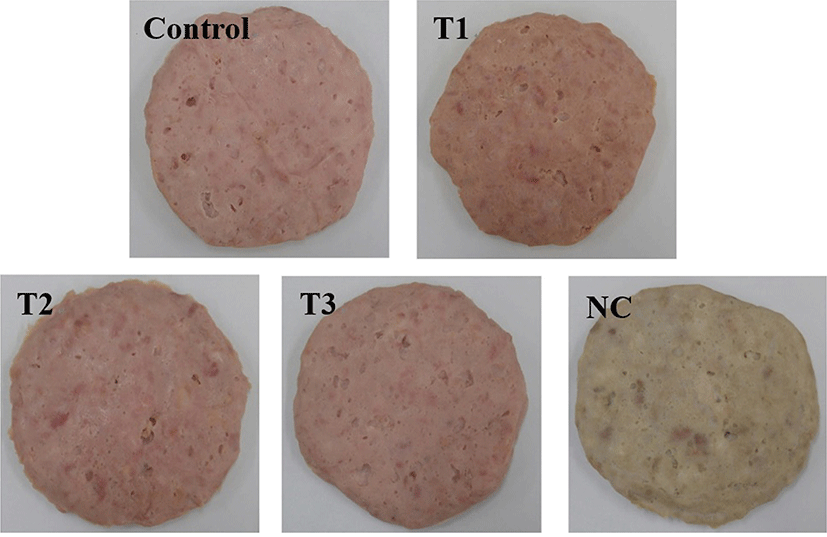
The change in the color of the cooked pork patties during refrigerated storage is shown in Table 4. The pink color of cured meat products is completely dependent on the formation of nitrosoheme pigment (Cornforth et al., 1986). Therefore, the higher nitrosoheme pigment content of the T1 and T3 samples might account for the higher redness values of T1 and T3. The redness value of the pork patties increased with an increase in the PS level (p<0.05). Similar results were obtained by Kim et al. (2017), showing that the redness of cured pork loins treated with fermented spinach extract increased with increasing levels of fermented spinach extract. According to Choi et al. (2011), a greater total color difference (∆E) and higher values of the hue angle (H˚) and chroma difference indicate more brown and more intensely red, respectively. The T3 sample had the lowest color difference (p<0.05). The color difference and hue angle of the T1 sample were the highest (p<0.05) among those of the treated samples, except for the NC sample. The color difference and hue angle of the pork patties differed significantly based on the level of PS due to the yellowness of PS. The T1 sample had the highest chroma difference (p<0.05). These results are in accordance with the redness value of T1. Similarly, Calvo et al. (2008) reported that tomato peel affected all color parameters for dry fermented sausages. Thus, pre-converted nitrite from Swiss chard powder could positively affect the color formation in cooked pork patties.
a-cMeans within a row with different letters are significantly different (p<0.05).
A-DMeans within a column with different letters are significantly different (p<0.05).
1)Treatments: Control-pork patties with 120 ppm sodium nitrite; T1-pork patties with 2% PS; T2-pork patties with 2% PC; T3-pork patties with 60 ppm sodium nitrite and 1% PS; NC-nitrite-free.
2)Traits: CIE L*-Lightness; CIE a*-redness; CIE b*-yellowness; ∆E-color difference; H˚-hue angle; C*-chroma. ∆E, H˚, and C* values were calculated using the following equations: ∆E= [(L*− L0*)2 + (a*− a0*)2 + (b*− b0*)2]1/2, H˚ = tan(b*/a*) and C* = [(a*)2 + (b*)2]1/2.
3)SEM: Standard error of the mean (n=8).
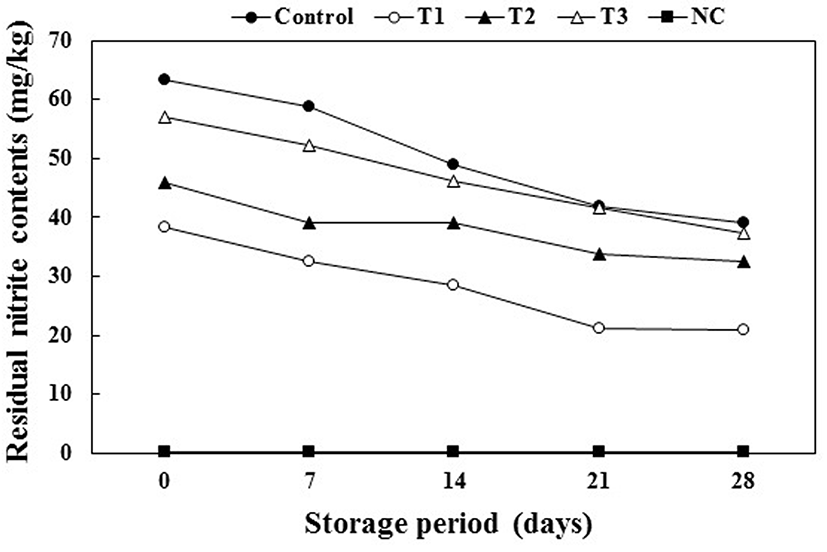
Fig. 3 shows that the residual nitrite content was significantly affected by the storage period and all treatments (p<0.05). The initial residual nitrite content obtained with all treatments ranged from 63.29-38.36 ppm, with the content markedly decreasing from 39.21 to 20.93 ppm after 28 d; however, the initial residual nitrite content of NC was less than 0.3 ppm for all storage periods. The residual nitrite content of the control was higher than that of the T1, T2, and T3 samples during refrigerated storage. Furthermore, the residual nitrite content of the T1 sample was lower than that of the T2 and T3 samples. The residual content of synthetic nitrite was higher than those obtained with nitrite from vegetable sources and the lower pH and additional antioxidant compounds derived from the vegetable extracts, which provide reducing conditions for forming nitric oxide, led to lower residual nitrite contents (Gabaza et al., 2013; Li et al., 2013). Thus, the lower pH (5.39±0.01) and the antioxidants such as syringic acid and kaempferol from PS may be associated with the decrease in the residual nitrite levels.
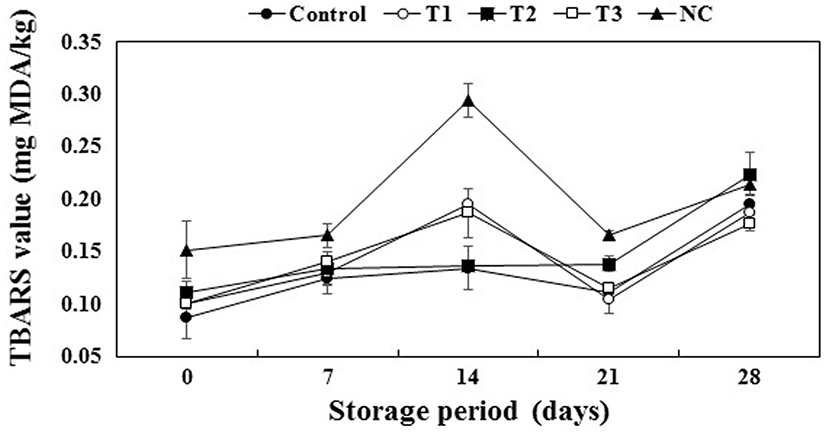
The TBARS values of the cooked pork patties stored for 0, 7, 14, 21, and 28 d is shown in Fig. 4. All treatments and storage times significantly affected the TBARS values (p<0.05). All treatments led to an increase in the TBARS values for the initial 14 d, followed by a decrease up to 21 d, with a subsequent increase again on the final day. Similar findings have been reported for restructured beef steak with walnut (Serrano et al., 2006). This pattern of variation of the TBARS values during storage has been attributed to the interaction between the meat proteins and TBA-reactive substances (Bhattacharya et al., 1988). Interestingly, the addition of PS in treatments T1 and T3 led to lower TBARS values than the other treatments after 28 d (p<0.05). Syringic acid, the main phenolic acid in Swiss chard, possesses strong free-radical scavenging capacity (Espín et al., 2000; Pyo et al., 2004). Sánchez-Escalante et al. (2001) demonstrated that beef patties with rosemary had lower TBARS values due to the presence of carnosine that possesses free-radical scavenging ability. Hwang et al. (2011) indicated that lipid oxidation was suppressed by the antioxidant effects related to phenolic compounds and flavonoid. According to Nuutila et al. (2003), kaempferol derived from onion extracts influenced the inhibition of lipid oxidation. Furthermore, the combination of PS and nitrite (T3) was effective for inhibiting lipid oxidation in pork patties. Thus, the combination of PS and nitrite as antioxidants might result in synergistic inhibition of lipid oxidation in meat products (Donald et al., 1980).
The change in the sensory properties of the cooked pork patties during refrigerated storage was expressed in terms of the color, flavor, off-flavor, tenderness, juiciness, and overall acceptability as summarized in Table 5. There were no significant differences in the color, tenderness, and juiciness during storage, except in the case of NC. The flavor, off-flavor, and overall acceptability of the T3 sample were higher than achieved with the other treatments. According to Sindelar et al. (2007), level of celery powder increased with decreased flavor of ham due to vegetable aroma and flavor increased. The flavor, off-flavor, and overall acceptability of the T2 sample were maintained for 14 d, followed by a decrease on the final day, whereas these traits were maintained in the T3 sample until the end of storage. Similarly, Lee et al. (1999) demonstrated that natural antioxidants were available to minimize the generation of warm-over flavors derived from lipid oxidation. Further, the lower TBARS values for the samples with added Swiss chard powder positively affected the off-flavor of the cooked pork patties (Choe et al., 2011). Thus, the addition of PS with a high content of antioxidants such as syringic acid and kaempferol might be effective for suppression of flavor deterioration.
a-cMeans within a row with different letters are significantly different (p<0.05).
A-DMeans within a column with different letters are significantly different (p<0.05).
1)Treatments: Control-pork patties with 120 ppm sodium nitrite; T1-pork patties with 2% PS; T2-pork patties with 2% PC; T3-pork patties with 60 ppm sodium nitrite and 1% PS; NC-nitrite-free.
2)Color, flavor, off-flavor, tenderness, juiciness, and overall acceptability: 1 = extremely unacceptable, 3 = moderately unacceptable, 5 = between acceptable and unacceptable, 7 = moderately acceptable, and 9 = extremely acceptable.
3)SEM: Standard error of the mean (n=10).
The total viable bacterial count for the pork patties treated with PS was below 1 Log CFU/g for all treatments, and the presence of E. coli and coliform bacteria was not detected in any of the cooked pork patties during refrigerated storage (data not shown). Similar results have been reported by Jeong et al. (2010). The microbes present in low-fat sausage (which was considered cooked when the core temperature reached 72°C during treatment in a water bath) was below 2 Log CFU/g. Fernández-López et al. (2005) reported that safe practices with heat treatment can limit the growth of bacterial groups such as coliform during storage.
Conclusions
In recent years, more consumers concern about health effects of nitrite although the level of nitrite in meat products is known to be safe in human body. Consumers also consider nitrite from natural source is healthier than synthetic nitrite. This is because synthetic nitrite has negative health image while the nitrite from natural source such as vegetables receives the perception as natural and healthy. Therefore, the concept of adding natural compounds to processed meat products can be a possible solution to improve both the actual healthiness and the health image of meat products (Hung et al., 2016). Thus, in this context, studies about replacing synthetic nitrite to natural nitrite in the production of meat products have been performed. Our study also aimed to improve health image of processed meat products as well as to evaluate functional properties of cooked pork patties added with pre-converted nitrite from Swiss chard powder (PS). Addition of PS to cooked pork patties improved the color stability. Shelf-life was also improved through E. coli inhibitory effects. PS increased the redness value of the cooked pork patties as a result of higher contents of nitrosoheme pigment. The lower pH of PS resulted in a lower residual nitrite content in the cooked pork patties. Moreover, PS inhibited lipid oxidation in the cooked pork patties. This is probably due to Swiss chard contains antioxidant phenolic acids and flavonoids such as syringic acid and kaempferol (Pyo et al., 2004). As a result, cooked pork patties with PS had higher scores for flavor and overall acceptability.
Some vegetables (e.g., celery, Chinese cabbage and radish) have been used as a natural nitrite source in previous studies (Santamaria, 2006; Sebranek and Bacus, 2007). Since these vegetable powder is mostly imported and expensive, a new vegetable with better application potential is necessary to be investigated (Jeong et al., 2016). In this regard, Swiss chard can be a cheap source of natural nitrite because it is a domestic and inexpensive product.
Taken together, the results obtained from the current study suggest that pre-converted nitrite from Swiss chard powder can be used as an alternative and natural source of nitrite in the processed meat products.