Introduction
Yogurt containing probiotics and prebiotics is known to have many beneficial effects such as strengthening the immune system, assisting lactose digestion and the intestinal system, inflammatory bowel disease, and allergies (Adolfsson et al., 2004). In addition to these health benefits, the physical properties, texture, appearance, and flavor of yogurt are also important factors for consumer acceptability. Yogurt texture, flavor, and acid production by yogurt starter cultures and bacteria during fermentation and storage can be modified by altering process conditions or by the addition of supplementary ingredients. The ingredients that determine the nutritional variations and/or technical applications of yogurts include milk protein (Peng et al., 2009), prebiotics (Bruno et al., 2002), and herbs (Amirdivani and Baba, 2011). Additionally, interest in plant-derived food additives has grown in recent years, since plant extracts have been shown to possess health-promoting properties such as antimicrobial and antioxidant effects. The effects of Korean plant have been reported extensively the water-soluble extract from leaves of Morus alba and Cudrania tricuspidata on inhibition of lipid peroxidative formation (Cha et al., 2000), the extracts of Nelumbo nucifera leaf and Curcuma Longa L. on preventing hypercholesterolemia (Jung et al., 2015), and Cudrania tricuspidata fruit essential oil on antibacterial efficacy (Bajpai et al., 2013). However, the study of plant extracts for application to food was hardly found. The sensory characteristics of fermented milk products play important roles in consumer acceptance. The various cultures and additives are strongly linked with yogurt aroma. Several flavor compounds have been isolated from yogurt, major compounds of which were acetaldehyde, ethanol, acetone, diacetyl, and 2-butanone (Kneifel et al., 1992). The quality of yogurt aroma also depends on the relationship between other volatile compounds; for instance, an acetaldehyde-to-acetone ratio of 2.8 is considered optimum (Accolas et al., 1980).
Plant extracts selected for this study may serve as a good source of functional nutrients and prebiotics for yogurt starter culture to improve microbial, physicochemical, and hence sensory properties. The study therefore investigates the effects of yogurt supplementation with plant extracts on acid production and the growth of yogurt starter cultures during fermentation. In addition, various properties of the final product immediately after production and during 28 d of refrigerated storage were evaluated to investigate the effects of Korean plant extracts on fermentation properties.
Materials and methods
Two plant leaves (Diospyros kaki THUNB. leaf; DK, and Nelumbo nucifera leaf; NN) were obtained from local market (Sunchang, Jeollabuk-Do). Leaves were washed and then soaked in distilled water for 9 h in a water bath (100℃) with occasional shaking, followed by harvest of the supernatant through the filter paper. The clear solution obtained was concentrated by evaporation to dryness under vacuum at temperatures not higher than 50℃. The concentrated water plant extracts were used after freeze drying in the making of yogurt.
Plain yogurt and plant extract supplemented yogurts were prepared on the same day. The powdered plant extracts (0.2% [g/g]) were added into pre-warmed (60℃) milk and the mixtures were pasteurized (85℃, 15 min) and cooled to 41℃. Prepared milks with or without plant extract, were inoculated with the ST-BODY-1 and LB-12 DVS cultures (Chr. Hansen, Denmark) at a dose of 0.0002%. The incubation proceeded at 41℃ to achieve a pH of 4.6. Subsequently fermented milks were cooled and kept at 4℃. The samples were collected to analyze directly after production and after 7, 14, 21, and 28 d of refrigerated storage.
The pH was measured after calibration of the pH meter with standardized pH buffer solutions 4.0, 7.0, and 10.0 (Fisher Scientific) prior to the analysis. The TA of the homogenized samples (9 g) in water (40 mL) was read using a digital titratable acidity titrator (848 Titrino plus, Switzerland). TA was determined by titration using 0.1 N NaOH under continuous stirring. TA was expressed as % lactic acid.
For each run of the experimental design, samples were analyzed immediately after fermentation and after four weeks of storage at 4℃. Yogurt samples were serially diluted using sterilized 0.1% peptone (Bacto, Becton Dickinson and Company, USA) water. Streptococcus thermophilus was counted on M17 agar (Oxoid) plates after aerobic incubation at 37℃ for 48 h. Lactobacillus delbrueckii subsp. bulgaricus was enumerated on MRS (DB) at pH 5.2 with acetic acid after incubation at 45℃ for 72 h anaerobically in an anaerobic jar with anaerobe gas packs (MGC, Japan).
Total phenolic compounds were determined by an assay from Maksimović et al. (2005). TPC was expressed as ug gallic acid equivalent (GAE)/mL using a regression of known concentrations of gallic acid which was determined every time total phenolic assay was carried out.
The antioxidant activity of natural compounds is dependent on the experimental procedures due on their different reaction mechanisms. Therefore, we evaluated the antioxidant activities of yogurt samples by DPPH radical scavenging activity, Ferric-reducing antioxidant power assay, and ABTS radical scavenging activity using the method of Oh et al. (2013).
The syneresis (%) of yogurt samples was determined by centrifugation procedure according to the method described by Aprodu et al. (2012).
Flavors and volatile compounds of yogurt samples were analyzed by HS-GC/MS according to Güler and Gursoy- Balci (2011) with slight modification. Ten gram of sample was transferred into a 20 mL headspace vial (Agilent, USA), which was sealed with PTFE/Si headspace septa (Agilent, Germany) and Al crimp cap (Agilent, USA). The vials with samples were held at 80℃ for 1 h, and subsequently headspace (100 L) was injected onto the GC column using split mode (15:1). The temperature of the loop and transfer line of headspace was kept at 90℃ and 100℃, respectively. Flavors and volatile compounds were analyzed using a Agilent model 7890B gas chromatography (GC) and mass selective detector (MS) (Agilent, USA) with selected-ion monitoring (SIM) mode. Column was a HPINNOWAX capillary column (60 m × 0.25 mm id × 0.25 mm film thickness). Peak identification was carried out by comparing the mass spectra with the W8N05ST (Wiley, version 8.0 and NIST, version 5.0) library, and quantification of compounds was calculated by external standard technique. For this purpose, standards of acetaldehyde, diacetyl, acetoin, ethanol, and acetic acid were accurately weighed and dissolved in 10 g of double distilled water. Six sets of concentrations were prepared in the range of 0.1-100 g/mL. All collections were made in triplicate and consequently the amounts of each compound in sample were calculated over known amount of standard and its peak area.
A sensory analysis was used to evaluate differences among the three types of yogurt samples by forty untrained panelists using a 9-point hedonic scale. Panelists were asked to rate samples for sourness, bitterness, flavor, viscosity, firmness, and overall preference with the words “low” (1-3), “medium” (5), and “high” (7-9) for each yogurt sample. Sensory evaluation was conducted in individual booths to prevent rate score bias. Scores are presented as mean±standard error. Sensotool (Sensometrics. Co., Ltd, Korea) was used for statistical analysis of sensory analysis.
All data were expressed as means±SD. Statistical significance for the differences between the groups was assessed using Duncan’s multiple range tests. IBM SPSS statistics software version 22 (IBM Corp., USA) was used to perform all statistical tests. Values of p<0.05 were considered to indicate a significant difference.
Results and Discussion
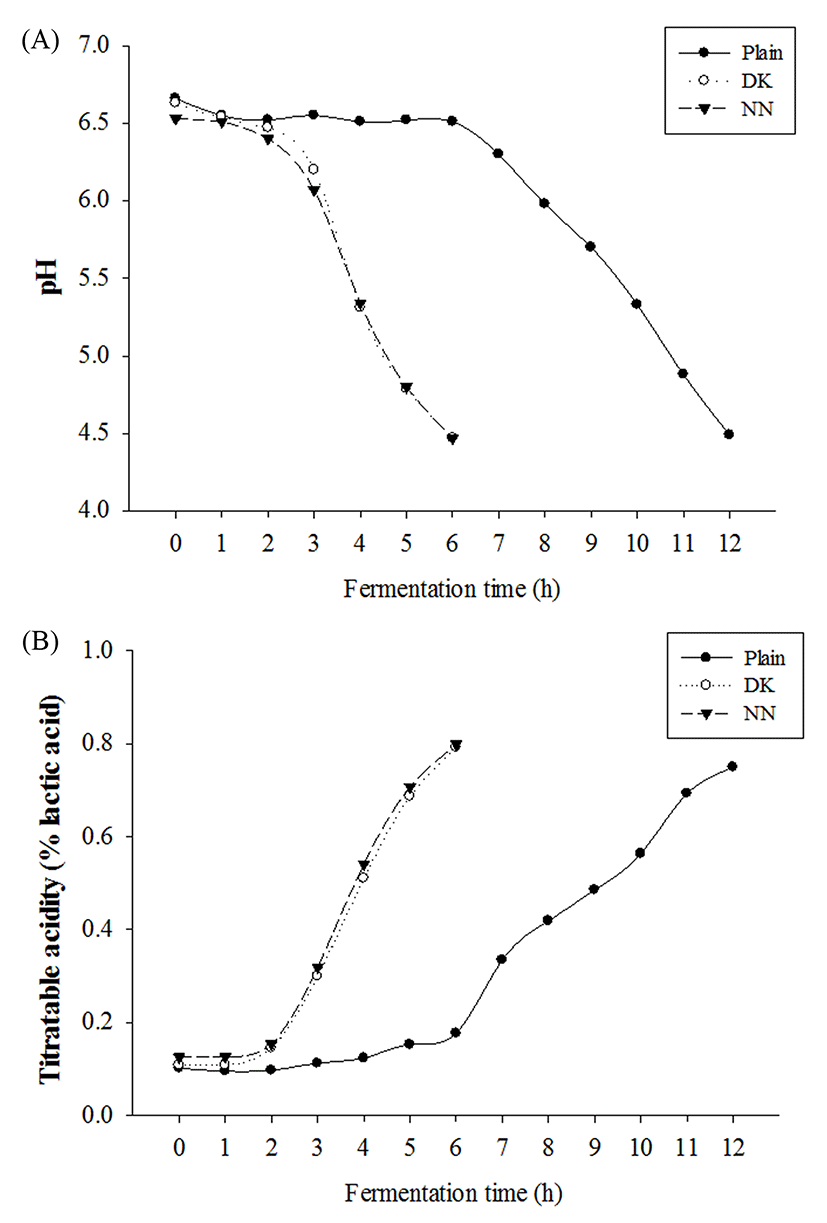
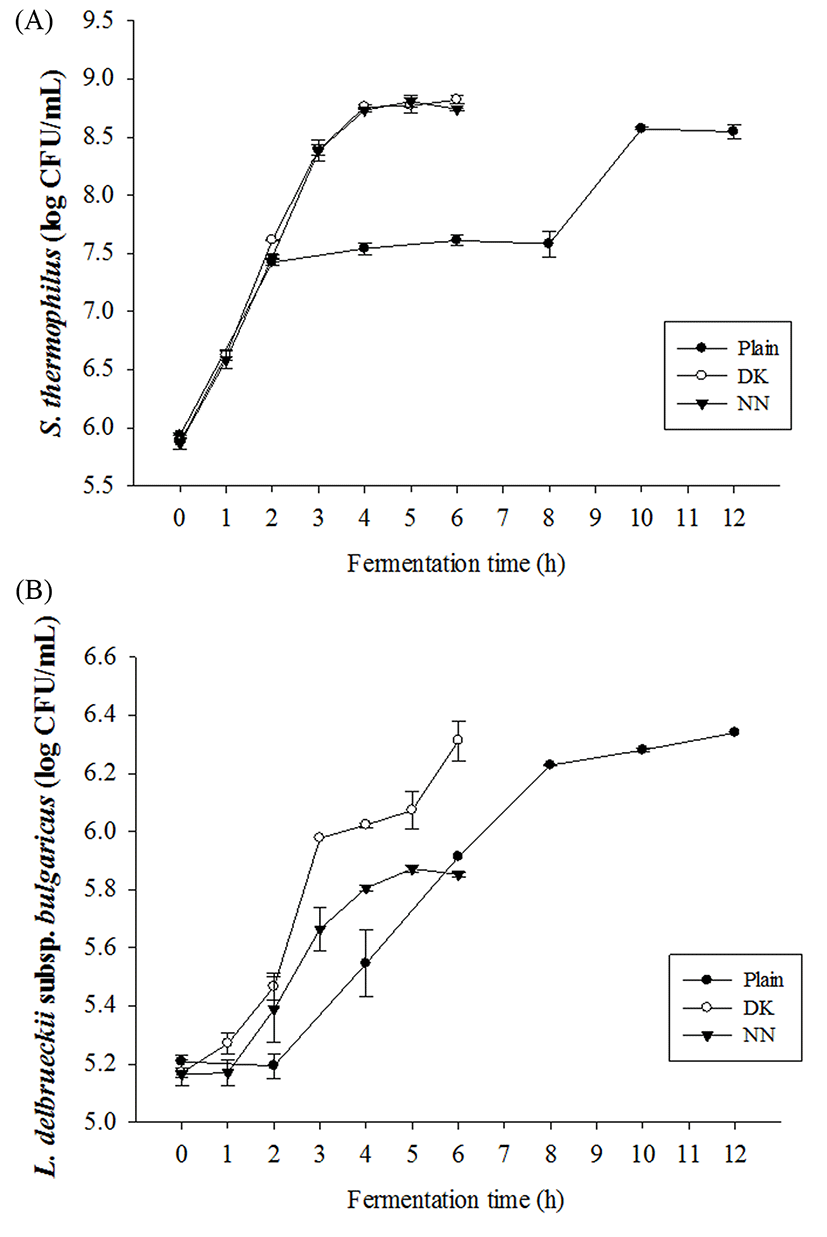
The fermentation properties of yogurt samples were determined by measuring pH and TA (% of lactic acid). Supplementation of plant extracts did not affect the initial pH and TA, which ranged from 6.51 to 6.66 and from 0.10 to 0.13%, respectively (Fig. 1). The fermentation of yogurts was stopped at pH 4.5. Fermentation time was significantly decreased to 6 h for all yogurts supplemented with plant extracts, compared with 12 h for plain yogurt as negative control. All supplemented yogurts showed more rapid pH decrease and TA increase than plain yogurt. Amirdivani and Baba (2011) reported that fermentation with herbs enhanced the metabolic activity of yogurt bacteria and that pH was decreased due to increased production of organic acids by lactic acid bacteria (LAB). Moreover, amino acids and small peptides produced by LAB stimulate the growth of S. thermophilus (Sandine and Elliker, 1970), whereas metabolites of S. thermophilus such as carbon dioxide and formic acid stimulate the growth of Lactobacillus spp. (Phillips et al., 2006). Supplemented yogurts showed a higher rate of TA, at 0.80% (0.75% for plain yogurt). Billard et al. (2007) reported that TA is more relevant in the evaluation of fermentation capacity of microbes, as organic acids produced in yogurts are linearly related with the accumulation of TA. As shown by the results for pH and TA in this study, supplementation with plant extracts may enhance the metabolic activities of yogurt starter cultures, resulting in dramatically shorter fermentation times than those for plain yogurt. Furthermore, the viability of S. thermophilus and L. delbrueckii subsp. bulgaricus in yogurts during fermentation is presented in Fig. 2. Collaborating with the results for pH and TA, all yogurts supplemented with plant extracts showed more rapid increases in microbial counts than those for plain yogurt. The viabilities of S. thermophilus and L. delbrueckii subsp. bulgaricus in DK yogurt increased the most by about 2.95 log units and 1.14 log units respectively from initial of fermentation. At the end of fermentation, the starter cultures in all yogurt samples remained viable, exceeding 8.5 Log CFU/mL for S. thermophilus and 5.8 Log CFU/mL for L. delbrueckii subsp. bulgaricus. The S. thermophilus counts in the DK and NN yogurts were significantly higher than for plain yogurt, whereas the counts of viable L. delbrueckii subsp. bulgaricus at the end of fermentation followed the sequence: plain yogurt > DK > NN. Based on these results, it is concluded that plant extracts have potential applications in yogurt manufacture as prebiotics to shorten fermentation time, increase the viability of starter cultures, or to target beneficial LAB.
The pH of all yogurt samples tended to decrease during the first 14 d of storage and then to decrease again from days 21 to 28 (Table 1). Generally, yogurts supplemented with plant extracts showed lower pH (within the range 0.02 to 0.09 units) compared with plain yogurt. Yogurt pH was decreased during cold storage, and NN yogurt showed the lowest pH among the yogurts supplemented with plant extracts. On the other hand, yogurt TA tended to increase at the initial of storage then to maintain the value of TA until day 28. All yogurts containing supplementary plant extracts showed higher TA than plain yogurt; moreover, the TA value of NN yogurt is higher than for DK yogurt. The results of pH, TA, and microbial analysis (described below) suggest that supplementary plant extracts supported the production of lactic acid by yogurt starter cultures. Furthermore, extended refrigeration for 28 days decreased the pH and increased the TA of yogurts, possibly as a result of accumulation of acetic acid, acetaldehyde, formic acid, and lactic acid (described below).
1Diospyros kaki THUNB. leaf extract.
2Nelumbo nucifera leaf extract.
Values are presented as mean±SEM (n=3).
Data followed by a different lower-case letter were significantly different (p<0.05) for the storage period.
*p<0.05 in comparison to plain.
The viability of S. thermophilus and L. delbrueckii subsp. bulgaricus was significantly affected by the addition of plant extracts, the type of plant extract, and storage period. S. thermophilus counts tended to increase until day 14 of storage then decrease, such that counts on day 28 were not less than the initial levels in all yogurt samples. The degree of increase in S. thermophilus counts in plain yogurt were greater than for supplemented yogurts, as S. thermophilus was more sensitive to lactic acid accumulation and supplemented yogurts showed high TA values. Furthermore, Robinson (2002) reported that under pH conditions of around 4.3-4.5, which most of the yogurts reached after 3 and 4 wk of cold storage, the growth and metabolism of S. thermophilus was usually inhibited. On day 28, S. thermophilus counts in all yogurts exceeded 8.77 Log CFU/mL, but there was no significant difference between yogurt samples. On the other hands, yogurts with plant supplements showed significantly higher counts of L. delbrueckii subsp. bulgaricus compared with plain yogurt throughout the storage. The L. delbrueckii subsp. bulgaricus counts in all yogurt samples showed the identical tendency of significant increase from day 0 through day 14. Especially, the L. delbrueckii subsp. bulgaricus counts of supplemented yogurts were sharply increased in initial of storage. In addition, NN yogurt had the highest L. delbrueckii subsp. bulgaricus counts (7.48±0.02 Log CFU/mL; on day 14). According to Michael et al. (2010), the presence of some prebiotics or sodium acetate in plant extracts could account for the improved L. delbrueckii subsp. bulgaricus viability in yogurt. This result corresponded with the results for TA during refrigerated storage. Consequently, both of the yogurt starter cultures were found to be present at sufficiently high levels at the beginning and the end of 28-d cold storage period, and some even showed increased counts.
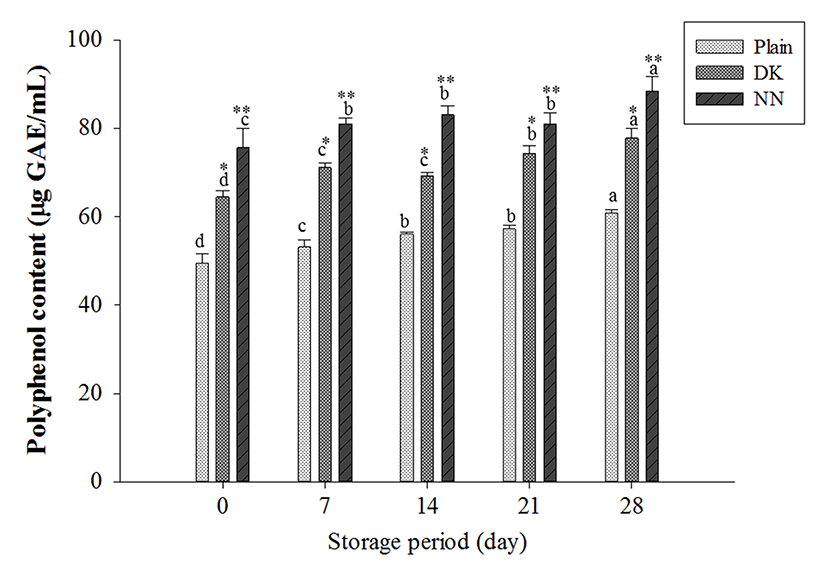
TPC in yogurts with plant extracts was significantly higher than for plain yogurt both after fermentation and during cold storage (Fig. 3). TPC content gradually increased for all yogurt samples during storage. Particularly, NN yogurt showed the highest TPC (88.4±3.4 g GAE/mL), followed by DK yogurt (77.8±2.2 GAE/mL) at the end of storage. This is agreement with the result that the NN yogurt showed the higher values of pH, TA and microbe viability than for DK yogurt. The increase of the TPC of yogurts may be explained by the action of microbes and indigenous phytochemical compounds (flavonoid and phenolic compounds) in plant extracts during refrigerated storage (Dalling, 1986). It is possible for the microbial utilization of phenolic acids such as ferulic acid and p-coumaric acid during fermentation and post acidification to lead to the production of others such as vanillic and p-hydroxybenzoic acids before the aromatic ring structure is broken down (Blum, 1998). Moreover, the breakdown of milk protein by yogurt microbes might also contribute to the increased TPC content of yogurts, since the amino acid tyrosine, for instance, has a phenolic side chain (Shah, 2000).
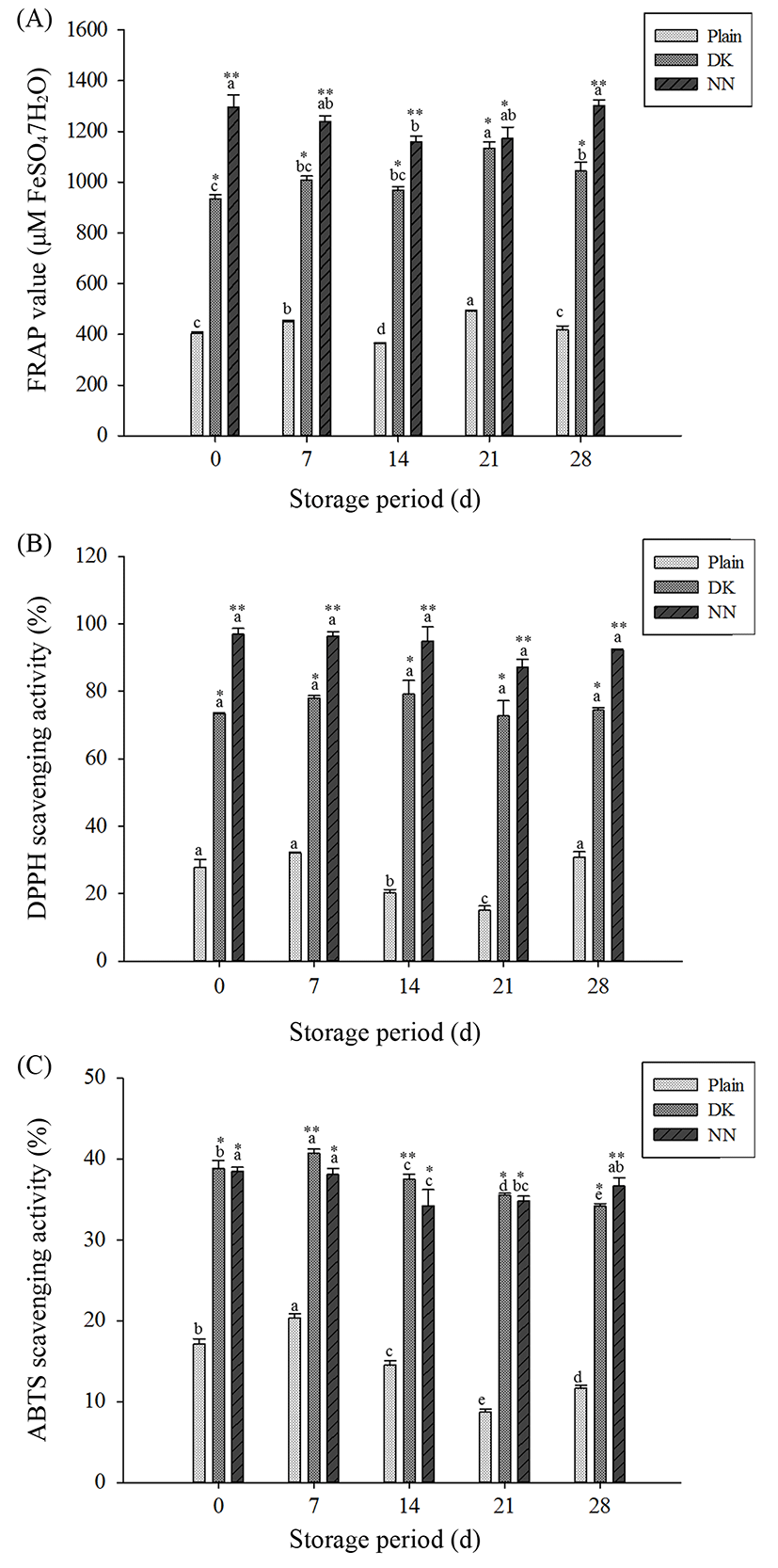
The antioxidant activities of yogurt samples determined by three assays with different mechanisms were shown in Fig. 4. Although antioxidant activities showed slight fluctuation during 28 d of storage, yogurts with plant extracts had significantly higher activity in all three antioxidant tests than plain yogurt. At the end of storage, supplemented yogurts showed antioxidant activities approximately 1.9-5.7 times greater than plain yogurt, depending on the type of plant extract. In particular, NN yogurt had the higher antioxidant activity (1295.6±50.0 M FeSO47H2O for FRAP, 97.0±1.7% for DPPH, and 38.5±0.5% for ABTS) than for DK yogurt as the initial value on the storage period. Moreover, the yogurts with plant extracts maintained this greater antioxidant activity during cold storage. In this study, the yogurts supplemented with plant extracts were characterized by high polyphenol content. According to Thompson et al. (2007) and Velioglu et al. (1998), the higher antioxidant activities of yogurts by addition of plant extracts were most likely a result of the phytochemical contents of plant extracts and microbial metabolic activities. Continued microbial growth during storage may have altered some of the phenolic compounds and hence increased antioxidant activities (Blum, 1998). On the other hand, previous studies attributed the fluctuations in microbial activity during storage to increasing degradation of phenolic compounds (Yildiz et al., 2009) and/or increasing milk protein/polyphenol interaction (Yuksel et al., 2010). Consequently, the consumption of yogurts supplemented with plant extracts has potential health benefits associated with high antioxidant activity and live bacterial contents.
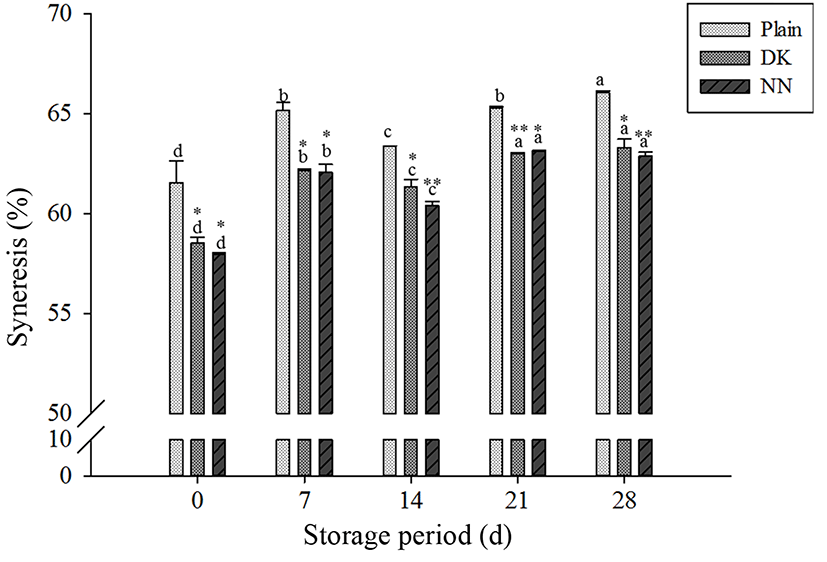
Syneresis was evaluated to investigate the physiological characteristics of yogurt samples during refrigerated storage. Syneresis of yogurts varied significantly with supplementation of plant extracts and type of plant extracts. Syneresis of plain yogurt was significantly greater than yogurts added plant extracts (Fig. 5). In the case of supplementation of plant extracts, the best water holding capacity was obtained in NN yogurts on 28 d of storage, as 62.90%, respectively. The acidification process of the yogurts supplemented plant extracts allowed obtaining gels with increased firmness, lower permeability, finer protein networks and improved whey drainage (Ozer et al., 2007). Moreover, water holing capacity decreased with the increase of storage period, which have been reported the similar result by Salvador and Fiszman (2004). Syneresis is one of the most important factors influencing consumer’s acceptance (Gürsoy et al., 2010).
The profiles of volatile compounds in yogurts during refrigerated storage are presented in Table 2. In agreement with previous reports (Gardini et al., 1999, Güler and Gursoy-Balci, 2011), the major volatile compound (by quantity) in yogurt appeared to be acetaldehyde. Supplementation, type of plant extract, and storage period significantly influenced the production of acetaldehyde. NN yogurt contained the highest amount of acetaldehyde during the storage period (92.03±0.13 mg/kg at the end of storage). This result was consistent with changes in the values of pH, TA, and microbe viability (Table 1), in which yogurts with high acidity contained high levels of acetaldehyde. Diacetyl is derived by the fermentation of citric acid present in milk (Vedamuthu, 2006), with gives a buttery taste that may be considered to improve the yogurt flavor. The typical concentrations of diacetyl in yogurt ranged from 0.2 to 3 mg/kg (Georgala et al., 1995, Pourahmad and Assadi, 2005), as a result of this study ranged from 0.63 to 1.71 mg/kg. Diacetyl contents were decreased from initial to day 14 then increased to day 28 as the result of viability of S. thermophilus. According to the previous study (Pinto et al., 2009), diacetyl is mainly resulting from the metabolic activity of S. thermophilus, and this result was consistent with the result of Cruz et al. (2013). The highest diacetyl concentration was detected in NN yogurt throughout storage period. As a previous study (Tamime and Robinson, 2001), diacetyl contents were considerably lower than those of acetoin. According to Urbach (1995), diacetyl was degraded to acetoin by starter bacteria in yogurt. Supplemented yogurts were determined to have significantly lower level of acetoin than for plain yogurt. Acetoin contents were lower at the beginning than the end of storage. The concentration of ethanol steadily increased in all yogurt samples during storage. Supplemented yogurts produced less ethanol than plain yogurt. Ethanol is produced from pyruvic acid in cell membrane with acetic acid (Pan et al., 2014), thus the level of ethanol and acetic acid are compensatory in fermented milk. In agreement with the previous studies (Donkor et al., 2007; Martínez-Villaluenga et al., 2006), acetic acid content fluctuated slightly during storage with the range of 9.38±0.45 to 19.64±1.99 mg/mL. The supplemented yogurts were particularly observed that the amount of acetic acid was decreased in initial then increased in the late of storage. Moreover, the amount of acetic acid in yogurt with plant extract was significantly higher than that of the control. Acetic acid is produced by starter cultures from several complicate biochemical pathways including degradation of sugar and organic acid sources which were contained in the plant extract (Guler, 2007; Ostlie et al., 2003). As the storage period increased, biochemical metabolism proceeded thus acetic acid was steadily produced in the late of storage as a result of this study (Gaafar, 1992). The profile of volatile compounds in yogurts may influence sensory characteristics such as flavor and taste.
1Diospyros kaki THUNB. leaf extract.
2Nelumbo nucifera leaf extract.
Values are presented as mean±SEM (n=3).
Data followed by a different lower-case letter were significantly different (p<0.05) for the storage period.
*p<0.05 in comparison to plain.
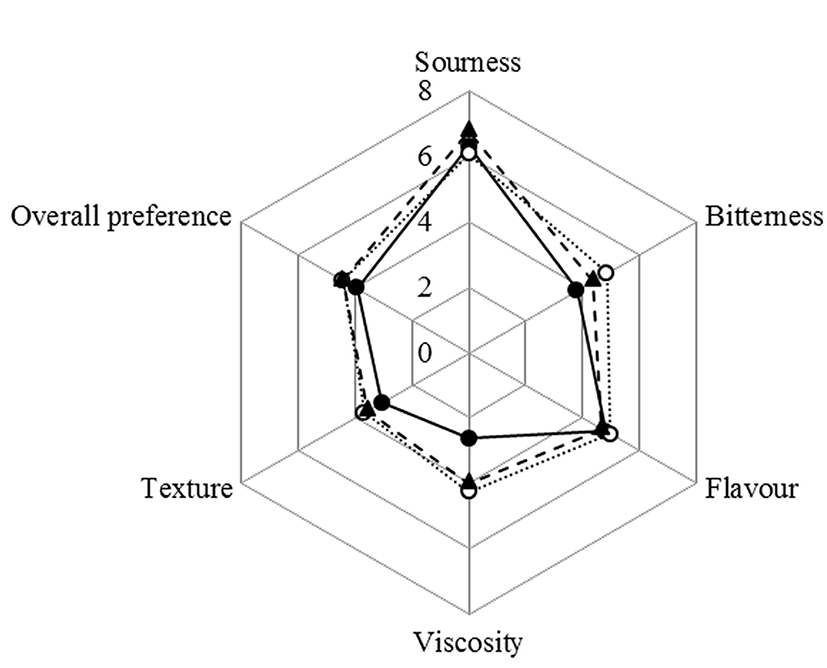
The results of sensory evaluation showed conspicuous differences in almost factors, between plain and supplemented yogurts (Fig. 6). The yogurt samples were tested a day after manufacturing for sensory evaluation. In comparison to plain yogurt, plant extracts added yogurts scored higher for viscosity, bitterness, texture, and flavor. Analysis of volatile compounds indicated that DK yogurt had a rather high level of flavor compounds such as acetaldehyde, acetoin, and diacetyl as it showed the highest score of flavor in sensory evaluation. Otherwise, NN yogurt showed the highest amount of acetic acid on volatile compound analysis and the highest score of sourness. As expected, the addition of plant extracts increased bitterness, since each plant extract contains several aromatic compounds. The evaluation for viscosity and texture were better of plant extracts supplemented yogurts than of plain yogurt, which was consistent with the water holding capacity of yogurt. In consequence, the plant extracts supplemented yogurts were judged to have a better organoleptic property than for plain yogurt. Yogurt may become a good carrier for plant extracts because its presence improved yogurt organoleptic property, such as complemented sourness by increased bitterness, and increased favored flavor, viscosity, and texture. Moreover, based on the results of the antioxidant test, the addition of plant extract contributed yogurt to have antioxidant activity as health-promoting characteristics.
Conclusions
Supplementation of plant extracts, as prebiotics, obviously decreased fermentation time and increased the viability of yogurt starter cultures, compared with plain yogurt. An addition of two plant extracts significantly increased the content of targeted L. delbrueckii subsp. bulgaricus at the end of storage. Although the syneresis was increased with increased storage period in all yogurt samples, plant extracts supplemented yogurts had a higher water holing capacity than for plain yogurt, contributing to better evaluation for viscosity and texture of yogurt. Moreover, substantially higher antioxidant activities were determined in the yogurts with plant extracts than for non-supplemented yogurt, in order of total polyphenol content in plant extracts. Among the plant extracts supplemented yogurts, NN yogurt showed the greatest antioxidant activities. Furthermore, both of plant extracts increased production of volatile compounds such as acetaldehyde, diacetyl, acetoin, and acetic acid in comparison with plain yogurt, but the patterns differed during cold storage depending on each compound. On the other hand, DK and NN extracts increased the acceptability of yogurt in sensory evaluation tests. Therefore, the addition of DK and NN extracts to yogurt is recommended, as this has the potential to be further developed for consumers as a functional yogurt with antioxidant properties.













