Introduction
Cardiovascular and cerebrovascular diseases seriously harm human health and have become major causes of human deaths (WHO, 2009). Cardiovascular and cerebrovascular diseases result mainly from high cholesterol concentration in serum (Steinberg et al., 1989; Yu et al., 2013; Zheng et al., 2013). Therefore, cholesterol intake and serum cholesterol levels are necessary to reduce. Bifidobacterium, the most beneficial microbe in the gastrointestinal tract of human and animals, has antibacterial, antiaging, anticancer, and immunity-enhancement functions (Nishida et al., 2004). Moreover, many bifidobacteria can reduce cholesterol levels (Oh and Lee, 2000; Liong and Shah, 2005; Ziarno et al., 2007). Thus, screening methods for cholesterol-lowering Bifidobacterium are gaining increased attention.
Guizhou Xiang Pig is a rare animal in China and is also an ideal animal model with a similar organ structure to the human body. This animal can be used as a new resource for screening new cholesterol-lowering Bifidobacterium strains. The new strains should have excellent properties with good tolerance to acid and bile salt because probiotic strains need survive acid and bile stress in the gastrointestinal tract. The new strains should also endure oxygen stress, which is an important characteristic for maintaining strain viability in manufactured products (Dunne, 2001).
This study aimed to obtain excellent cholesterol-lowering Bifidobacterium by screening from Guizhou Xiang Pig and evaluate the screened strain’s tolerance to oxygen, acid, and bile. Results can lay a preliminary foundation for the potential industrial applications of this strain.
Materials and Methods
Samples for the separation of bifidobacteria were collected from the feces of 32 Guizhou Xiang pigs from the Agricultural Science and Technology Demonstration Park, Huaxi, Guiyang, Guizhou province, China. Cholesterol with purity above 99% as standard was purchased from Sigma. Analytical grade cholesterol with purity above 95.5% as a substrate for biotransformation was purchased from Sinopharm Chemical Reagent Co., Ltd, China. All other chemicals used in this work were analytical grade and commercially available. Mupirocin lithium (Li-MUP) was from QingDao Hopebio-Technology Co., Ltd., China. Peptone and beef extract were from Shanghai Bio-way Technology Co., Ltd., China. ʟ-Cysteine hydrochloride was from Beijing Solarbio Technologies Co., Ltd., China. Xgal was from Beijing Dingguo Changsheng Biotech Co., Ltd., China. Carbon dioxide incubator (WJ-185I) was from Shanghai Santn Instrument Co., Ltd., China, biochemical incubator (SPX-250B) was from Shanghai Ke Heng Industrial Co., Ltd., China, and UV-visible spectrophotometer (TU-1810) was from Beijing Purkinje General Instrument Co., Ltd., China. All other chemicals used in this work were from Sinopharm Chemical Reagent Co., Ltd., China.
MRS agar medium was prepared by adding 20 g of agar per liter MRS broth medium (De Man et al., 1960). Li-MUP-modified MRS agar medium (MUP-MRS) was prepared by supplementing MRS agar medium with 50 mg/L Li-MUP. Modified PTYG medium contained the following: tryptone, 5 g/L; soy peptone, 5 g/L; yeast extract, 10 g/L; glucose, 10 g/L; Tween 80, 1 mL; ʟ-cysteine hydrochloride, 0.05 g/L; fructooligo-saccharides, 5 g/L; and salt solution, 4 mL. pH was adjusted to 6.5. Salt solution contained the following (in g/L): CaCl2, 0.2; K2HPO4, 1.0; KH2PO4, 1.0; MgSO4·7H2O, 0.48; NaCO3, 10; and NaCl, 2. Modified PTYG agar medium (PTYG-F) was supplemented with 10 g/L CaCO3 and 20 g/L agar. PTYG containing X-gal medium (PTYG-X) was prepared by supplementing PTYG agar medium with 40 mg/L X-gal.
To screen oxygen-tolerant Bifidobacterium, all microbial cultures were performed at 37℃ in 20% CO2-80% atmospheric air, unless otherwise stated.
The oxygen-tolerant microbes with similar colony to bifidobacteria were screened through three steps. First, diluted sample was spread in MUP-MRS agar plate and incubated for 72 h. Second, Gram-positive microbes with small, smooth, convex, opaque, white, or milky colonies with neat edges and soft texture were further sequentially spread in PTYG-F and PTYG-X agar plate for incubation of 48 h. Mcrobes both with transparent circles on PTYGF plate and with dark-blue colonies with a lytic circle on the PTYG-X plate were then selected to observe their morphology by microscopy. Third, microbes with V-type, irregular rod, long rod, short rod, slender rod, or stick shapes were selected for subsequent experiments.
To select cholesterol-lowering strains from oxygen-tolerance microbes with similar colony to Bifidobacterium, 5% (v/v) fresh culture from modified PTYG was inoculated with modified PTYG containing 0.1 mg/mL cholesterol and incubated for 48 h. At the same time, a control group without inoculation was prepared. The yield of cholesterol-lowering strain was determined using o-phthalaldehyde (Rudel and Morris, 1973).
To evaluate the acid tolerance of cholesterol-lowering strains with oxygen tolerance, 5% (v/v) fresh enriched culture with modified PTYG was inoculated in phosphate-buffered saline (pH 3 and 7) and incubated for 2 h. The viable bacteria population was measured by spreading PTYG-F plates, which were then incubated for 48 h. Acid survival rate NA was calculated according to the following formula (1):
where N1 denotes surviving bacteria (CFU/mL) after incubation at pH 3 for 2 h, and N0 denotes surviving bacteria (CFU/mL) after incubation at pH 7 for 2 h.
To evaluate the bile-salt tolerance of bacteria from cholesterol-lowering strains with oxygen and acid tolerance, 5% (v/v) fresh enriched culture with modified PTYG was inoculated in modified PTYG containing 0.3% (w/v) oxgall for 24 h. Living bacteria were measured at 0 and 24 h by spreading PTYG-F plates, which were then incubated for 48h. Bile-salt survival rate NC was calculated according to the following formula (2):
where N2 denotes starting bacteria (CFU/mL) after incubation in 0.3% (w/v) bile salt for 0 h, and N3 denotes surviving bacteria (CFU/mL) after incubation for 24 h.
Gram staining, catalase, and oxidase tests were carried out for BZ25. The fermentation of carbohydrates was conducted using BZ25 through the API 50 CH system.
BZ25 was further identified by China General Microbiological Culture Collection Center (CGMCC) through its 16S rRNA gene-sequence analysis. A phylogenetic tree was constructed using the neighbor-joining algorithm through the maximum composite likelihood method in MEGA 5.0.
Bifidobacterium BZ25 was cultured in modified PTYG medium at 37℃ in 20% (v/v) CO2-80% (v/v) and 100% atmospheric air, respectively. After incubation for 0, 6 h, 12 h, 18 h, 24 h, and 30 h, samples were collected and subjected to absorbance analysis at 600 nm using spectrophotometry to plot the growth curve. Then, BZ25 was spread and incubated in modified PTYG agar medium at 37℃ under the two environmental conditions.
Results and Discussion
The vast majority of bifidobacteria are strict anaerobes, i.e., they cannot grow on plates under aerobic conditions. However, some bifidobacteria with enzymes that metabolize oxygen can survive from 0.1% (w/v) to 21.0% (w/v) oxygen environment (Ahn et al., 2001; Shin and Park, 1997). To identify bifidobacteria with resistance to oxygen, all microbial incubation was conducted in 20% (w/v) CO2-80% (w/v) atmospheric air (unless otherwise stated). (Li-MUP can inhibit the majority of lactic-acid bacteria but not bifidobacteria. In addition, >90% (N/N) of bifidobacteria may decompose 5-bromo-4-chloro-3-indolyl-β-🇩-galactopyranoside (X-Gal) to generate a blue color, so X-Gal was added to PTYG-F. Accordingly, MUP-MRS, PTYG-F, and PTYG-X were used to screen bifidobacteria. Then, 27 Gram-positive and rod-shaped bacteria with round, soft, and creamy colony morphology with convex central and smooth edges were selected form Guizhou Xiang Pig because their cell and colony characteristics were similar to those of bifidobacteria. Moreover, all of them may grow in 20% (w/v) CO2-80% (w/v) atmospheric air. This finding suggests these strains are likely to be bifidobacteria and resistant to oxygen.
To select cholesterol-lowering strains from the 27 oxygen-tolerant bacteria, cholesterol-lowering tests were carried out, and the results are shown in Table 1.
| Cholesterol-lowering rate | <10 | 10-20 | 20-30 | >30 |
|---|---|---|---|---|
| Strains (quantity) | 8 | 9 | 8 | 2 |
The cholesterol-removal rates of the 27 strains were between 4.30% (w/w) and 36.32% (w/w) (Table 1). Among them, eight strains had cholesterol-lowering rates of 20% to 30%, and two strains had cholesterol-removal rates exceeding 30% (w/w). Thus, for subsequent experiments, the following 10 strains were selected: BZ8, BZ12, BZ13, BZ14, BZ17, BZ22, BZ24, BZ29, BZ10, and BZ25 (with the highest cholesterol-removal rate of 36.32% (w/w)).
The screened strains should have excellent properties with good tolerance to acid and bile because probiotic strains need survive acid and bile stress in the gastrointestinal tract. To simulate the gastrointestinal condition, the 10 isolated strains were subjected to pH 3 for 2 h and 0.3% (w/v) bile salts for 24 h. The results are shown in Table 2. All 10 strains had good tolerance to low pH (Table 2), which suggested that acid-tolerance response occurred under acidic conditions. Some researchers (Sánchez et al., 2006; Sánchez et al., 2007) have also observed this phenomenon and identified acid-resistant B. longum and B. animalis subsp. lactis strains with higher F0F1-ATPase (atpA and atpD) activity after acid exposure. Acid-tolerance response in bacteria may be induced through an assemblage of inducible molecular mechanisms involving a modulation in gene expression (Liu et al., 2015; Sánchez et al., 2006).
Bile-tolerance test showed that most of the 10 bacteria may be tolerant to 0.3% (w/v) bile salt because their survival rate exceeded 90% (N/N), except for BZ12, BZ17, and BZ22. BZ25 also had the highest survival rate of 101% (N/N). Bifidobacteria can reportedly develop stable bile resistance phenotypes upon exposure to bile salt, regardless of its intrinsic tolerance (Sánchez et al., 2008).
The bile-salt tolerances of different bacteria are related to their biological characteristics. Andriantsoanirina et al. (2013) studied the acid and bile-salt tolerance of Bifidobacterium bifidum, Bifidobacterium adolescentis, Bifidobacterium breve, Bifidobacterium longum, and Bifidobacterium pseudocatenulatum and found that Bifidobacterium adolescentis was the most resistant to bile salts.
The above experiments indicated that BZ25 had the highest cholesterol-lowering rate and survival rate, as well as good tolerance to acid and oxygen. Thus, the physiological characteristics, biochemical characteristics, molecular biology, colony, and cell morphology of BZ25 were further examined.
Table 3 shows that BZ25 was gram-positive bacteria and negative for catalase and oxidase tests. The strain could utilize 🇩-ribose, 🇩-galactose, 🇩-glucose, α-methyl-🇩-glucoside, amygdalin, esculin, salicin, cellobiose, maltose, lactose, melibiose, sucrose, raffinose and gentibiose to produce acid. However, BZ25 could not metabolize glycerol, 🇩-mannose, erythritol, 🇩-arabinose, ʟ-arabinose, 🇩-xylose, ʟ-xylose, adonitol, β-methyl-🇩-xyloside, 🇩-fructose, ʟ-sorbose, ʟ-rhamnose, dulcitol, mannitol, sorbitol, α-methyl-🇩-mannopyranoside, N-acetyl-glucosamine, arbutin, trehalose, inulin, melezitose, starch, glycogen, xylitol, 🇩-turanose, 🇩-lyxose, 🇩-tagatose, 🇩-fucose, ʟ-fucose, 🇩-arabinitol, ʟ-arabinitol, gluconate, 2-keto-gluconate, and inositol to produce acid.
Note: * + and − represented positive and negative, respectively.
Moreover, the phylogenetic tree of BZ25 was constructed based on 16S rRNA gene sequence by neighbor-joining algorithm. Numbers at the nodes represent percentage bootstrap values based on 1,000 replicates. The horizontal scale bar indicates a distance of 0.005 (Fig. 1). This result indicated that the newly isolated strain BZ25 was closely related to Bifidobacterium animalis subsp. lactis AD011 strain and B. animalis subsp. lactis YIT 4121 (Fig. 1).
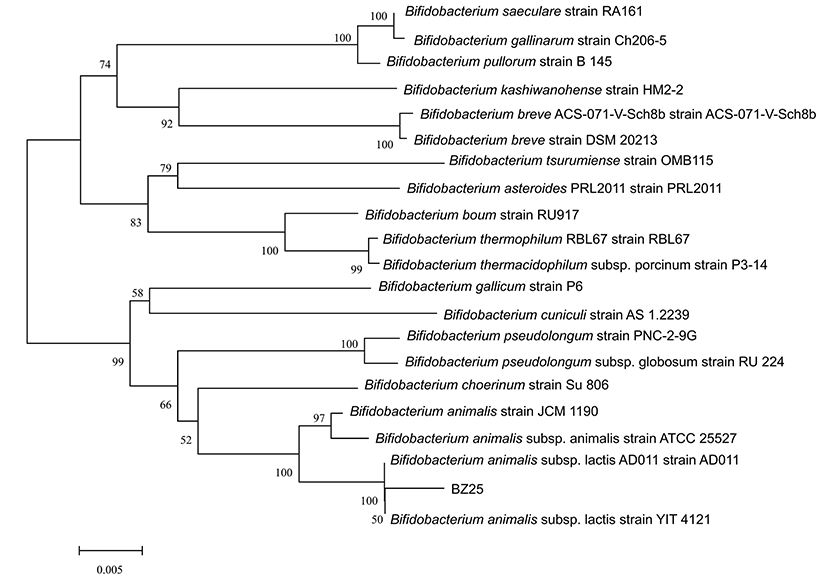
In addition, BZ25 colony with convex central and smooth edge was small, round, soft, and creamy shaped in a modified PTYG plate, whereas BZ25 cell was V-type or irregular rod. Thus, BZ25 was identified as B. animalis subsp. lactis based on analyses of physiological and biochemical characteristics, 16S rRNA gene sequence, colony morphology, and cell morphology. BZ25 is now registered in CGMCC, numbered as CGMCC No. 10225.
The name of B. animalis subsp. lactis is currently undergoing a correction process (Jungersen et al., 2014). It was first considered to belong to Bifidobacterium bifidum (Jungersen et al., 2014), then to B. animalis (Jungersen et al., 2014), to a new species Bifidobacterium lactis s (Meile et al., 1997), and to B. animalis as a subspecies (Cai et al., 2001). Based on its cha- racteristic, BZ25 belonged to B. animalis subsp. lactis.
The growth characteristics of BZ25 were then evaluated by incubation in liquid medium and in an agar plate.
Fig. 2 shows that BZ25 was in a slow growth phase in the first 6 h, entered the logarithmic growth phase, and then became stable after 30 h. Overall, growth in 100% atmospheric air was slightly less than that in 20% (v/v) CO2-80% (v/v) atmospheric air, whereas the growth ratio exceeded 0.8:1 at 30 h. Cell concentrations in the two growth environments were also both over 108 CFU/mL. Moreover, the same cell concentration of BZ25 was spread and incubated in modified PTYG agar medium at 37℃ under the two environmental conditions. The number of colonies in agar medium in 100% atmospheric air was 75% of that in 20% (v/v) CO2-80% (v/v) atmospheric air, indicating that BZ25 had high oxygen tolerance.
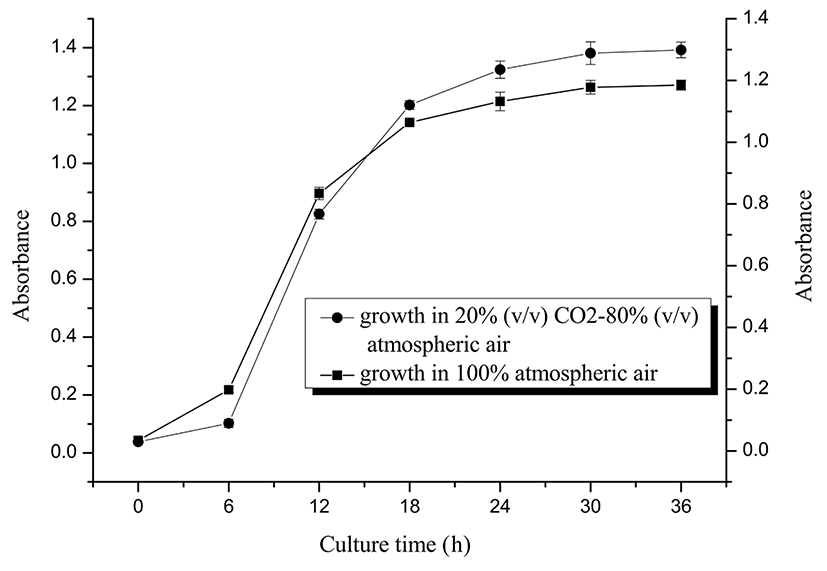
Meile et al. (1997) found that a moderately oxygen tolerant Bifidobacterium lactis sp. can tolerate 10% of oxygen in headspace atmosphere. Kawasaki et al. (2006) have also reported that Bifidobacterium globosum and Bifidobacterium thermophilus may grow under atmospheric conditions in an air/CO2 (9:1) mixture. By comparison, BZ25 grew well in a plate under 100% atmospheric air condition, indicating that BZ25 was aerotolerant. Thus, BZ25 was our target strain with a cholesterol-removal rate of 36.32% (w/w), as well as tolerance to acid, bile salt, and oxygen.
Many commercial dairy strains with probiotic relevance belong to B. animalis subsp. lactis (Mayer et al., 2007). For example, B. animalis subsp. lactis Bb-12® is the world’s most documented probiotic Bifidobacterium with proven beneficial effects on gastrointestinal health and immune function (Jungersen et al., 2014; Nishida et al., 2004). BZ 25 also belongs to B. animalis subsp. lactis. Thus, BZ25 may have excellent characteristics in addition to lowering cholesterol and tolerance to acid, bile and oxygen. These characteristics will be the focus of our next research.
Conclusions
Bifidobacterium with excellent characteristics was screened from Guizhou Xiang Pig by analyzing cell and colony morphology, cholesterol-removal rate, and tolerance to acid, bile, and oxygen. Twenty-seven strains with similar colonies to bifidobacteria were isolated. Among them, the target strain was BZ25, which was found to have the highest cholesterol removal rate (36.32%, w/w), as well as tolerance to acid, bile, and oxygen. BZ25 was identified as B. animalis subsp. lactis by colony morphology, cell morphology, physiological characteristic, and biochemical characteristic, and 16S rRNA gene analyses. BZ25 also had the maximum growth, reaching an absorbance of 1.185 at 600 nm at cell concentrations exceeding 108 CFU/mL in 100% atmospheric air.













