Introduction
Petroleum-based synthetic plastics used in the food industry have many advantages such as low cost and good mechanical and barrier properties (González and Igarzabal, 2013). However, driven by environmental problems caused by plastic incineration and disposal in landfills, many studies on biodegradable polymers have been performed (Lim et al., 2015; Tawakkal et al., 2014). Polylactic acid (PLA) is a biodegradable polymer obtained by polymerization of lactic acid following fermentation of renewable natural resources such as corn, potato starch, and sugar beets (Hughes et al., 2012). In addition, PLA is biocompatible and widely applied as an alternative to plastic for the packaging of food products and beverages (bottles, overwraps, yogurt containers, etc.) (Jin and Zhang, 2008).
Recently, antimicrobial packaging has emerged as a new strategy to prevent foodborne illnesses caused by pathogen contamination during processing, shipping, or handling of food products (Lantano et al., 2014; Wang et al., 2015). For this reason, the antimicrobial activity of materials such as essential oils and plant extracts into packaging films has been recently studied (Ahmed et al., 2016). Among essential oils, lemongrass oil is widely used as an antimicrobial agent and biodegradable films or coatings containing lemongrass oil have antimicrobial activities ideal for the packaging of minced meat (Barbosa et al., 2009; Moore-Neibel et al., 2012).
Pork sausages are ready-to-eat meat products popularly consumed worldwide. However, Listeria monocytogenes contamination often occurs during post-cooking storage, handling, and packaging of sausages, and even some sausage products in Korea have been recently reported to be contaminated with L. monocytogenes (Korea Consumer Agency, 2014; Siripatrawan and Noipha, 2012). Therefore, antimicrobial packaging represents a useful and new technology to prevent L. monocytogenes contamination. The aim of this study was to determine optimum condition for making a PLA film and develop an antimicrobial packaging using lemongrass oil to prevent the contamination of sausages with pathogenic bacteria.
Materials and Methods
PLA resin (4032D) manufactured by NatureWorks (USA) was purchased from Green Chemical Co. Ltd. (Korea). Chloroform (Chl) and ethanol (EtOH) were purchased from Daejung Chemical & Metals Co. Ltd. (Korea). Lemongrass oil (LO) was purchased from The Certification Academy for Holistic Aromatherapy (Korea). Pork sausages (Samda, 98% lean pork, 2% other ingredients, such as salt, wiener seasoning, and sugar) were purchased from a local market.
The PLA resin was dried in a drying oven at 40℃ for 48 h to remove moisture. The dried resin (8 g) and LO (1, 2, and 3%) were mixed with 80 mL of Chl-EtOH (9:1, v/v) and stirred for 17 h until dissolved completely. The film-forming solutions were then cast on a Teflon-coated glass plate and kept in a hood at room temperature for 5 h to let the solvent evaporate. The dried films were peeled off from the glass plate.
To determine the physical properties of the films, film thickness was measured using a micrometer (Model No. 2046-08, Mitutoyo, Japan) and tensile strength (TS) and percent elongation at break (E) were measured using an Instron Universal Testing Machine (Model 4484, Instron Co., USA). The films were cut to a size of 2.54×10 cm, and the measurement of TS and E was performed at the initial grip separation of 5 cm and at the crosshead speed of 50 cm/min. The water vapor permeability (WVP) of the films was evaluated at constant temperature and humidity (25℃ and 50% RH) using the method described by Yang et al. (2016). A polymethylacrylate cup was filled with 18 mL of distilled water and covered with a piece of each film sample (2×2 cm/piece). The WVP was calculated by measuring the weight of the cup every hour up to 8 h.
Scanning electron micrographs (SEM) of the films with different amounts of LO were acquired using a focused ion beam scanning electron microscope (LYRA3 XMU, Tescan, USA) to examine surface and cross-section morphologies. Each film was coated with platinum using a Cressington Scientific Instruments sputter coater 208HR (USA) for 2 min, and measurements were performed at an accelerating voltage of 5 kV.
Antimicrobial activity of the PLA films against L. monocytogenes was examined by disc diffusion and viable cell count. L. monocytogenes (ATCC 19111) was grown in brain heart infusion (BHI) broth (Difco Laboratories, USA) at 37℃ for 24 h. For the disc diffusion assay, all films were cut into 10 mm diameter discs, and each disc was sterilized under UV light for 30 min. A bacterial suspension was then spread on the surface of Oxford Medium Base (OMB, Difco Laboratories) using a sterilized cotton swab, and each sterilized disc was put onto the inoculated medium. After 24 h of incubation at 37℃, the antimicrobial activity of LO was determined by measuring the diameter of the inhibition zone. Viable cell count was performed according to the method described by Lee et al. (2015). The cultured broth of L. monocytogenes was centrifuged for 15 min, and the pellet obtained was mixed with sterile BHI broth. After dilution with 0.1% peptone water, UV-sterilized films (5×5 cm) were dipped into the diluted broth and incubated for 12 h under shaking at 37℃. Every 6 h, serially diluted aliquots were plated onto BHI agar (Difco Laboratories), and plates were incubated at 37℃ to estimate the population of L. monocytogenes.
Prior to sausage packaging, the PLA film and PLA film containing 2% LO were cut into a proper size (7.5×15 cm) to wrap the samples. L. monocytogenes was cultured in BHI broth at 37℃ for 24 h up to approximately 109 colony forming units per mL (CFU/mL), and l mL of the bacterial suspension was added to 99 mL of 0.1% peptone water. Sausage samples (30 g each) were sterilized under UV light for 30 min and dipped into the diluted bacterial solution for 5 min. After air-drying (approximately 45 min) on a clean bench, sausage samples were wrapped with the PLA films, in direct contact with the surface of the sausage. All samples were enclosed in a low-density polyethylene (LDPE) bag, and control samples were enclosed in a LDPE bag without being wrapped in PLA. All samples were subsequently stored at 4℃ for 12 d.
Sausage samples were cut into small pieces (10 g), placed into a sterile Stomacher bag with 90 mL of 0.1% peptone water, and homogenized for 3 min using a Stomacher (MIX 2, AES Laboratoire, France). The mixtures obtained were serially diluted and plated onto OMB to evaluate the L. monocytogenes population. All plates were incubated at 37℃ for 24 h, and bacterial counts were determined as Log CFU/g.
Results and Discussion
PLA is more rigid than other polymers, and for this reason, its application in the food industry as a packaging material is limited (Ahmed et al., 2016; Qin et al., 2015). Therefore, a proper solvent is needed for the preparation of a film-forming solution to improve the flexibility of PLA (Kang et al., 2013; Qin et al., 2015). The PLA film prepared with 100% Chl had a TS of 35.2 MPa and an E value of 14.3%. To improve E, other organic solvents such as EtOH were additionally used. When a Chl:EtOH mixture (9:1, v/v) was used, the TS decreased from 35.2 to 33.0 MPa while the E increased from 35.2% to 180.8%. These results suggest that a relatively hydrophilic solvent like EtOH has a plasticizing role in the PLA film matrix (Saidi et al., 2014), affecting the physical properties of the films. In agreement with our study, Erdohan et al. (2013) reported that TS decreased and E increased as the ratio Chl:EtOH increased from 10:0 to 6:4 (v/v). In particular, the increase of E is mainly due to the reduction in attractive forces between polymers by the plasticizing effect of EtOH as well as volatilization of ethanol. Consequently, based on the TS and E of the PLA films, Chl: EtOH at a ratio of 9:1 (v/v) were chosen for the preparation of the PLA film containing LO.
The physical properties of the PLA films containing various amounts of LO are shown in Table 1. E and TS decreased with increasing concentrations of LO. In particular, the E of the PLA film containing 3% LO was 21.9%, whereas the control film displayed an E of 205.9%. It is apparent that the incorporation of LO into the film affected the arrangement of the PLA molecules. Similarly, Jin et al. (2009) observed that a PLA/pectin composite film had a decrease in TS and E compared to the pure PLA film. Sánchez-González et al. (2009) also reported that a hydroxypropyl- methylcellulose film containing tea tree essential oil had inferior mechanical properties, due to the interference of the tea tree oil with the arrangement of the PLA film structure. In general, when essential oils are incorporated into the films, their physical properties have the tendency to increase the E and decrease the TS of the films by reducing interactions between the film base materials (Ahmed et al., 2016; Qin et al., 2015). In contrast, our study showed different results, such as a decrease in E. The reason for the decrease in E could be due to the crystallization of oil and the size of oil droplet in the film matrix. Further research should be performed in order to elucidate this process at a molecular level.
Data are expressed as mean±standard deviation.
a-dValues in the same column followed by different letters are significantly (p<0.05) different by Duncan's multiple range test.
The WVP of the PLA films increased with increasing LO concentrations. The PLA film without LO exhibited the lowest WVP of 0.35×10−9 g m/m2s Pa, whereas the PLA films containing 3% LO showed the highest WVP of 0.74×10−9 g m/m2s Pa. According to the report by Maizura et al. (2007), WVP is associated with structural changes in the film caused by additives, and the resulting structure leads to an increased water penetration. Similarly, Qin et al. (2015) also reported that PLA/polytrimethylene carbonate composite films containing cinnamaldehyde had an increase in WVP due to the modification of the polymer structure by cinnamaldehyde. Therefore, the increase in the WVP of the PLA films containing LO might be attributed to the deformation of the PLA film structure. Although the water barrier properties of the PLA films containing LO were decreased, their values are still higher in comparison with the majority of other biodegradable films such as starch or protein films.
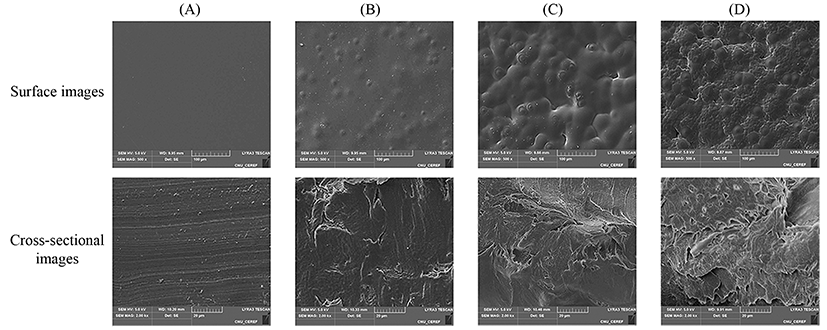
The SEM images of the PLA films containing different amounts of LO are shown in Fig. 1. The PLA film without LO showed a clean surface, and its cross-section also had a homogeneous structure. Conversely, the PLA films containing LO had a less homogeneous structure compared with the control film. In addition, as LO concentration was increased, an uneven and porous structure became more prevalent. The presence of heterogeneous surfaces and cross-sections is likely to be caused by the evaporation of organic solvents during the preparation of the PLA films (Qin et al., 2015). Among the films tested, the PLA film containing 3% LO had an extremely fragmented structure. It can be hypothesized that there were uneven distribution of an excessive amount of LO and probable crystallization of LO, causing poor compatibility and increased WVP.
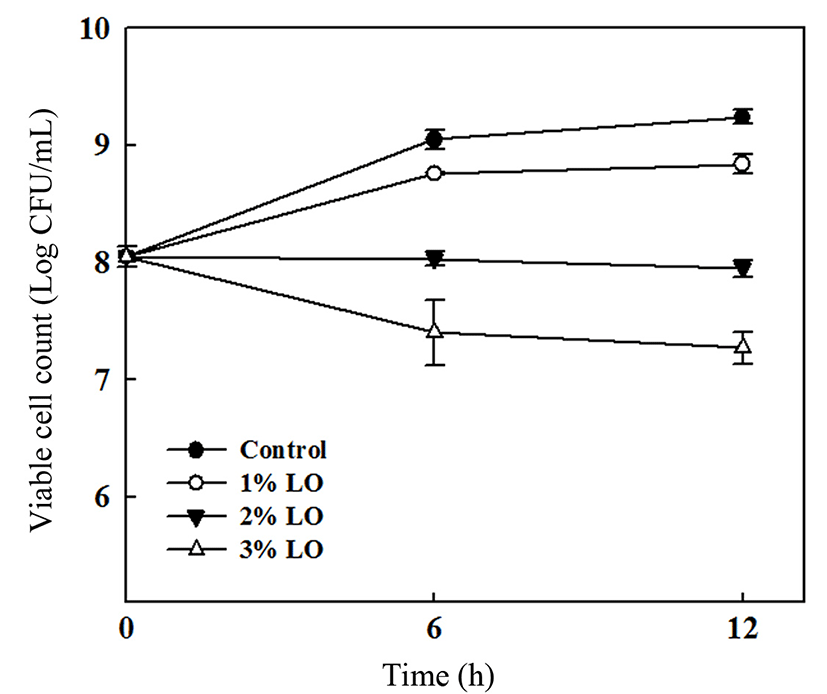
The antimicrobial activities of the PLA films containing various amounts of LO against L. monocytogenes were determined using disc diffusion and viable cell count. The PLA film without LO showed no inhibition zone, whereas the PLA films containing LO had inhibition zones proportional to the concentration of LO (Table 2). To evaluate the population of L. monocytogenes after different incubation times, a viable cell count was conducted (Fig. 2). During the initial 6 h of incubation, the L. monocytogenes populations of the PLA film without LO and the film with 1% LO increased, whereas growth on the PLA film with 3% LO was significantly slower. After 12 h, no significant variation was identified for the PLA films containing LO, whereas the population of the PLA film without LO gradually increased. The antimicrobial activity of essential oils is performed via disruption of the bacterial cell wall, which leads to protein denaturation and cell lysis (Emiroglu et al., 2010). Essential oils also affect membrane permeability and induce leakage of K+ ions, a process that results in cell death (Ahmad et al., 2012a). Based on the physical properties and antimicrobial activities of the PLA films tested, the PLA film containing 2% LO was chosen for the antimicrobial packaging of sausages.
| Lemongrass oil (%) | Inhibition zone (mm) |
|---|---|
| 0 | - |
| 1 | 11.78±0.55c |
| 2 | 20.42±1.01b |
| 3 | 30.75±1.94a |
Data are expressed as mean±standard deviation.
a-cValues in the same column followed by different letters are significantly (p<0.05) different by Duncan's multiple range test.
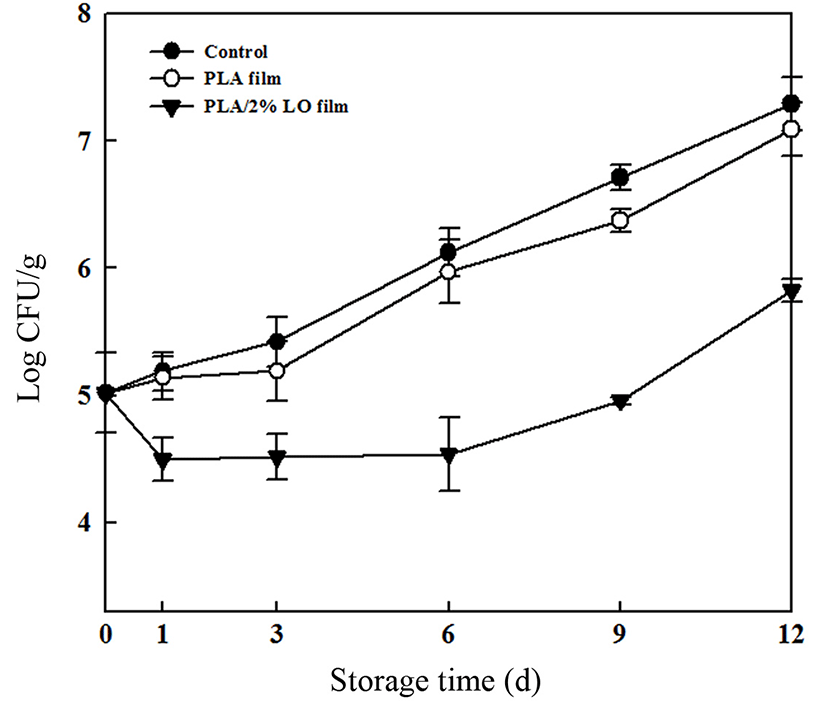
The antimicrobial effect of the PLA film containing 2% LO against inoculated L. monocytogenes was evaluated during storage of sausages at 4℃ for 12 d (Fig. 3). The initial population of inoculated L. monocytogenes was 5.02 Log CFU/g, and the populations in the control sample and in the sample wrapped with the PLA film steadily increased. In contrast, the sample wrapped with the PLA film containing 2% LO showed a reduced population, which did not undergo significant growth for 6 d. During the entire storage time, the sample wrapped with the PLA film containing 2% LO had a smaller L. monocytogenes population than those of other samples. These results indicate that the incorporation of LO into the PLA film inhibits the growth of microorganisms. Similarly, Ahmad et al. (2012b) reported that the total viable cell and psychrophilic bacterial counts in sea bass slices wrapped with a gelatin film containing LO decreased compared with unwrapped samples and a sample wrapped with gelatin alone during the storage time tested. In this study, the population of the sample wrapped with the PLA film containing 2% LO was reduced by 1.47 Log CFU/g compared with the control sample after 12 d of storage.
Conclusions
We prepared a PLA film in Chl-EtOH, and found that its antimicrobial activity increased with increasing LO concentrations. Among the films tested, the PLA film containing 2% LO exhibited the most desirable physical properties and antimicrobial activity for sausage packaging. As a result of its application in the packaging of sausages, the inoculated sausage samples wrapped with the PLA film containing 2% LO had 1.47 Log CFU/g less than that of the control as a consequence of the antimicrobial efficacy of LO against L. monocytogenes. Therefore, our study demonstrates that PLA films containing 2% LO can be used as a new quality improving method for the packaging of sausages.













