Introduction
The utilization of agro-industrial byproducts is gaining traction, spurred by their potential to minimize waste, reduce environmental damage, stimulate economic growth, and improve human health, primarily when incorporated as key ingredient in global food production (Morone et al., 2019). The food industry, a significant contributor to the global economy, faces pressing waste management challenges. Indeed, environmental regulations have become increasingly strict since the 1970s–1980s, resulting in higher costs for disposing of organic residues from both technological and valueless processes (Chapoutot et al., 2018). Investigations into novel applications for these “by-products,” such as their incorporation into animal feed, have demonstrated improvements in the overall productivity of production systems, particularly in terms of protein and energy outputs (Laisse et al., 2019).
Wet brewers’ spent grain (WBSG), a byproduct sourced from barley (Hordeum Brew vulgare L.), retains outer pericarp-seed coat layers following the mashing process, which involves hot water extraction at temperatures between 65°C–70°C (Jaeger et al., 2024). In Algeria, annual WBSG production, primarily driven by northeastern and western regions including Béjaïa, Tizi Ouzou, Algiers, and Oran, approximates three million tons, predominantly channeled into beer production. Employing these residues in livestock nutrition, particularly for ruminants, could significantly diminish the environmental footprint of this industry, given the high fiber and protein content of WBSG. Reintegrating these agricultural by-products into the food chain not only adds value to what might otherwise be considered waste but also enhances food quality. The bioactive substances present in these natural residues play a crucial role in strengthening and improving food quality. Comprehensive research into the nutritional characteristics of brewers’ spent grain (BSG) underscores its viability as a valuable dietary additive. BSG is recognized for its high protein and fiber content, as well as its appreciable levels of lipids, minerals, polyphenols, and vitamins (Mussatto et al., 2006). BSG is considered a lignocellulosic material commonly used as animal feed, particularly for cattle (Steiner et al., 2015). The potential sources of dietary fiber in BSG are polysaccharides, such as cellulose, lignin, hemicellulose in the form of arabinoxylans, which can be fermented into short-chain fatty acids (FAs) and function as a prebiotic. Additionally, (1-3, 1-4)-β-D-glucan may help lower cholesterol levels (Steiner et al., 2015). Incorporating WBSG into the diet has the potential to enhance overall health and well-being. WBSG is rich in several vitamins, including folic acid, biotin, niacin, choline, riboflavin, thiamine, pantothenic acid, peroxidase, and vitamin E (Ikram et al., 2017). Devnani et al. (2023) suggest that the nutritional composition and unique functional properties of WBSG can be leveraged to improve the texture and sensory qualities of various food products. Additionally, the most common lipids identified in WBSG are triglycerides (55%–67% of all identified lipid compounds), followed by linoleic (18:2(z,z)n-6), palmitic (16:0), and oleic (18:1n-9) acids as the primary FAs (Fărcaş et al., 2015). These compounds may offer multiple health benefits, as they help reduce the risk of developing diabetes, obesity, coronary heart disease, cancer, and gastrointestinal disorders (Fărcaş et al., 2014).
Dietary management of cattle significantly influences the quality and properties of milk and associated dairy products like cheese (Chapoutot et al., 2018; Laisse et al., 2019). These feeding strategies are meticulously formulated to fulfil the nutritional needs of cattle while considering environmental limitations and land availability. Incorporating brewers’ grain into the diet of lactating dairy cows has been found to enhance milk quality, particularly in relation to C18:2 conjugated linoleic acid (CLA) cis-9, trans-11 (c9t11), and n-3 FAs. Kilcawley (2017) observed a clear correlation between the amount of fresh grass consumed and the levels of CLA in cheese, which are also influenced by the type and quality of milk used.
Cheese production has emerged as a significant value-added outcome for the expanding volume of milk in Algeria, particularly after the discontinuation of milk quotas by the European Union in 2015 (Hafla et al., 2013). A variety of factors impact the quality, composition, and public acceptance of cheese, including the methods of cheese production, the initial composition of the milk, and the quality of the raw milk used (Hafla et al., 2013). Feeding BSG for cattle-induced plays a crucial role in enhancing nutraceutical qualities of milk and dairy products, specifically by increasing polyunsaturated fatty acids (PUFAs) levels (Difonzo et al., 2023; Jaeger et al., 2024). Supplementing feed with essential lipids increases the content of vital FAs in milk, which are important for human health. These include rumenic acid (cis-9 trans-11 C18:2), a CLA isomer, and n-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (Wang et al., 2012). Consequently, producers aim to consistently deliver fresh cheese with health-enhancing properties year-round, especially during seasons when pasture is scarce, such as winter or summer. Jben, a soft cheese variety, is crafted using traditional methods involving rennet coagulation of raw, whole cow’s milk. This type of cheese is highly regarded by consumers for its distinctive organoleptic qualities. The sensory characteristics of cheese, which define its eating quality, are attributes perceived by the human senses during consumption, including appearance, flavor, and texture. However, cheese is a complex food product made from milk derived from animals raised under diverse farming systems and processed using various techniques (Xia et al., 2022). Nowadays, dairy cattle farming increasingly seeks alternative protein-rich resources, such as brewer’s grains, due to the high cost of concentrated feed, the low nutritional quality of basic rations typically used by breeders in Algeria, and the poor quality of pasture soils. This study investigates the potential effects of incorporating wet brewer’s spent grain on the FA composition and sensory characteristics of Jben, a traditional fresh cheese made from cow’s milk
Materials and Methods
WBSG was obtained from a commercial brewery located in the province of Oran, in western Algeria. The pH of the spent grain was measured at 5.7. Multiple 45 kg bags were carefully loaded onto a truck, ensuring safe transportation. The bags were then delivered to the farm, where they were stored at ambient temperature and wrapped in plastic to preserve their quality.
In this research, 100 female Prim-Holstein cattle, aged between 3 and 5 yr and averaging a live weight of 450±50 kg, were acquired for the period from February 2022 to early March 2023 by Djilalli Keroum, the Livestock Breeding Farm Manager for Fattening and Improving Quality (LBFFIQ-code 28865, delivered in 02/2024). Originating from intensive Holstein breed farming and entering the 2nd lactation phase, these cows were selected based on the availability of the Holstein breed, with ten heads per batch, incorporating spent brewery grain into their diets. The herd was split into two groups; each consisted of 50 dairy cows. In the first group, the diet comprised hay (1 kg), silage (41 kg), and concentrate (6 kg). The second group received an identical diet to the first, with the addition of 9 kg of brewer’s grain per cow per day, as per the feeding levels recommended by Thai et al. (2022), Westendorf and Wohlt (2002). An adaptation and transition period of 15 to 21 d was provided for the cattle to develop the bacteria needed to degrade the WBSG, under strict veterinary supervision.
Milk samples were collected during the early morning milking session (5:00 AM), using sterile containers clearly marked with each cow’s identification number to ensure sample traceability. The milking process adhered to strict hygienic protocols, involving comprehensive cleansing of both udders and milking equipment. Raw whole cow’s milk underwent pasteurization at 65°C for 30 min and was coagulated at 45°C using lemon juice. The curd was subsequently placed in perforated molds for drainage. No additional salt, fats, or other ingredients were added during the cheese-making process, which was uniformly maintained across all samples. The final products were wrapped in aluminum foil and kept at a chilled temperature of 4°C.
Cheese yield was quantified as actual yield (YA, kg/100 kg) and dry matter cheese yield (YDM, kg/100 kg). YDM was derived using the equation:
where, MD =moisture content of cheese.
This calculation facilitates accurate measurement of solid cheese recovery, factoring in milk fat content while eliminating influence of moisture variation commonly seen in actual yield figures. Projected cheese yield (YP) was calculated using the well-established Van Slyke and Price (1952) formula:
F=fat percentage in milk; CN=casein percentage in milk determined using CN% total protein rate (TP) from raw milk and CN% TP from starter culture as earlier described; 1.09 is a multiplier to account for non-fat non-protein cheese solids (SNFP); and Md=reference moisture level, which, in this investigation, was average moisture content for a given cheese fat category.
To assess dry matter (DM) and crude ash contents, samples were heated in a drying oven at 103°C for 24 h and subsequently at 550°C for 4 h, in accordance with AOAC (1990) standards. Total lipid extraction was carried out utilizing a method outlined by Folch et al. (1957), while crude fiber content was determined using the technique described by Van Soest et al. (1991). Crude protein (CP) levels were examined through the Kjeldahl method (AOAC, 1990). Total phenolic content in feeds was measured following the protocol established by Miliauskas et al. (2004). Analysis of free sugars in fresh cheese was conducted using gas-liquid chromatography (GLC) and mass spectrometry following the method described by Honda (1989). Antioxidant activity was assessed via the DPPH assay (Blois, 1958).
A 10 g sample of fresh cheese was thoroughly blended with 20 mL of distilled water, and its pH was determined using a pH meter (SevenEasy, Mettler Toledo, Columbia, MD, USA).
To determine Dornic acidity, a drop of 1% phenolphthalein alcoholic solution was used as an indicator for 1 g of cheese. The sample underwent titration in 0.01 mL increments of N/9 sodium hydroxide (NaOH) until a persistent color transition from white to light pink was obtained. Each 0.01 mL increment of NaOH equates to 1° of Dornic acidity (°D), where 1°D represents 0.1 g of lactic acid per L, as detailed by Accolas et al. (1977).
Dry matter content (DMC) was determined by removing moisture from a specified volume of cheese. This process involved warming sample in a water bath at 70°C, followed by additional drying for 5 h in an incubator at 103±2°C (AFNOR, 1980). To directly compute DMC, a recognized equation links cheese’s DM with its density and fat content, initially developed by Vuillaume (1942):
DMD content was measured by: DMD=DM–fat.
Lipids were solubilized in hexane, and FA esters were transformed into their methyl ester forms using 2N potassium hydroxide in methanol. Individual FAs were isolated and quantified using GLC on an Agilent Technologies 7890A system (Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and a split ratio of 20:1 (Morrison and Smith, 1964). The system used a DB-23 column (60 m, 0.25 mm i.d., 0.25 mm film thickness, Agilent Technologies), with injector and detector temperatures set at 250°C and 280°C, respectively. Oven temperature was initially set at 50°C for 1 min. It was then increased at a rate of 25°C per min until reaching 175°C. After that, the temperature was raised at a rate of 4°C per min until it reached 220°C, where it was held steady for 18 min. Helium was used as the carrier gas at a steady pressure of 230 kPa. Chromatographic air and hydrogen were supplied to FID at flow rates of 400 mL/min and 35 mL/min, respectively. Methyl esters were identified by matching their retention times with those of standard fatty acid methyl ester (FAME) references (Supelco 37 component FAME Mix, Supelco, St. Louis, MO, USA). Relative amounts of each FA in samples were reported as percentages of the total FAMEs detected. The analysis included totals of saturated, monounsaturated, polyunsaturated, and highly unsaturated fatty acids (UFAs), as well as the ratio of n-3 to n-6 FAs.
α-Tocopherol was isolated employing the technique outlined by Wang et al. (2010) and measured via high-performance liquid chromatography (HPLC). HPLC setup included a Beckman Coulter model 114-M pump (Beckman Coulter, Barcelona, Spain), a manual injector, a System Gold® interface, a Jasco fluorescence detector FP1520 (Jasco, Madrid, Spain), a Kromasil Silica 150×4.6 (5 μm) column KR100-5-150 (Symta, Turkey), and a Kromasil Silica guard column (10 mm) KR100-10-10C5 (Symta, Turkey). Mobile phase consisted of isooctane and tetrahydrofuran (90:10), with a flow rate of 1 mL/min. Detection occurred at λem 297 nm and λex 321 nm. α-Tocopherol isomer was quantified using a dl-α-tocopherol standard for calibration (Sigma-Aldrich, Madrid, Spain). Results were reported in mg of α-tocopherol per kg of both diet and fresh cheeses.
To perform the sensory descriptive analysis, 15 internal panelists from the Milk Transformation Unit GIPLAIT-West Algeria, Mostaganem (LE LITTORAL; IRB number: 000448B016256540) conducted a hedonic test on the cheese samples. The panelists were selected based on their availability and willingness to participate in the study, in accordance with International Organization for Standardization (ISO, 1993) guidelines. The panelists were required to be nonsmokers and capable of performing sensory tasks. They were given 15 min to record their evaluations of the sensory qualities on a tasting sheet. Each panelist evaluated two cheese samples, each identified by a unique three-digit random code, presented simultaneously in a blinded manner. The evaluation took place in a controlled sensory analysis lab maintained at 18°C±1°C. Each panelist was presented with 5 g of each cheese sample in a randomized sequence and was asked to choose their preferred sample. Before evaluating each sample, the panelists were instructed to cleanse their palate by eating bread and rinsing their mouths with plain water.
Following ISO (1993) guidelines, the panelists were asked to rate the degree of difference perceived between the two fresh cheese samples as “None,” “Slight,” “Moderate,” “Much,” or “Extreme” after determining which sample they preferred. These qualitative ratings were then converted into quantitative scores: None-1, Slight-2, Moderate-3, Much-4, and Extreme-5. The acceptability scores for color, texture, taste, odor strength, and overall impression were calculated using these values. The results were subsequently summarized through descriptive statistical analysis.
The assays were conducted in three replicates (n=3) and the results were expressed as mean±SD. The assays were compared using one-way analysis of variance, followed by post-hoc Tukey test for mean comparison. The analyses were conducted using Minitab® 12.1 for Windows. FAs characteristics were analyzed using the Kruskal–Wallis test (multiple pairwise comparison) in R software (version 4.2.2). The same software was also used to generate the graph of sensory attributes. Statistical differences with a p-value lower than 0.05 were considered significant.
Results
Nutritional characteristics of bovine diets are outlined in Table 1. WBSG feed is noted for its elevated levels of crude moisture, CP, total fat (TF), and crude fiber compared to other feed components (p<0.001). In contrast, the concentrate recorded the lowest crude ash content, with 2.42% and the highest levels of oleic acid C18:1 n-9c and monounsaturated fatty acid (MUFA) content. At the same time, the linolenic acid (LA; C18:2c n-6) level was observed to be considerably higher in silage (p<0.001) compared to WBSG and concentrate feed types. However, pasture is noted for its higher polyphenol content (1,525.23 mg Eq gallic acid/g DM) and greater concentrations of PUFA (76.04%), particularly for ALA (C18:3n-3) amount, as shown in Table 1. The data presented in Table 1 reveal that WBSG exhibited the highest levels of vitamin E, surpassing those found in pasture (PM), silage (S) and concentrate (C; p<0.001). Furthermore, the findings demonstrate more highly significant enhancement (p<0.001) in the antioxidant capabilities of the Jben cheese studied, attributable to WBSG inclusion in diet.
As shown in Table 2, the results reported on the cheese manufacturing yield analysis indicate significant differences (p<0.05) and predominance for the cheese WBSG group. Replacing part of the concentrate feed with wet brewer’s spent grain significantly altered the composition of fresh cheese, though it had a highly significant effect on the pH of fresh cheese (Table 2). Results showed significantly higher acidity values, moisture, and defatted dry extract contents in the Jben cheese group from cows that grazed pasture and received concentrated feed without WBSG in their diet compared to cheeses from the other group. However, the analysis of both group’s fresh cheeses protein shows a predominance for group 2 rearing system (58.44% vs. 50.20%, p<0.001). Findings demonstrate a highly significant influence of dietary patterns on TF levels, with a notable increase in the cheese from the WBSG group. Fresh cheese derived from cows fed a diet including WBSG was enriched in vitamin E relative to those from other feeding systems (p<0.01).
Fig. 1 details FA profiles of fresh cheeses. The addition of WBSG markedly enhanced (p<0.001) the FA concentrations in Jben, especially in terms of UFAs, MUFAs, and the UFA/saturated fatty acid (SFA) ratio. Conversely, SFAs predominated in cheese from cows fed a diet of pasture silage and concentrate. Gamma-LA (C18:3 n-6) was highly significant (p<0.001), followed by linoleic acid (C18:2 n-6), which was also significant (p<0.01) in the fresh cheese compared to the cheese from the WBSG group. However, no significant effect was observed for LA (C18:3 n-3; Fig. 1A). Furthermore, the ratios of total LA/alpha-linolenic acid (ALA; p<0.05) and n-6/n-3 (p<0.001) were substantially decreased in cheese from the WBSG group (Fig. 1B).
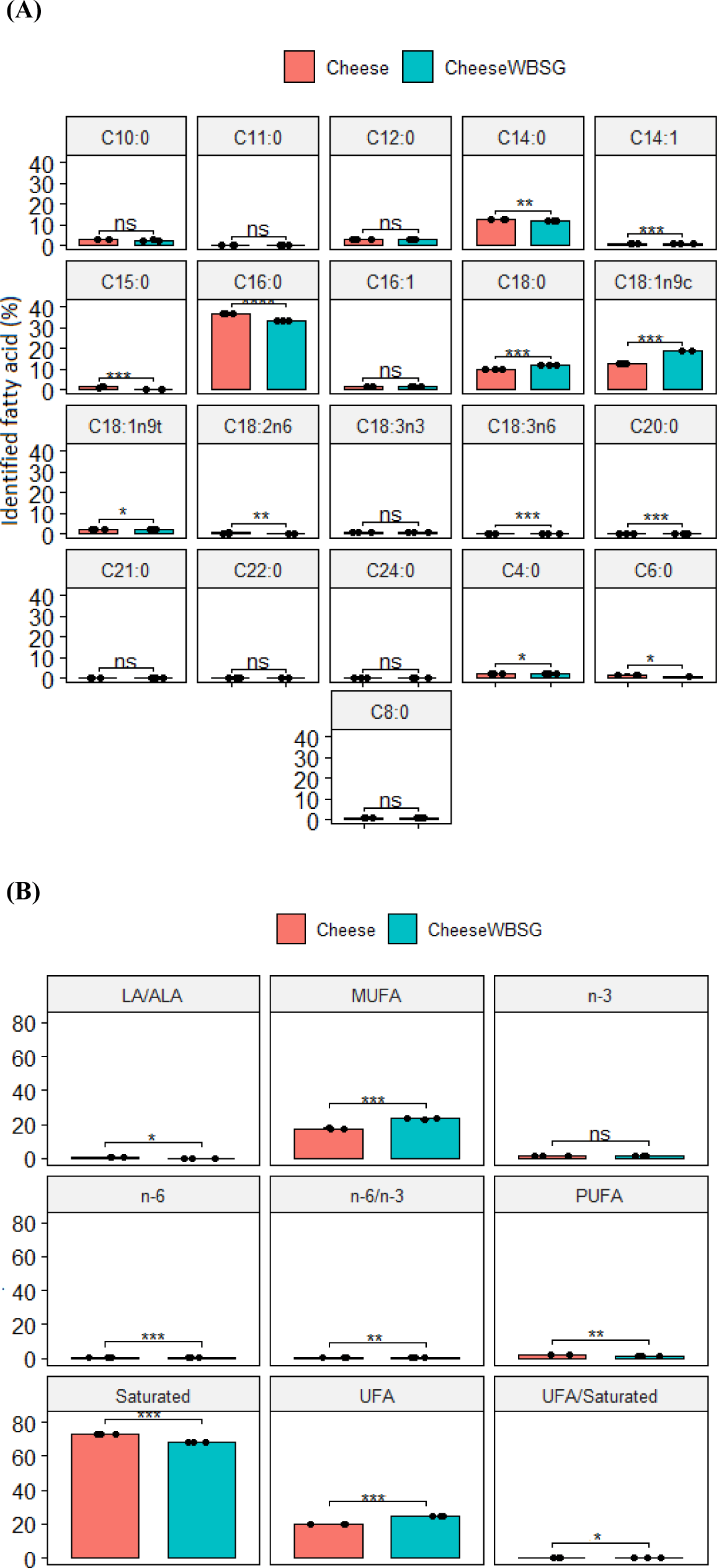
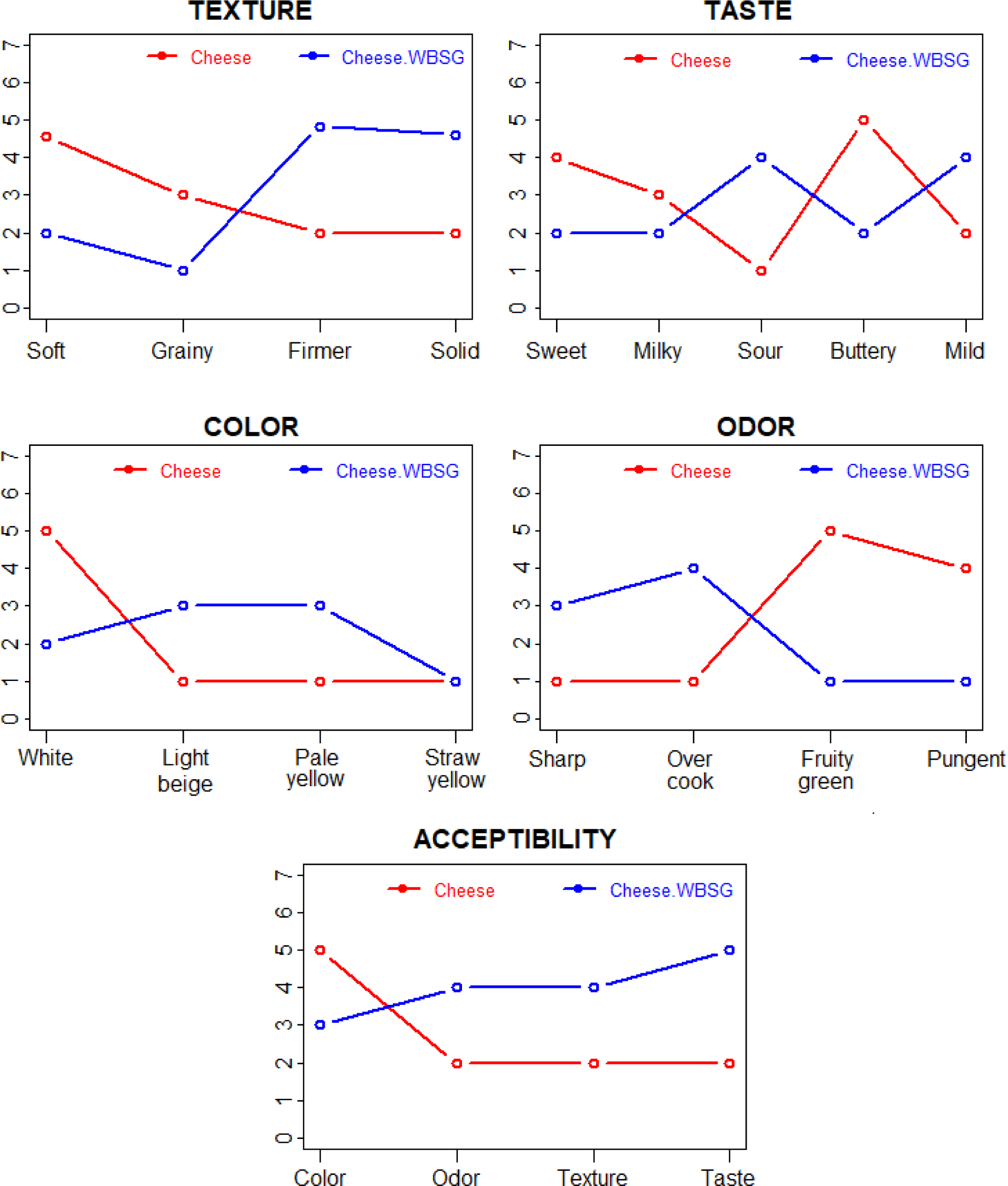
Fig. 2 shows the mean scores attributed to each of the parameters evaluated: colour, texture, odor, taste and overall impression. All the cheeses received qualitative ratings above 3.0 for all the attributes evaluated, showing that they were well accepted by the panellists. Significant differences in sensory characteristics were more pronounced in cheese from the WBSG group with higher scores of soft textures, fruity, green vegetable odor and a more buttery taste. However, the cheese group had a white colour, firmer texture and lower score of acceptance than the cheese of the other group.
Discussion
The results for the diets are summarized in Table 1. Significantly, WBSG registered the highest moisture content at 79.07%, aligning with the findings of Ikram et al. (2017), who reported moisture levels of 77%–81% in brewers’ grains post-brewing. This elevated moisture content renders grains prone to rapid fermentation and deterioration due to microbial proliferation. Various techniques have been investigated and refined to preserve vast amounts of WBSG for extended periods of time for use as animal feed. The most popular method of preserving WBSG used is quick drying applied in a tunnel set at a temperature of 60°C to 80°C for 2 to 5 min for a conservation of brewers up to 6 mon. It does not alter its composition as the freezing technique reduces the volume of the product, thereby lowering transport and storage costs (Faccenda et al., 2021). Using superheated steam is an alternate drying technique that is crucial for reducing energy costs. It offers several benefits, including a reduced environmental impact, increased drying efficiency, no risk of fire or explosion, and improved recovery of valuable organic compounds (Terefe, 2022). It has been suggested that BSG be utilized to increase the protein content in solid-state fermentation methods (Canedo et al., 2016). Furthermore, Al-Hadithi et al. (1985) revealed that chemical preservatives including potassium sorbate, lactic, formic, acetic, and benzoic acid can be used to preserve the quality and nutritional value of BSG. The characteristics of WBSG are affected by the age of the brewery, processing techniques used, and any additives introduced during the brewing process (Chapoutot et al., 2018; Laisse et al., 2019). Protein and ash contents reported for WBSG in Table 1 are slightly below the figures presented by Öztürk and Akin (2018), which were 36.9% and 6.36%, respectively. Variations in these metrics may stem from differences in the barley variety, malting procedures, adjuncts, and processing methods employed. As indicated in Table 1, WBSG provides a substantial source of crude fiber compared to pasture and concentrate, corroborating findings by Radzik-Rant et al. (2019). Its high fiber content, consisting mainly of structural carbohydrates such as cellulose and hemicellulose left after starches and sugars are extracted during malting, makes brewers’ grain an effective dietary supplement for ruminants (Difonzo et al., 2023).
Polyphenol analysis revealed the predominance of these compounds in the pasture. As common components of plant secondary metabolism, phenolic compounds are utilized to evaluate the quality of animal feed during grazing (Bertelli et al., 2021). Notably, BSG contains higher levels of polyphenols than both silage and concentrate (Table 1). Given that corn silage is a staple in cattle diets globally, WBSG could serve as an alternative polyphenol source in animal nutrition. Its rich fiber and protein content not only supports this potential use but also suggests additional benefits for ruminants (Mussatto et al., 2006).
Birsan et al. (2019) highlighted the benefits of substituting higher-cost dietary components with agro-industrial byproducts like WBSG, which, despite its low market value, is a rich source of polyphenols exhibiting higher antioxidant activities than corn silage. The lipid content is notably higher (p<0.001) in WBSG compared to pasture and concentrate feed. Zhang et al. (2023) have identified that WBSG contains a fat percentage between 7%–10%, with essential FAs constituting more than half of these lipids. According to Khanal et al. (2005), the reduced total FA levels in corn silages result from the oxidation actions of lipoxygenases (LOX) during early ensiling stages. Conversely, concentrates demonstrated the lowest fat content relative to pasture (p<0.001). Whetsell and Rayburn (2022) state that total FA concentrations in grasses and legumes peak in spring, decline over summer, and rebound in autumn. They also note that CP content closely correlates with total FA levels and effectively indicates forage maturity.
Silage is characterized by elevated concentrations of ALA (p<0.001), which influences the biohydrogenation processes in rumen and may impede synthesis of new FAs in the mammary gland. These observations align with prior studies on sheep and cows (Salfer et al., 2018). Pasture, however, significantly enhances (p<0.001) ALA accumulation and reduces n-6/n-3 ratio in lean tissue compared to other feed types, potentially offering health benefits due to elevated desirable FA levels. Khanal et al. (2005) observed that FA profiles in grasses vary significantly and are influenced by the predominant FA. Salfer et al. (2018) noted that long-chain omega-3 PUFA, like EPA, are especially beneficial for long-term health maintenance, with EPA levels being substantially higher in pasture-fed animals, likely due to increased rainfall and greener pastures.
The correlation between CP and FA content in forages, as discussed by Khanal et al. (2005), is attributed to their co-location in the photosynthetic tissues of leaves. Cheese yield measurements, which are important for determining milk pricing, assessing processing efficiency, and evaluating new cheese-making ingredients, showed more highly significant variations as indicated in Table 2. Significantly, cows fed on WBSG produced more cheese (p<0.001), likely due to enhanced coagulation capabilities stemming from elevated casein levels rather than the overall protein content, as indicated by Olsen et al. (2023). The production of Jben cheese is affected by a multitude of factors such as cow’s energy requirements, capacity for DM consumption, stages of lactation physiology, attributes of milk composition and quality, procedures for milk handling, practices of cold storage, standardization and pasteurization of milk, as well as the variables related to cheese-making, including the equipment and technology used (Olsen et al., 2023).
As shown in Table 2, the results revealed that there was more highly significant (p<0.001) increase in fresh cheeses yield (%) when WBG was included in the lactating dairy cows ration at 9 kg per animal compared to the other group. These results corroborated those of Imaizumi et al. (2015), who observed higher milk yields in lactating Holstein dairy cows fed a diet that included WBSG. According to Garg et al. (2016) and Stasinakis et al. (2022), the two main factors limiting milk production are energy and protein. Increasing rumen microbial protein synthesis leads to more optimal rumen function, and supplementing these nutrients into the diets of lactating ruminants increases milk yield. WBSG inclusion in cattle feed modified the nutritional characteristics of fresh cheese, as demonstrated in Table 2. Data analysis showed that the differences between the two fresh cheeses were statistically highly significant, with the highest value for the cheese from the WSBG group while the lowest pH value for the other group (p<0.01). This is likely due to the prebiotic qualities of WBSG, which are attributed to its rich fiber and protein content (Lao et al., 2020). Such a shift in pH is linked to changes in lactic acid concentrations during storage. Abd EL-Moneim et al. (2018) who observed that adding spent grain to the standard diet of cows considerably affects the pH of fresh cheese. The acidic condition in cheese results from action of lactic acid bacteria in milk, which transform lactose into lactic acid, thereby lowering the pH— an essential element in cheese manufacturing (Lao et al., 2020).
Information on the titratable acidity of various fresh cheeses is recorded in Table 2, indicating more highly significant differences (p<0.001), especially in cheeses derived from cows that grazed on ryegrass silage and concentrate.
Naibaho et al. (2021) observed that high-protein diets have a significant impact on the acidity of fresh cheese. Moreover, the ability of cheese to produce lactic acid is affected by several factors including moisture, protein, and mineral content (Olsen et al., 2023). Due to its specific composition, which includes polyphenols, cellulose and dietary fiber, WBSG influences pH values and lactic acid production during cheese storage. This results in cheese with elevated pH and reduced lactic acid concentration. Oancea et al. (2023) also highlight WBSG’s potential to influence lactic acid variability in cheese production.
Jben fresh cheese derived from a WBSG-based diet exhibits low moisture content (p<0.001). The surface moisture, likely influenced by the melting points, could be associated with higher PUFA content found in pasture-rich cheese. This aligns with findings from Wang et al. (2012), which showed similar characteristics in cheese from grazing cows. Simopoulos (2004) found that moisture and protein levels in cheese increase as fat content decreases. Cheeses from cows fed WBSG exhibited significantly higher (p<0.001) total dry extract (TDE) levels and reduced defatted dry extract compared to other cheeses group. Abd EL-Moneim et al. (2015) noted that processing adjustments could enhance the nutritional value of WBSG in dairy by-products. WBSG increases the health benefits of food products by altering their chemical composition and biological activity. Notably, incorporating WBSG into the diet of cows reduced the protein concentration in the resulting cheese while having no effect on its fat and ash content. Although there is no information available on how BSG affects the techno-mechanical qualities of yogurt processing, BSG has a major impact on cheese block rheological behaviour. WBSG leads to a more compact texture by interfering with the formation of networks, making it chewier. As a result, it makes things more chewy, gummier, cohesive, firm, and sticky. According to Soares et al. (2019) changes in TDE directly correlate with variations in TP and total butter rate, both of which are influenced by diet.
Incorporation of WBSG into the diet had a highly significant effect (p<0.001; Table 2) on the CP, lactose (L; p<0.01), and TF (p<0.01) contents in fresh cheese as shown in Table 2. These results may be attributed to the unique composition of the ingredient-based diet (Table 1). WBSG improves digestibility, which affects total DM intake and the nutrient accessibility for rumen microbiota, thereby influencing the livestock (Faccenda et al., 2018). Fresh cheeses produced from cows fed a diet of ryegrass silage and concentrate exhibited a reduced total lipid content (p<0.01) compared to those from group receiving WBSG supplement. TF content in cheese is influenced by the physical and chemical characteristics and the raw material composition, which are significantly affected by the dietary habits of the cows (Aguiar et al., 2017). Table 2 shows highly significant differences (p<0.01) in average concentrations of vitamin E and antioxidant activities in the cheese from the WBSG group. These differences likely stem from variations in the original milk composition and the characteristics of the dietary ingredients, primarily due to the high vitamin E content in brewer’s spent grain compared to pasture, silage, and concentrate (Table 2). Prior research has shown that dietary choices have a more pronounced impact on the levels of vitamins E than species variations. The transfer of α-tocopherol to the fats in ruminant milk and dairy products is directly influenced by their dietary concentrations (Lynch et al., 2016). Antioxidant activity is characterized by the ability to inhibit nutrient oxidation, especially targeting lipids and proteins, via oxidative chain reactions. Öztürk and Akin (2018) showed significant differences between the groups. Fresh cheese from cows fed on WBSG exhibited significantly (p<0.05) higher amount of antioxidant activity compared to the jben from the other group. This activity is facilitated by various antioxidant compounds such as polyphenols and the amount of vitamin E contents found in the wet brewer’s grain (Table 1). According to Naibaho et al. (2021) one of the key properties of polyphenols is their potent antioxidant activity.
Examination of FA profiles was conducted to determine the influence of WBSG on cheese fat quality. The dietary input distinctly affected the dominant FAs in fresh cheese, specifically palmitic acid (C16:0; p<0.001), followed by oleic acid (C18:1 n-9; p<0.001), myristic acid (C14:0; p<0.01), myristoleic acid (C14:1; p<0.001) and pentadecanoic acid (C15:0; p<0.001; Fig. 1A). These FAs are considered more efficient energy sources for humans, owing to their smaller molecular structures and the pathways they utilize for bodily transport (Simopoulos, 2004). As indicated in Fig. 1B, the SFA levels were predominant in the cheese group (p<0.001). Incorporating WBSG resulted in a notable decrease (p<0.05) in PUFA levels and a significant rise (p<0.001) in UFA and MUFA levels. Existing literature lacks data on WBSG’s effects on the FA profile of fresh cheeses. These variations are likely due to the lipolysis process undergone by the cheese during refrigerated storage for 1 wk.
The introduction of WBSG in group 2 significantly reduced (p<0.001) ω-6 FAs content in cheese, which consequently led to a notable decrease in the ω-6/ω-3 ratio (p<0.05). Such a low ω-6/ω-3 ratio in dietary lipids is beneficial for health, potentially aiding in prevention of autoimmune, inflammatory, and cardiovascular diseases (Abd EL-Moneim et al., 2018). ALA, a key component of n-3 FAs, was found in higher concentrations in cheese group, but there was no significant difference between the two groups studied. These variations are likely due to the higher ALA levels in pasture (Table 1). This finding aligns with Corazzin et al. (2019) who reported an enhanced n-3 FA profile in cheese from cows fed on pasture. The diminished levels of n-6, n-3, and LA/ALA ratios in the fresh cheese from cows fed wet brewer’s spent grain (Fig. 1B) are linked to an elevated fat content in Jben. Although natural pasture contains high amounts of n-3 PUFA, and despite ruminal biohydrogenation, a portion of dietary unsaturated FA escapes digestion in the rumen. Therefore, cows fed with forages rich in n-3 PUFA (Table 1) have higher levels of these FAs in the fresh cheeses (Fig. 1), resulting in a higher n-6/n-3 ratio.
Regarding the UFA/SFA ratio, Jben cheese from cows fed on WBSG exhibited significantly greater amounts (p<0.05) compared to those from cows not fed WBSG. This effect may be attributed to the bioactive compounds in WBSG, such as vitamin E (primarily α-tocopherol) and polyphenols (Table 1), which act as natural antioxidants and are transferred to the milk and dairy products. The FA profile of cheese is strongly affected by milk FAs and beneficial molecules are transferred from milk into the dairy products (Nudda et al., 2021). Hence, the breeding system plays a crucial role in the transfer of functional fat components from cow’s milk to dairy product and increases their nutritive value.
Lynch et al. (2016) demonstrated that low-molecular-weight compounds released during proteolysis contribute to an increase in pH values of cheese. This increase in pH leads to ionized carboxyl groups, which results in higher repulsion between proteins as well as in enhanced solubilization, with the consequent weakening of the protein matrix, and consequently reduces the cheese’s textural properties.
In the sensory analysis for color, the fresh cheese group obtained higher scores than the other one, although no statistically significant differences were observed. For dairy products enriched with BSG, no color analysis findings are provided. However, the inclusion of BSG led to a decrease in the hedonic evaluation score, indicating the impact of the color change (Abd EL-Moneim et al., 2015; Abd EL-Moneim et al., 2018). Regarding the taste attribute, fresh cheeses from the group receiving WBSG showed significantly higher values for taste intensity notably for sweetness, and bitterness (Fig. 2). The bitterness in cheese from the WBSG group is frequently observed due to the release of hydrophobic peptides of medium or small size (Corazzin et al., 2019; Lynch et al., 2016). Concerning the texture, both cheeses had high significant scores, however, those of the fresh cheese were higher. This could be attributed to higher content of SFAs (Fig. 1B), which gives cheese a more solid structure. Odor intensity was significantly more pronounced in the WBSG group and exhibited pungent aromas, along with fruity and green vegetable scents. These were perceived orthonasally and were significantly influenced by the type of feeding diet (Fig. 2). These olfactory attributes could be linked to elevated levels of polyphenols and vitamin E in diet, corroborating findings by Aprea et al. (2016). Variations in the pungent aroma and the sharper taste of sour cheese among the Jben varieties may be attributed to differing concentrations of free FAs, specifically acetic acid and propionic acid (Abd EL-Moneim et al., 2018), or possibly from excessive moisture content. Cheeses group were noted for their sharper and overcooked odors. Factors such as genetic traits of the animals, the dietary regimen, milk quality, and the cheese-making process, including the cooking phase, are known to affect the odor characteristics of dairy products (Difonzo et al., 2023). The influence of brewer’s spent grain on odor intensity is well-documented in various cheese studies.
All fresh cheeses evaluated were well-received, scoring highly in overall acceptability, with no significant differences in average scores across both types of cheese. However, Jben cheese from the WBSG group received especially positive feedback from taste testers, attributed to its enhanced odor, and textural properties.
Conclusion
WBSG by-product has shown potential as a functional ingredient in processed cheese production. This study found that cows grazing on natural pastures with the incorporation of WBSG, particularly in the Mazouna region, produced fresh cheese with altered composition, notably exhibiting a significant reduction in Dornic acidity (31°D). The addition of WBSG led to notable differences in chemical properties, cheese yield, and sensory evaluations. Remarkably, fresh cheese from group 2 (WBSG group), exhibited enhanced health benefits, highlighted by significant differences (p<0.05) in lactose content, total lipids, and CP levels. This improvement is linked to the energy-dense constituents of spent grains, such as dietary fiber, phenols, and Vitamin E, all known for their antioxidant properties. The research demonstrates that diets incorporating WBSG considerably influence FA composition of fresh cheese, specifically affecting the n-6/n-3 and UFA/SFA ratios, with clear distinctions noted in cheese from cows nourished on grass silage and concentrate.
The feeding regimen also influenced the color of fresh cheese, with those fed WBSG appearing darker. This change is attributed to variations in n-3 content and is closely linked to the antioxidant compounds in the diet, such as polyphenols and vitamin E. Sensory evaluations confirmed the viability of using brewers spent grain as a by-product in processed cheese manufacturing due to its chemical composition and essential qualities. Additionally, this product could be considered a novel item with functional properties and health advantages. The findings of this research were positively received by sensory evaluation panels, who were intrigued by all the fresh cheeses produced. Sensory analysis revealed that some sensory evaluation panels favored the intense odor and the soft, grainy texture of the fresh cheese, while others preferred its white color and sour flavor.













