Introduction
Tenderness is a crucial quality attribute that directly affects the palatability and overall eating experience of beef meat. Factors such as age of animal, post-mortem proteolysis and aging methods, muscle structure, content of connective tissue and fat, cooking process have important role in the development of meat tenderness (Arshad et al., 2016; Dikeman et al., 2013; Kemp et al., 2010). Aging allows natural enzymes to break down connective tissues and proteins, resulting in increased meat tenderness and flavour development (Kim et al., 2020; Terjung et al., 2021). Aging method and time are primary factors for meat tenderization. Among these, mechanical tenderization and enzymatic treatments are preferred to tenderize meat in a reduced time (Bhat et al., 2018).
Enzymatic treatments, using proteases derived from plants or microbes, accelerate the breakdown of proteins and collagen, leading to tenderized meat (Ashie et al., 2006; Ha et al., 2012). The use of plant proteases for meat tenderization is increasingly favoured by the meat industry due to their lack of food safety risks, efficient utilization, easy accessibility, acceptance by a wide range of consumers and lack of ethical or animal welfare concerns (Fernández-Lucas et al., 2017; Mohd Azmi et al., 2023). Microbial proteases, which work similarly to plant proteases by breaking down proteins into smaller peptides and amino acids to tenderize meat, are also used for enzymatic tenderization of beef (Arshad et al., 2016). This enzymatic effect increases the tenderness of meat, making it easier to chew, while releasing savoury and umami compounds that contribute to a richer and more complex flavour (Botinestean et al., 2020; Gagaoua et al., 2021; Pooja et al., 2022). Enzymes accelerate this protein degradation, facilitating the process and leading to faster aging, potentially reducing the time required for traditional aging methods. This accelerated aging results in beef with improved tenderness, juiciness and flavour in a shorter time frame (Botinestean et al., 2020; Mohd Azmi et al., 2023; Zhao et al., 2020).
Enzyme treatments improve the consistency of beef quality depending on the enzyme type and the processing conditions from tender and buttery to firmer and chewier texture. This allows meat to be tailored to specific culinary applications and consumer preferences (Zhao et al., 2020). Plant proteases such as papain, ficin and bromelain, and microbial proteases such as bacterial and fungal enzymes (Bacillus subtilis, Aspergillus oryzae, Aspergillus niger), which are defined as GRAS (Generally Recognised as Safe) category, are commonly used for meat tenderization (Bekhit et al., 2014; Gagaoua et al., 2021; Ha et al., 2012). In addition to plant-derived cysteine proteases such as papain, bromelain and ficin, there are other plant enzymes utilized for the tenderization of meat, including actinidin (from kiwi fruit) and zingibain (ginger protease) which have not yet been approved as GRAS. These are less investigated enzymes than papain and bromelain and target myofibrillar proteins moderately. However, since the effects of these plant proteases on collagen are not strong, they do not provide effective tenderisation of connective tissue-rich meats (Gagaoua et al., 2021).
Papain (E.C 3.4.22.4), a non-specific cysteine protease obtained from the extract of Carica papaya, is the most commercial tenderizer. The enzyme of this tropical plant causes a major degradation of the Z disk in the muscle during the aging, tenderizing the meat in a shorter time (Arshad et al., 2016; Marques et al., 2010). Bromelain (E.C 3.4.4.4.24) is a proteolytic enzyme found abundantly in the fruits, leaves and stems of pineapple (Ananas comosus), has the ability to degrade myofibrillar proteins and collagen, resulting in tenderization of meat (Marques et al., 2010). Fungal proteases, which are enzymes of the aspartic protease derived from A. oryzae, do not maintain their tenderization effect in muscle tissue continuously unlike plant enzymes (Ashie et al., 2006). Being an enzyme that limits its own activity, it ensures sufficient breakdown of the protein structure, preventing the formation of a mushy texture in the meat and resulting in a more palatable product (Calkins and Sullivan, 2007).
Barekat and Soltanizadeh (2017) reported that the application of 0.1% papain enzyme solution significantly reduced the shear force value of longissimus lumborum meat. Meanwhile, Nadzirah et al. (2016) stated that bromelain reduced the hardness of beef round cuts. Similarly, Whetstone et al. (2014) indicated that 20 mg/mL bromelain solution was effective in improving the tenderness of beef steaks. Ha et al. (2013) highlighted that bacterial enzymes are more efficient at degrading myofibrillar and collagen proteins than papain.
In this context, this study was aimed to determine the effect of accelerated aging process provided by enzyme treatment on the quality characteristics of longissimus thoracis and longissimus lumborum muscles.
Materials and Methods
Longissimus dorsi thoracis (LT, ribeye) and Longissimus dorsi lumborum (LL, striploin) muscles, from twelve cattle (male, 1.5–2 years old, 450±50 kg weight) fed with a same regime, were obtained from a commercial plant at 24 h post-mortem and stored at 4°C until enzyme treatments.
Papain (Carica papaya powder, ≥3 U/mg, P76220), bromelain (Bromelain from pineapple steam, ≥3 units/mg protein, B4882) and fungal protease (Protease from A. oryzae, ≥500 U/g, P6110) enzymes were purchased from Sigma Chemical (St. Louis, MO, USA). All the reagents and chemicals used were of analytical grade.
Stock enzyme solutions were prepared by dissolving 1 g papain, 1 g bromelain and 1 g fungal protease in 100 mL each of 0.9% sterile saline water. Each of these enzymes was applied at concentrations of 0.01 g/kg, 0.02 g/kg and 0.05 g/kg, using an 18-gauge single-needle syringe to inject the solution along the entire line at 12 different equally scaled points in each meat block to ensure consistent enzyme distribution. An amount of sterile saline equal to the enzyme applied to the samples was also injected into the untreated meat. In order to prepare meat samples for enzyme treatments, one pair of LT and one pair of LL meat from each animal were divided into 10 equal parts to be treated with papain (P), bromelain (B) and fungal protease (F) enzymes at concentrations of 0.01 g/kg, 0.02 g/kg and 0.05 g/kg, and control.
The enzyme-treated samples were vacuum packed into bags (90 μm thickness, polyamide/polyethylene, oxygen permeability of 30–60 cm3/m2/24 h at 23°C, 0% RH, water vapour permeability of 3–4 g/m2/24 h) by vacuum packaging machine (Orved VM 18, Orved, Musile di Piave, Italy) and kept at 2±1°C and were subjected to relevant analyses on days 1, 2, and 5 of the aging period.
The pH values of beef samples were determined by homogenizing 10 g of sample in 100 mL of distilled water using a digital pH meter (Hanna HI 9321; AOAC, 2005). The water activity (aw) values of samples were measured by an aw meter (Decagon AquaLab Series 4TE; ISO, 2017a). The moisture content was detected by drying 2 g of homogenized meat samples at 105°C in Sartorious MA45 Moisture Analyzer (AOAC, 2005).
The water holding capacity (WHC) was measured by the method of pressure in filter paper (Whatman No. 4, 150 mm diameter) as described by Aroeira et al. (2016), with minor modifications. Briefly, a piece of meat sample (300 mg) was placed onto a filter paper, and inserted between the plexiglass plates (6 mm thickness) for 20 min under a weight of 1 kg. Following the compression, the area of the pressed meat spread on the filter paper and the area of the total leaked liquid were determined using AutoCAD 23.1 and calculated with the formula:
For cooking loss (CL), meat samples were cooked in a water bath at 100°C for 10 min. After cooking, the samples were weighed, and the CL was calculated based on the formula:
Twenty grams of meat sample were homogenized by mixing with 50 mL of 20% trichloroacetic acid (prepared in 2 M phosphoric acid solution). After filling to 100 mL with deionised water, it was filtered, and 5 mL of the filtrate was mixed with 5 mL of freshly prepared 0.005 M thiobarbituric acid solution. The mixture was kept at room temperature in the dark for 15 h and the absorbance of the colour was measured at 530 nm in UV spectrophotometer (Shrestha and Min, 2006). Thiobarbituric acid reactive substances (TBARS) value was expressed as mg malondialdehyde (MDA)/kg.
Twenty-five grams of beef samples were taken into sterile bags under aseptic conditions and homogenized in a stomacher (Interscience, Saint-Nom-la-Bretèche, France) by adding 225 mL of sterile peptone water (CM0061, Oxoid, Basingstoke, UK). Serial dilutions were prepared from the main dilution and meat samples were analysed for the relevant microorganisms (ISO, 2017d).
The psychotropic bacteria counts were detected using Standard Plate Count Agar (CM0463, Oxoid) with incubation at 7°C for 10 days (ISO, 2019). For the enumeration of Enterobacteriaceae spp., Violet Red Bile Glucose Agar (VRBG - Oxoid, CM0485, Oxoid) was used and incubated at 37°C for 24 h (ISO, 2017c). To quantify the lactic acid bacteria, Man, Rogosa and Sharpe Agar (CM0361, Oxoid) was used with double layer and incubated at 30°C for 72 h (ISO, 1998). The Pseudomonas spp. and Brochothrix thermosphacta counts were determined in Pseudomonas Agar Base (CM0559, Oxoid) with CFC Selective Agar Supplement (SR0103, Oxoid) and Streptomycin Thallous Acetate Actidione Agar (CM0881, Oxoid) with ISO (2010) and ISO (2017b) protocols, respectively. The yeast-mould counts were detected using Yeast Extract Glucose Chloramphenicol Agar (PO5032A, Oxoid) and incubated at 25°C for 5 days (ISO, 2008).
CIE L*, CIE a* and CIE b* values of meat samples were determined using HunterLab ColorFlex Color Measurement System (Hunter Associates Laboratory, Reston, VA, USA). Colour measurements were obtained in “daylight” mode using diffuse illumination (D65 2° observer) with a viewing aperture of 8 mm and a port size of 25 mm (AMSA, 2012). The arithmetic averages of five measurements from each sample were recorded.
The metmyoglobin content of meat samples was determined according to the method described by Bekhit et al. (2003). Briefly, 5 g of samples were homogenized in 40 mM cold buffered phosphate solution for 10 s. The homogenate was kept at 4°C for 1 h and centrifuged at 4,500×g for 30 min. The supernatant was filtered through Whatman No. 1 filter paper and measured in UV spectrophotometer at 572, 565, 545 and 525 nm absorbance. The metmyoglobin content was calculated according to the following formula:
The tenderness of LT and LL samples was measured with Warner-Bratzler shear force (WBSF) Texture Analyzer (3343, Instron, Buckinghamshire, UK). The samples cooked for CL were chilled in a bag overnight and were cut parallel to the muscle fibres as a 1.27 cm diameter to determine the WBSF value using Warner-Bratzler blade with a 1,000 N load cell. The data of measurements was recorded with BlueHill 2 software and the average of ten different measurements was evaluated.
The organoleptic properties (colour intensity, odour intensity, tenderness, chewiness, juiciness, flavour intensity) of meat samples were evaluated by twelve panelists (age between 28 to 47 years) trained in accordance with ISO (2012). A preliminary session (1 h) discussing sensory attributes to familiarize panelists with the attributes and scale to be used was organized in two separate sessions, using a standard procedure (ISO, 2016).
Steaks of each treatment were divided in two main group and one of them was cooked in an electric oven at 170°C until internal temperature reached to 71°C. Then, each steak belonging to each treatment was cut into 12 equal pieces and cooked ones were kept with aluminium foil in an oven at 60°C until service. In each analysis day, two pieces of each of ten treatments were served monadically as raw and cooked, coded with a three-digit number in randomized order.
The colour, odour, tenderness, chewiness, juiciness and flavour (cooked samples only) attributes of meat samples were evaluated using an unstructured line scale (0: weak red/brown colour, no odour, extremely tough, easy to chew, extremely dry, no flavour; 10: very dark red/deep brown colour, predominant odour, extremely tender, hard to chew, extremely juicy, extremely flavourful or intense, respectively; ISO, 2007). Sensory panel was conducted in triplicate in two sessions.
Analysis of variance (ANOVA) in SPSS 21.0 (IBM, Armonk, NY, USA) was performed to determine the effects of enzyme treatments at different concentrations on the quality characteristics of LT and LL muscles during the aging process. A factorial design of 10 different treatment groups and aging time was used to explain significance of differences between and within groups and the significance was defined as p<0.05. Fisher’s least significant difference test (LSD) was used to analyse the data for sensory characteristics and mean separations were obtained using Duncan’s multiple range tests. Microbial counts were expressed as Log CFU/g. Each experimental trial was repeated in triplicate on different times.
Result and Discussion
The pH values of LT and LL samples treated with different concentrations of plant and microbial proteases increased during the 5-day aging period. The pH of the enzyme-treated samples was higher than untreated samples, and on the 5th day of aging, the highest pH value was determined in papain-treated samples, while the lowest pH value was recorded in the bromelain-treated ones. The pH of LT samples was higher than LL ones, while papain-treated meats at 0.01 g/kg were higher than in other treatments. Also, there was no significant difference in pH value among enzyme treated samples (p>0.05; Figs. 1 and 2).
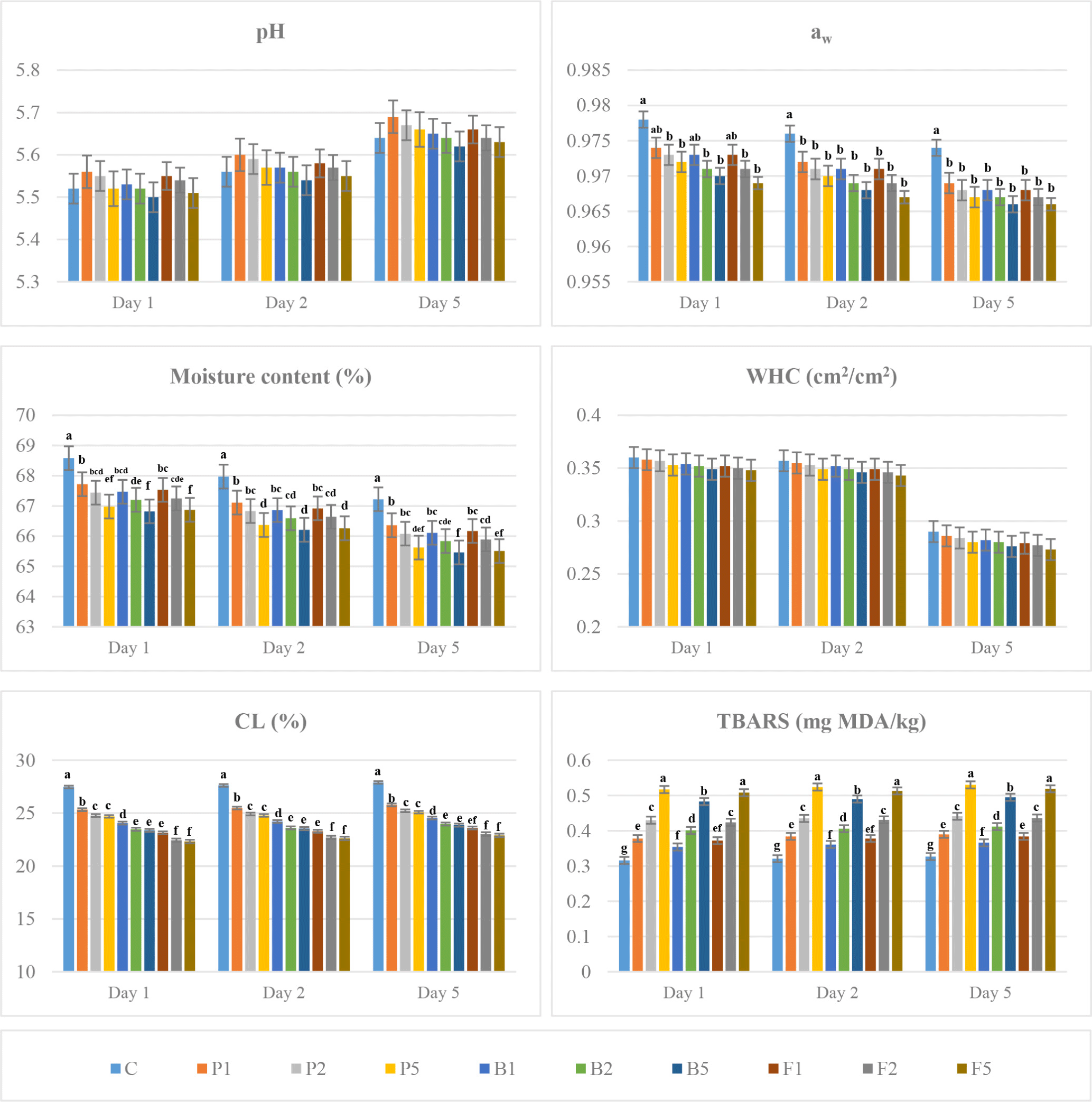
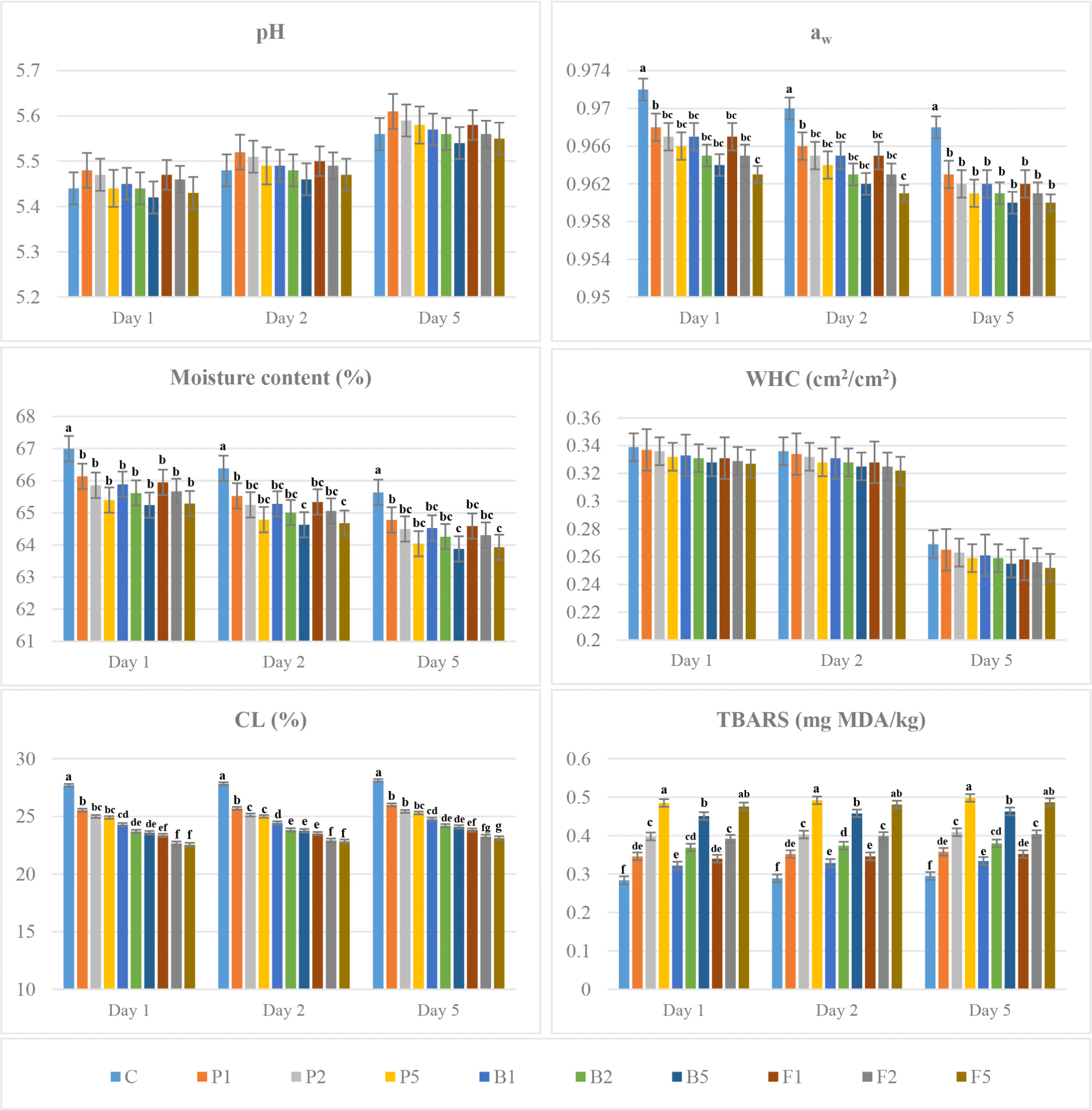
Protease enzymes affect the pH of beef by breaking down proteins into smaller peptides and amino acids. During the proteolytic process of meat aging, amino acids such as glutamic acid, aspartic acid and components with acidic side chains are released, increasing the concentration of hydrogen ions thereby decreasing the pH of the meat. However, as the aging period progresses and the aging day of enzyme activity increases, the alkaline components released in the advanced stages of protein degradation due to proteolytic activity cause a gradually increase in the pH value (Altan et al., 2024; Mohd Azmi et al., 2023). The higher pH value in the enzyme-treated meats compared to the control was associated with the advanced degradation reactions caused by proteolytic enzymes mentioned above. Moreover, due to the increased count of lactic acid bacteria in the control group, the pH values of the meat in this group were lower than the other enzyme-treated groups (Figs. 1 and 2). A decrease in pH due to protease-induced breakdown of proteins and the release of acidic by-products can help preserve meat by inhibiting the growth of spoilage organisms and pathogens. Falling pH interferes with the cell membrane potential and metabolic processes of microorganisms, leading to slow growth and even bacterial death.
The increase in the pH values of enzyme-treated beef is associated with an increase in the loss of free acidic groups, that directly affects the quality properties and shelf-life of meat (Huang et al., 2011). Contrarily, Ketnawa and Rawdkuen (2011) determined a significant decrease in the pH values of meat samples marinated with bromelain extract (BE) compared to the untreated sample and found that the pH value decreased further as the concentration of the enzyme increased due to the low pH level of BE.
In the present study, the water activity (aw) values of the ribeye and striploin samples showed a decrease during storage. This decrease was recorded more in the enzyme-treated samples, while the aw values of papain-treated samples were relatively higher than the other enzymes and a decrease in the aw value was determined with the increase in the enzyme concentration. Both enzyme-treated LT and LL samples were significantly different from untreated ones on all days of aging (p<0.05). However, among the LL samples in the first 2 days of aging, the samples treated with 0.05 g/kg fungal protease differed from the other enzyme treatments (Figs. 1 and 2).
Proteases can affect the water retention ability of beef by affecting free and bound water in the meat structure. As proteases break down muscle proteins, particularly myofibrillar proteins, muscle fibres can lose their ability to hold water tightly. This can lead to a reduction in bound water, making it more likely to be released during cooking or processing. The breakdown of collagen during ageing or enzymatic processes leads to softer tissue, but can also create gaps and spaces between muscle fibres, which can alter the distribution of water within the tissue. Over time, protease-induced degradation of muscle proteins leads to meat in the aging process losing moisture and thus lowering its water activity (Madhusankha and Thilakarathna, 2021).
Similarly, the moisture content of the samples decreased during the 5-day aging period and the moisture content of the untreated samples was higher than the enzyme-treated meat. There was a significant difference in moisture content between enzyme-treated and untreated LT and LL samples for all days of aging (p<0.05). While enzyme treatment decreased the moisture content of LT and LL samples, the lowest moisture content was found in the bromelain-treated samples. Also, the moisture content of samples decreased with increasing enzyme concentration. In LT samples, 0.05 g/kg enzyme treatments differed from the other treatments for each day of aging (Figs. 1 and 2).
Most of the water content in meat is retained in the muscle and muscle cell structure. Therefore, the reduction in moisture content is caused by the destruction of the muscle cell structure, which in turn is caused by the denaturation of myofibrillar proteins by the action of heat and meat tenderizing enzymes (Huang et al., 2011). Moreover, there is a negative correlation between the moisture content of meat samples, and the growth of total bacterial, mould and yeast. This is due to microbial degradation affecting the water retention ability of muscle tissue and the increase in weight loss during ripening, indicating a decrease in overall water content in this tissue (Gudjónsdóttir et al., 2015). Similar with our findings, Nadzirah et al. (2016) found that bromelain enzyme significantly reduced the moisture content of meat. Besides, Ketnawa and Rawdkuen (2011) reported that powdered BE absorbed more water in fresh meat samples, resulting in lower moisture content compared to the untreated meat. The moisture content of BE-treated samples was found to be significantly lower than those without the enzyme and an increase in BE concentration resulted in a decrease in moisture content, confirming that enzyme treatment improves hydrophilic properties.
WHC of meat is of great importance as it partially affects physical properties such as colour, texture and hardness. In this study, WHC of LT and LL samples decreased in all treatments during the aging process. The WHC values of the untreated samples were relatively higher than the enzyme-treated ones. However, there was no significant difference between enzyme-treated and untreated samples during aging period (p>0.05; Figs.1 and 2). Meanwhile, the WHC of LT samples was higher than LL samples.
Ketnawa and Rawdkuen (2011) and Nadzirah et al. (2016) found that the bromelain enzyme reduces the WHC, which the enzyme does by hydrolyzing proteins into small peptides or amino acids. Degradation of myofibrillar proteins by proteases can reduce the ability of muscle fibres to retain water, lowering WHC and leading to water loss during cooking or processing (Huff-Lonergan and Lonergan, 2005). The decrease in the WHC of enzyme-treated meat samples may be due to the low pH value (Ketnawa and Rawdkuen, 2011) and the decrease in the available WHC in protein reactive groups may be responsible for this decrease in pH value (Joo et al., 1999). Similarly, Hafid et al. (2020) reported that the WHC of meats treated with papain significantly decreased regardless of the muscle group. However, Akpan and Omojola (2015) stated that different concentrations (0%, 0.2%, 0.4%, 0.6%, 0.8% and 1.0%) of papain injection did not significantly affect the WHC of meat.
Since the longer the enzyme treatment process, the more extensive the protein degradation, prolonged exposure to the enzyme causes the meat to lose its textural integrity, reducing its WHC during aging process or cooking. Correspondingly, in the present study, it was noted that WHC decreased with increasing enzyme concentration and prolonged aging time.
The CL values of ribeye and striploin samples increased in all treatments during the aging period. CL values observed in untreated meat were higher than the papain, bromelain and fungal protease-treated samples. Among the enzyme treated meat, the highest CL were recorded in papain treatment, while the CL decreased with the increase in the applied enzyme concentrations. A significant difference was observed between enzyme-treated and untreated samples for all days of aging (p<0.001). There was a significant difference between enzyme-treated LT and enzyme-treated LL samples (p<0.001), while 0.02 g/kg and 0.05 g/kg concentrations within the same enzyme treatment had the same effect on CL (Figs. 1 and 2).
Botinestean et al. (2021) reported that the increased concentration of papain leads to greater CLs. Higher concentrations of proteases lead to faster and more extensive breakdown of muscle proteins and collagen. While this can increase tenderness, it also tends to increase CL due to the reduced ability of the meat to retain water. In this study, a decrease in CL values was found with increasing enzyme concentration. This was attributed to the fact that during the aging process, as a result of the degradation of proteins by the activity of proteolytic enzymes, muscle tissue fibrils become loose, and intercellular and intramuscular water leaks out of the meat mass. Therefore, since the amount of water remaining in the meat is reduced, the water loss during cooking is also less in meat treated with higher enzyme concentrations (Chang and Han, 2020; Xu et al., 2020).
During cooking process, changes in the water content in the myofibrils between the filaments and shrinkage of tissue matrices cause CL in meat (Murphy and Marks, 2000). It is thought that heat treatment can remove more water from enzyme-treated beef compared to other animal meats and this is a result of the fact that bromelain enzyme hydrolyses proteins in beef better than poultry and fish meats (Ketnawa and Rawdkuen, 2011).
TBARS value was used to determine the level of lipid oxidation that may occur during the aging process of beef. In this study, TBARS values of LT and LL samples in all treatments increased during the aging period. While the TBARS values of the enzyme-treated meat were higher than the untreated ones, significant increases in TBARS values were recorded with increasing enzyme concentration. In addition, TBARS values of LT samples were higher than LL ones. Enzyme treatments caused an increase in TBARS value depending on the increase in concentration. The highest TBARS value was determined in 0.05 g/kg papain-treated samples which were significantly different from bromelain-treated LT samples (p<0.001; Figs. 1 and 2).
Higher concentrations of protease enzymes further degrade muscle proteins, disrupting muscle structure and exposing more surface area for lipid oxidation. As proteins are broken down, lipids (especially those in membrane phospholipids) are released into the interstitial space of meat, where they are more readily oxidised by reactive oxygen species. The release of more lipids leads to higher levels of lipid oxidation, increasing the TBARS value with the formation of MDA and other TBARS compounds (Warner et al., 2022).
There is a close relationship between lipid and myoglobin oxidation, and the increase in oxidation is responsible for the negative colour changes in meat (Faustman et al., 2010). As high pH value reduces the myoglobin oxidation, the pH value, which increases with the prolongation of the aging period, can reduce this negative colour changes in meat (McKenna et al., 2005). Lipid oxidation can also negatively affect the flavour of meat. Although the limit value determined for TBARS value can be found in a wide range (Bingol and Ergun, 2011; Campo et al., 2006), many researchers have stated that TBARS value should be below 1 mg MDA/kg during storage and long aging period (Bingol and Ergun, 2011; Colle et al., 2015; McKenna et al., 2005).
The bacterial load of enzyme-treated LT and LL samples was lower than that of the untreated ones, and an increase was recorded in all bacteria counts during aging (Figs. 3 and 4). In addition, more inhibition was achieved in the enzyme-treated meat with the increase of the concentration. The difference between treatments was significant on the 5th day of aging in all analysed bacteria. Besides, Pseudomonas spp., B. thermosphacta and yeast-mould counts showed significant differences between enzyme-treated and untreated samples from the 2nd day until the end of aging only in LL. However, a significant difference was observed only in Pseudomonas spp. and yeast-mould counts between enzyme-treated and untreated LT samples on day 2 of aging (p<0.05), while fungal protease-treated samples differed from other enzyme treatments on day 5 of aging (p<0.001). This indicates that fungal protease enzyme at a concentration of 0.02 and 0.05 g/kg delayed the deterioration of meat during cold storage. These results also show the effectiveness of enzymes on microorganism load depending on time. A significant inhibition of 0.5 Log CFU/g on PsB counts was recorded in meat treated with fungal protease enzyme on the 5th day of aging, while no significant difference was observed in papain- and bromelain-treated samples during aging period (Figs. 3 and 4). The increase in the count of Enterobacteriaceae in fungal protease-treated striploins was lower than the other enzymes, and an increase in bacterial inhibition was observed in parallel with the increase in the enzyme concentration. In addition, the bacterial load in LT samples was found to be lower than LL muscles. Lactic acid bacteria increased in all groups during aging, but this level was lower in the enzyme-treated samples than in the untreated ones. The count of LAB in the LT samples was lower at the level of 0.3–0.6 Log CFU/g compared to the striploin samples. The counts of Pseudomonas spp. that forms the dominant flora in red meat stored at low temperature and B. thermosphacta that causes spoilage in meat and meat products were lower in enzyme-treated samples compared to untreated ones. Furthermore, this inhibition increased with increasing enzyme concentration. The mould and yeast count of LT and LL samples were lower in the samples treated with fungal protease than in the other groups (Figs. 3 and 4).
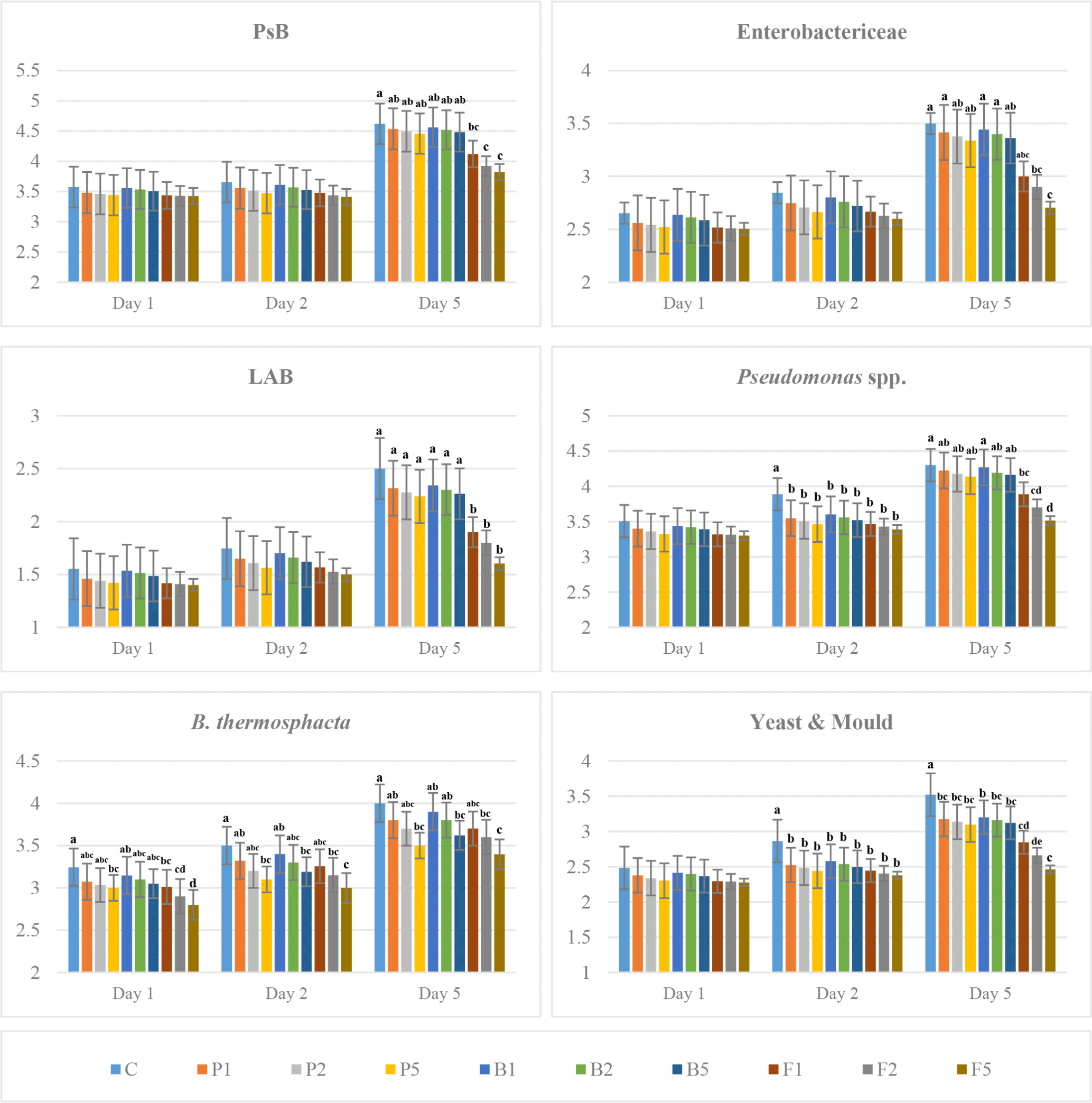
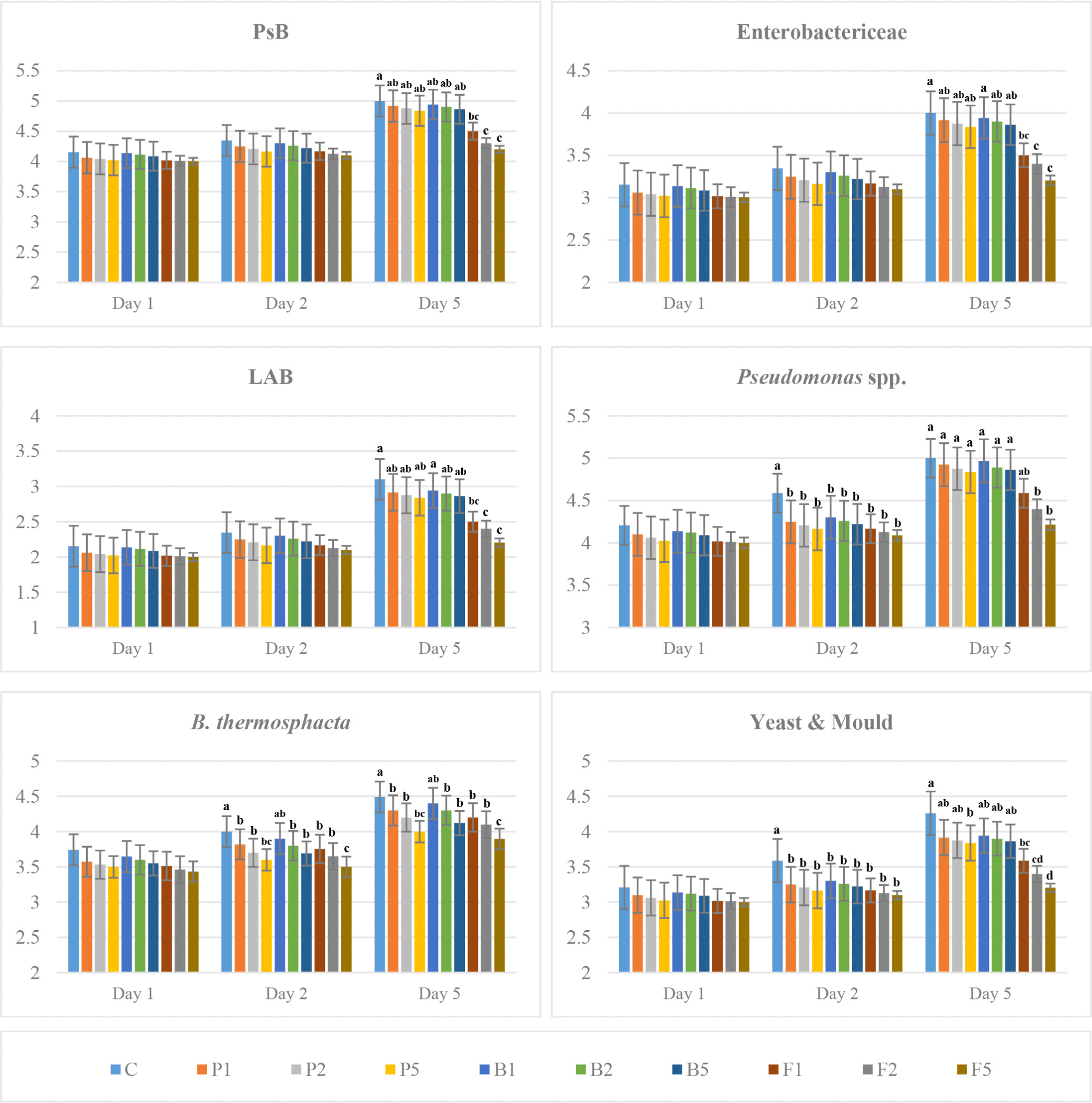
Proteolytic enzymes such as papain, bromelain and fungal protease can exert inhibitory effects on microorganisms by degrading bacterial structural proteins, membrane proteins or enzymes essential for bacterial cell functioning. Enzymes can disrupt the integrity of the bacterial cell membrane by degrading cell wall proteins or membrane proteins, leading to cell lysis or impaired bacterial function. Particularly, papain can interact with bacterial enzymes required for cell metabolism, replication or virulence. For example, some enzymes involved in protein synthesis or cell wall biosynthesis may be targeted by the proteolytic activity of papain, slowing or halting bacterial growth (Eshamah et al., 2014). In addition, fungal proteases, especially those produced by fungi like Aspergillus species, can have a lytic effect on the bacterial cell wall by breaking down peptidoglycan and surface proteins. Moreover, fungal proteases might degrade key membrane proteins, increasing permeability and making bacteria more susceptible to external factors (Ashie et al., 2006).
Significant differences were observed in the colour parameters of enzyme-treated and untreated LT and LL samples during aging period (p<0.001; Fig. 5). The CIE L* and CIE b* values of LL and LT samples treated with enzymes were lower than the untreated samples, while the CIE a* values of the enzyme-treated samples were higher than the untreated ones. Increasing enzyme concentration caused a decrease in CIE L* and CIE a* values and an increase in CIE b* values. The highest CIE a* values were determined in papain-treated samples, while CIE b* values in bromelain-treated samples were higher than other enzyme treatments. The difference between the CIE L* values of LT and LL samples treated with 0.05 g/kg enzyme and those treated with enzyme at 0.01 and 0.02 g/kg concentrations was found significant (p<0.001). A significant difference was observed in CIE a* value among enzyme-treated samples on the 2nd day of aging, while bromelain-treated samples were significantly different from the other enzyme treatments on the 5th day of aging.
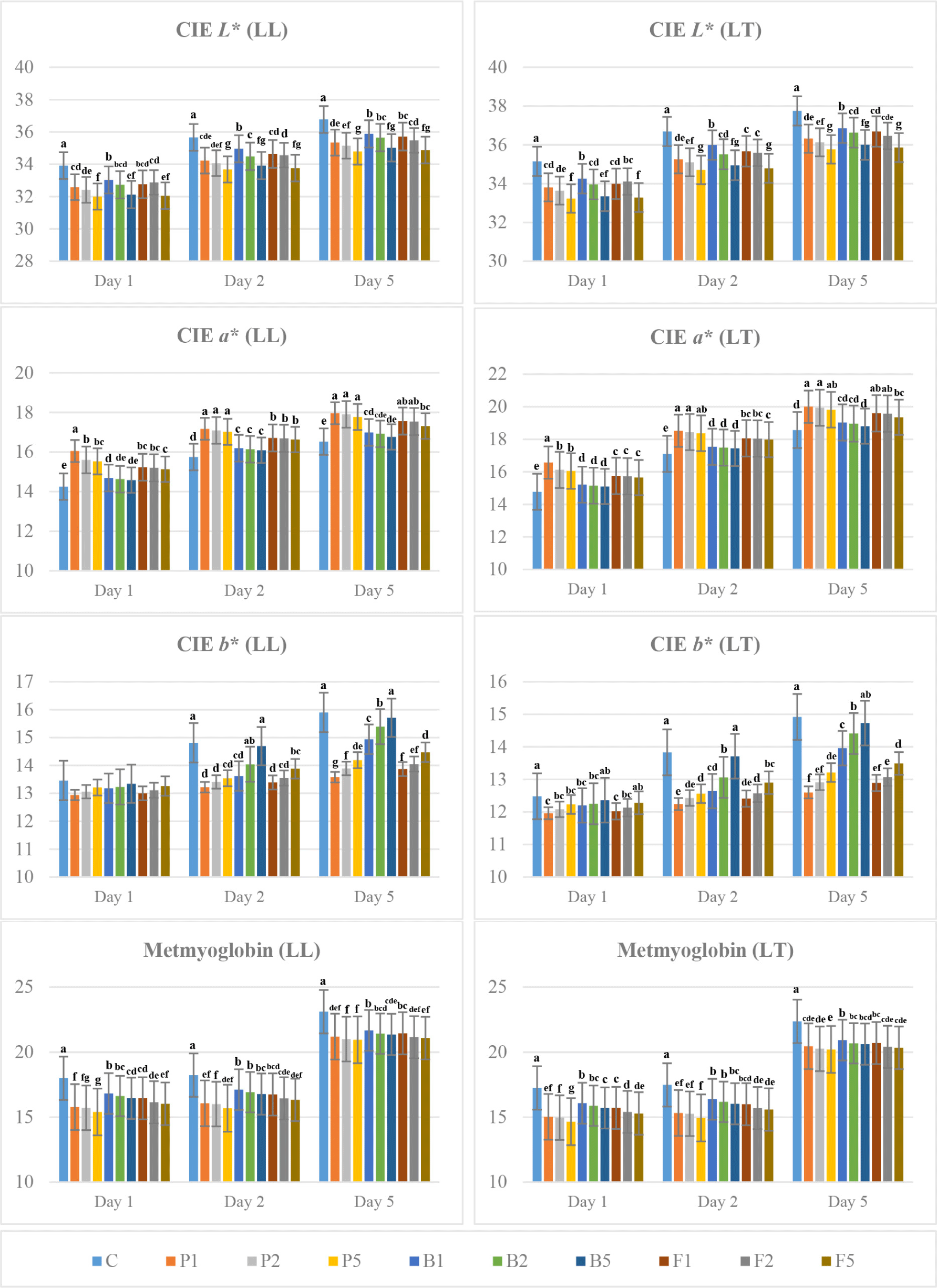
The metmyoglobin values of untreated samples were significantly higher than those enzyme-treated samples (p<0.001; Fig. 5). The metmyoglobin values decreased with increasing enzyme concentrations.
The colour of fresh meat varies depending on the amounts of myoglobin, oxyoglobin and metmyoglobin in its composition. With the formation of metmyoglobin, which is formed in environments where oxygen is not sufficient, a dull brown colour occurs in meat (Hernandez et al., 2006). The CIE a* value is related to the total pigment, myoglobin and iron ion concentrations. Therefore, changes in the CIE a* value are associated with the content of myoglobin, which undergoes oxidation to metmyoglobin, resulting in a browner colour (Chueachuaychoo et al., 2011). In the present study, no significant difference was observed in the CIE a* values of LT and LL samples between different concentrations of the same enzyme treatment except for papain treatment in first day of aging. Botinestean et al. (2021) reported that papain treatment had no effect on CIE L* and hue angle values, however affected the CIE a* value with an increase in CIE a* value depending on higher concentration. The CIE b* values of beef steaks increased with papain concentration, which was explained by the development of metmyoglobin resulting in a brownish colour in the cooked product.
A significant decrease was recorded in the shear force values of LT and LL samples treated with high concentrations of enzyme in the first 24 h of aging and this decrease continued in the following days. There was a significant difference between enzyme-treated and untreated samples during aging period (p<0.001; Fig. 6). The WBSF value of LT samples was lower than that of LL samples. Increasing concentration of enzyme resulted in a decrease on the WBSF values of meat samples. Papain, bromelain and fungal protease treatments with a concentration of 0.05 g/kg provided more tender meat, while the most effective enzyme treatment was observed in papain-treated samples.
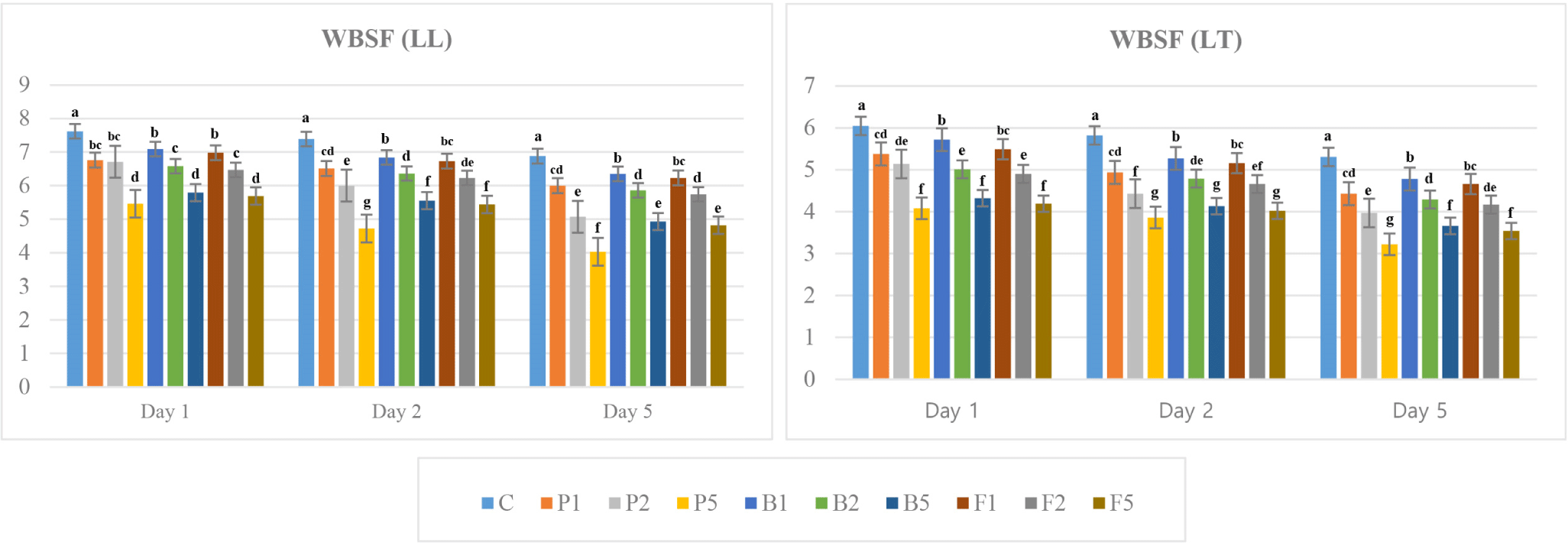
Sullivan and Calkins (2010) reported a significant effect of enzyme (papain, ficin, bromelain, homogenised fresh ginger, B. subtilis protease and A. oryzae protease) treatments on WBSF value except ginger compared to the untreated meat. Papain-treated samples had the lowest WBSF value (34.42 N), differing from the other treatments which is in line with our findings. These results agree with the view that papain has a great effect on the WBSF value, and the tenderness is improved compared to the untreated samples (Ashie et al., 2006; Barekat and Soltanizadeh, 2017; Botinestean et al., 2021; Istrati, 2008). In addition, a linear decrease in the WBSF value is provided with the increase in the applied enzyme concentration (Akpan and Omojola, 2015; Ketnawa and Rawdkuen, 2011).
Ketnawa and Rawdkuen (2011) stated that the reduction in meat hardness was caused by the effect of proteolytic enzymes on myofibrillar proteins. They indicated that the degradation of myofibrillar proteins and the formation of low molecular weight small peptides or proteins resulted in a decrease in the hardness of meat. Nadzirah et al. (2016) highlighted that the tenderness of beef increased due to the proteolysis effect of bromelain enzyme on muscle proteins, basing this on the study of Ketnawa and Rawdkuen (2011) who found that bromelain degrades myosin heavy chain.
Enzyme-treated LT and LL samples had higher values in terms of colour and texture properties compared to the untreated samples. Beef treated with low concentrations (0.01 and 0.02 g/kg) of enzyme were generally rated with higher score by the panelists, however, the flavour characteristics of samples were negatively affected by the high concentration (0.05 g/kg) of enzyme treatments that causes over-tenderization with mushy texture. Additionally, LT samples were scored higher than LL samples (Fig. 7).
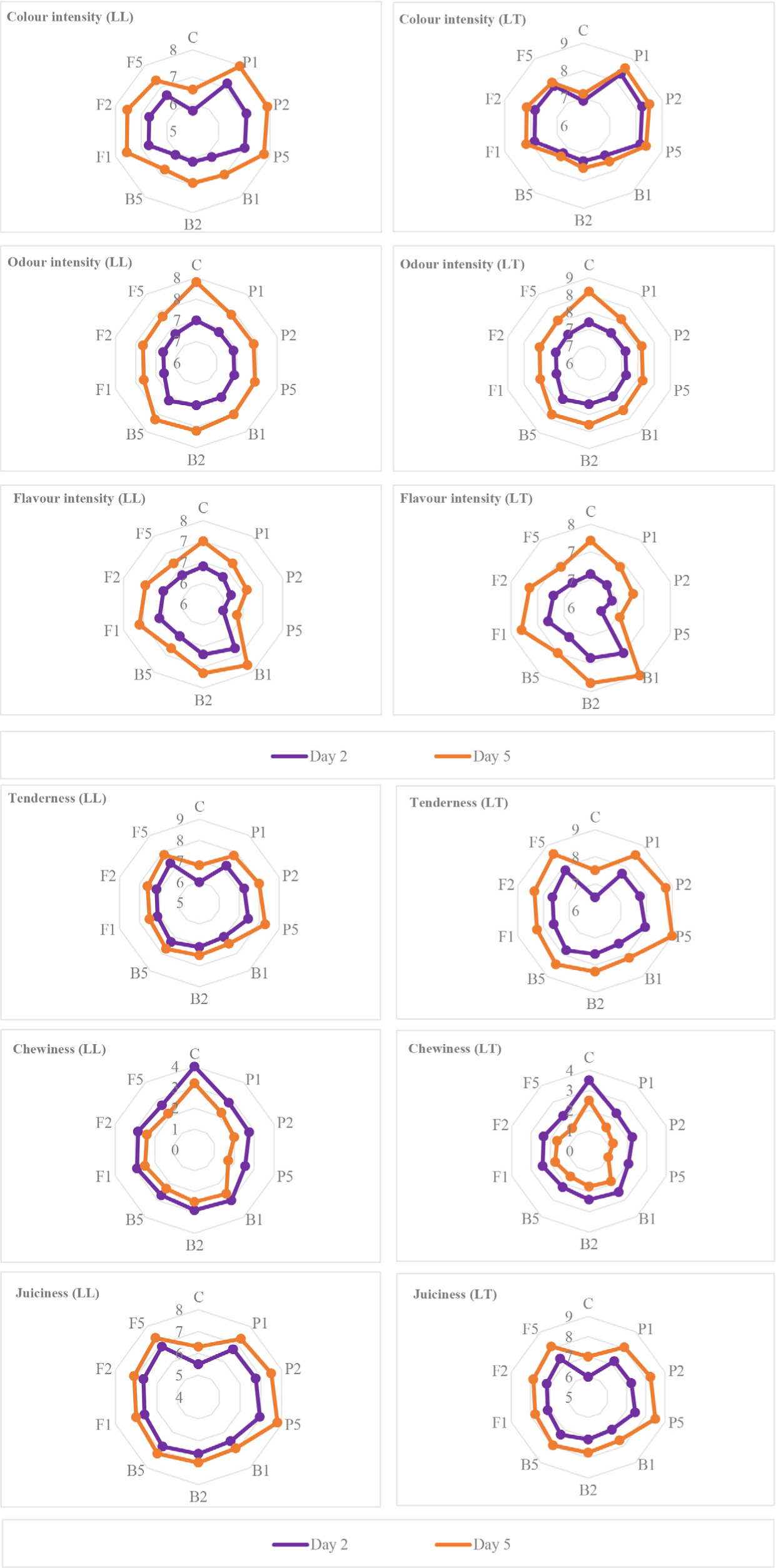
The values of textural properties such as tenderness, chewiness and juiciness of the beef treated with different enzymes were higher than the untreated ones, and papain was found to be the most effective enzyme treatment in improving the tenderness of the meat. However, it lagged fungal protease and bromelain enzymes in terms of flavour values. In particular, LT and LL samples treated with low concentrations of bromelain had the highest scores.
The consistency of meat is directly determined by the tenderness, chewiness and juiciness attributes that influence consumer preferences. According to Sullivan and Calkins (2010), enzyme treatment was significantly effective on the sensory tenderness of meat. The highest tenderness was observed in papain-treated meat, but the juiciness and textural changes were negatively affected. Also, microbial proteases were more effective on myofibrillar proteins than connective tissue (Sullivan and Calkins, 2010). It was also found in our study that textural properties such as tenderness, chewiness and juiciness were mostly improved by papain treatment (Fig. 7). Likewise, Ashie et al. (2006) found that the tenderness of meat samples increased with increasing papain concentration. However, increasing the dose above 0.01 AU/100 g resulted in an over-tenderization that cause the formation of a mushy texture. On the other hand, aspartic proteinase resulted increase in the tenderness of meat, while doses above 0.01 AU/100 g did not cause a rise in the tenderness. Moreover, Istrati et al. (2012) reported that papain-injected meats were more tender compared to bromelain-treated ones and the juiciness ratio was much higher in marinated meats. They also reported that the combined usage of papain and bromelain was much more effective on the proteolysis of connective tissues. The combined use of plant and microbial proteases may have a synergistic effect on meat tenderization (Arshad et al., 2016). The most effective enzyme on collagen hydrolysis was reported as papain, followed by ficin and bromelain (Calkins and Sullivan, 2007). Bromelain has the potential to tenderize meat by catalysing the degradation of both myofibrillar (actin and myosin) and connective tissue proteins (Ketnawa and Rawdkuen, 2011).
The proteolytic activity of papain is not very selective, affecting both myofibrillar muscle proteins (actin and myosin) and connective tissue proteins (collagen). When papain breaks the peptide bonds in actin and myosin proteins, the structural integrity of the myofibrillar proteins of the meat is lost and tenderization occurs. In addition, the breakdown of the connective tissue proteins collagen and elastin, which give the meat its toughness, also contributes to the tenderization, which is much more pronounced at high concentrations of enzyme treatment, resulting in a further weakening of the tissue structure (Bhat et al., 2018).
Microbial proteases show specific activity against meat substrates. For example, aspartic protease from A. oryzae has no effect on connective tissue, but it affects only myofibrillar proteins. Another important feature of some microbial proteases is that they limit their own activity to eliminate the risk of excessive tenderness (Ashie et al., 2006). In this context, an ideal enzyme used for meat tenderization should be proteolytic, specific for collagen and elastin in connective tissue, effective at relatively low pH of meat, and able to function at low temperatures during storage or high temperatures during cooking (Marques et al., 2010).
Conclusion
In present study, which comprehensively evaluated the quality characteristics of ribeye and striploins meat during accelerated aging period with enzyme treatments, revealed that enzyme treatment enhanced the quality parameters depending on the concentration applied. Papain treatment was found to be the most effective in improving the tenderness of meat. However, papain enzyme lagged the fungal protease and bromelain enzyme treatments in terms of flavour attributes. Treatment with 0.01 and 0.02 g/kg enzyme improved quality parameters such as tenderness, palatability and flavour, while 0.05 g/kg enzyme concentration caused over tenderization with mushy texture, leading to negative effects on some quality parameters that influence consumer preferences. In particular, low concentrations of bromelain-treated LT and LL samples had the highest sensorial scores. Considering muscle type, the improvement in textural and sensorial properties of LT samples was more than LL samples.
The results of this study showed that the quality parameters of beef treated with plant and microbial enzymes can be improved and supplied to the market and consumers in a shorter time with an accelerated aging process. Among the studied enzyme concentrations, the most effective results was obtained with the treatment of 0.01 and 0.02 g/kg enzyme. The recommended enzyme concentration to obtain a final product with acceptable quality characteristics for industrial application is 0.01 and 0.02 g of enzyme per kg of beef meat.
The limitation of this study is the presence of limited information on the mechanisms of action of proteolytic enzymes, which are widely used in meat tenderization, on the protein structures of meat. A number of reactions caused by enzymes in the tenderisation process, particularly on the myofibrillar and connective tissue structures of meat, may be inconsistent in some studies. This limited understanding and inconsistent results necessitate further studies to clarify the issue. Ongoing research is planned for the application of these enzymes together or with other enzyme combinations in different cuts of meat, as well as in meat from animals of different ages, breeds and sexes.













