Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease accompanied by cognitive impairment and memory loss that interfere with normal social life. AD is the most common cause of dementia, and the number of patients with AD is expected to grow to 13.8 million by 2060 (Sh Lapobersky and Neporent, 1945). Several studies have investigated the causes of AD. Among them, senile plaques composed of excessive accumulation of amyloid beta (Aβ) are known to be one of the direct causes of AD development by inducing damage to brain cells (Tiwari et al., 2019). Therefore, the majority of anti-AD drugs have focused on reducing the toxicity of Aβ, but have mostly shown unfavorable results in clinical studies (Cummings, 2018). Therefore, currently, only drugs such as donepezil, memantine, and rivastigmine, which can improve AD symptoms, are used to cure patients with AD (Livingston et al., 2020; Yiannopoulou and Papageorgiou, 2020).
In the brain tissues of patients with AD, a massive loss of neurons is easily detected, which could be partially caused by apoptosis (Behl, 2000). Neuronal loss in the cerebral cortex and hippocampus is a major cause of cognitive impairment in patients with AD (Lu et al., 2022). Since the damaged brain tissue in these patients cannot be recovered by currently available anti-AD drugs, attenuating the process of apoptosis could be an effective way to prevent AD progression. Studies have revealed the molecular regulators that control the activation of apoptosis-related genes in the AD brain (Sharma et al., 2021). Among them, mitogen-activated protein kinases (MAPKs) have been suggested as master regulators controlling the abnormal expression of apoptosis-related genes, such as caspases and the bcl-2 family in the brain tissue of AD-like animal models (Kwon and Lee, 2020; Kwon and Lee, 2021; Kwon and Lee, 2023). Therefore, ameliorating MAPKs activation induced by Aβ could be an attractive way to prevent or cure AD progression.
To develop effective drugs for patients with AD, the need to identify new regulators of AD progression is rapidly increasing (Zhang et al., 2021). Recently, the gut microbiota has received much attention as a key regulator of the progression of various diseases, such as diabetes, atopic dermatitis, and neurological disorders (Fang et al., 2021; Homayouni et al., 2020; Socała et al., 2021). Studies using AD-like animal models have shown key regulatory roles of the gut microbiota in improving cognitive impairment, indicating the importance of using functional probiotics to activate the bidirectional interaction between the gut microbiota and the brain (Kwon and Lee, 2022). Therefore, if we find effective and safe microorganisms that can modulate the gut microbiota of patients with AD in a healthy state, we believe that they can be developed as potential microbial-based therapeutic agents that can modulate AD progression.
Bifidobacteria are gram-positive anaerobic bacteria that are believed to provide positive benefits to their hosts (Hidalgo-Cantabrana et al., 2017; Picard et al., 2005). Therefore, the use of various Bifidobacterium strains as probiotics to treat various diseases, including AD, has been actively studied (Jakubczyk et al., 2020; Kwon and Lee, 2022; Shu et al., 2020; Wieёrs et al., 2019). However, to develop Bifidobacterium-based drugs for AD, the use of appropriate AD-like animal models and a clear evaluation of their effects on molecular changes in the brain should be accompanied by assessments of each strain. In the present study, we evaluated the regulatory roles of Bifidobacterium lactis CBT BL3 (BL) in cognitive deficits and molecular changes in the brain of an AD-like animal model.
Materials and Methods
Rabbit anti-phospho-ERK1/2 (9102S), rabbit anti-ERK1/2 (9101S), rabbit anti-phospho-p38 MAPK (9212S), rabbit anti-p38 MAPK (9211S), rabbit anti-phospho-JNK (9251S), and rabbit anti-JNK (9252S) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse anti-caspase-9 (9508P), rabbit anti-caspase-3, and rabbit anti-cleaved poly (ADP-ribose) polymerase (PARP) antibodies were purchased from Cell Signaling Technology.
BL isolated from infant feces (KCTC 11904BP) was provided by Cell Biotech (Gimpo, Korea) and cultured in BL medium (Difco, Becton Dickinson, Bergen County, NJ, USA) for 24 h at 37°C under anaerobic conditions using a gas pack EZ pouch system (BD Biosciences, Bergen County, NJ, USA). Live BL (2×109 CFU/mL) was centrifuged and washed twice with phosphate-buffered saline (PBS) and orally administrated to mice (100 μL/mouse/d) for 6 wk.
Aβ peptide (Aβ25–35) was purchased from Abcam (Cambridge, UK) and dissolved in DMSO solution (10%, w/v) and then aggregated in a 37°C shaking incubator (JEIO TECH, Daejon, Korea) for 1 week. Finally, Aβ solution was diluted with PBS (410 μmol/5 μL) and intracerebroventricularly (ICV) injected into the mouse brains according to a previously described method (Kim et al., 2016).
C57BL/6 male mice (4 wk old) were obtained from RaonBio (Yongin, Korea). After adaptation (1 week), the mice were divided into three groups (n=10): control, Aβ, and Aβ+BL. The control group was ICV injected with PBS (5 μL) and gavaged with PBS (100 μL). Aβ group was ICV-injected with Aβ peptide (410 μmol/5 μL) and gavaged with PBS (100 μL). The Aβ+BL group was ICV-injected with Aβ peptide (410 μmol/5 μL) and gavaged with BL (2×109 CFU/mL, 100 μL/mouse/d) for 6 wk. Animal experiments were conducted in a room maintained at 22±3°C with 55±10% humidity, and a 12-h light/12-h dark cycle. The mice had access to food and water ad libitum. Three days after Aβ or PBS ICV injection, the Y-maze and Morris water maze (MWM) tests were sequentially performed.
To estimate the effects of BL in an AD-like mouse model, the short-term memory deficit of each mouse group was estimated using the Y-maze test according to a previously described method (Kwon and Lee, 2020). Briefly, the mice were introduced to the center of the Y-shaped maze with three white plastic arms and allowed to explore the arms. The number of arm entries for each mouse was counted for 8 min to estimate the percentage of alterations. The rate of alternation (%) was estimated as follows: alternation behavior (%) = (actual alternations) / (possible alternations) × 100. Possible alterations were calculated as the total number of arm entries – 2. The movements of each mouse were recorded using the SMART 3.0 video-tracking system (Harvard Apparatus, Holliston, MA, USA).
The MWM test was performed according to a previously described method (Kwon and Lee, 2023). Briefly, during a training session, mice were allowed to swim three times to escape to a visible platform (30 cm in height and 6 cm in diameter) located in the southwest (SW) zone of the pool. In the MWM test, the mice were gently dropped into three different zones (north, east, and south), and the time taken to reach the submerged platform was in the SW zone. The MWM test was repeated for 4 d. For the probe test (5 d after the MWM test), the mice were allowed to swim for 60 s in a pool without a platform, and the time spent in the SW zone was calculated. All movements were recorded and calculated using the SMART 3.0 video-tracking system (Harvard Apparatus).
After completing the MWM test, the mouse brain tissue (10 mg) was isolated and homogenized in ice-cold cell lysis buffer (5 mL; Kwon et al., 2020) using a homogenizer (Huanyu Instrument, Zhejiang, China). After centrifugation (13,000×g, 4°C, 10 min), the supernatant was used for western blotting according to a previously described method (You et al., 2022). Briefly, each protein sample (30 μg) was separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was incubated in nonfat milk (5%, w/v) at 4°C for 15 h to block nonspecific binding, and then incubated with the primary antibodies (all 1:2,000) at 4°C for 18 h. After washing three times with Tris-buffered saline (TBS)-T buffer [136 mM NaCl, 20 mM Tris, Tween 20 (0.4%, w/v), pH 7.4], the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (1:3,000; Abcam). After washing three times with TBS-T, the membrane was incubated with an enhanced chemiluminescence detection reagent (Bio-Rad, Hercules, CA, USA) at room temperature for 5 min and then exposed on an X-ray film (Agfa, Motsel, Belgium). The bands were visualized using a film developer (TAEAHN Machine, Incheon, Korea).
Statistical differences between groups were analyzed using two-way ANOVA, followed by Tukey’s post-hoc multiple comparison test. Data were expressed as mean±SEM and GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses. p<0.05 was considered statistically significant.
Results
To investigate the effects of BL on AD progression, live BL was orally administered to the mice for 6 wk, and then the Aβ peptide was ICV-injected into the mouse brain (Fig. 1A). We found that changes in mouse body weight did not differ among the groups (p>0.05) during the experiment (Fig. 1B). This suggests that the administration of BL may not have induced toxic effects in the mice in this experiment.
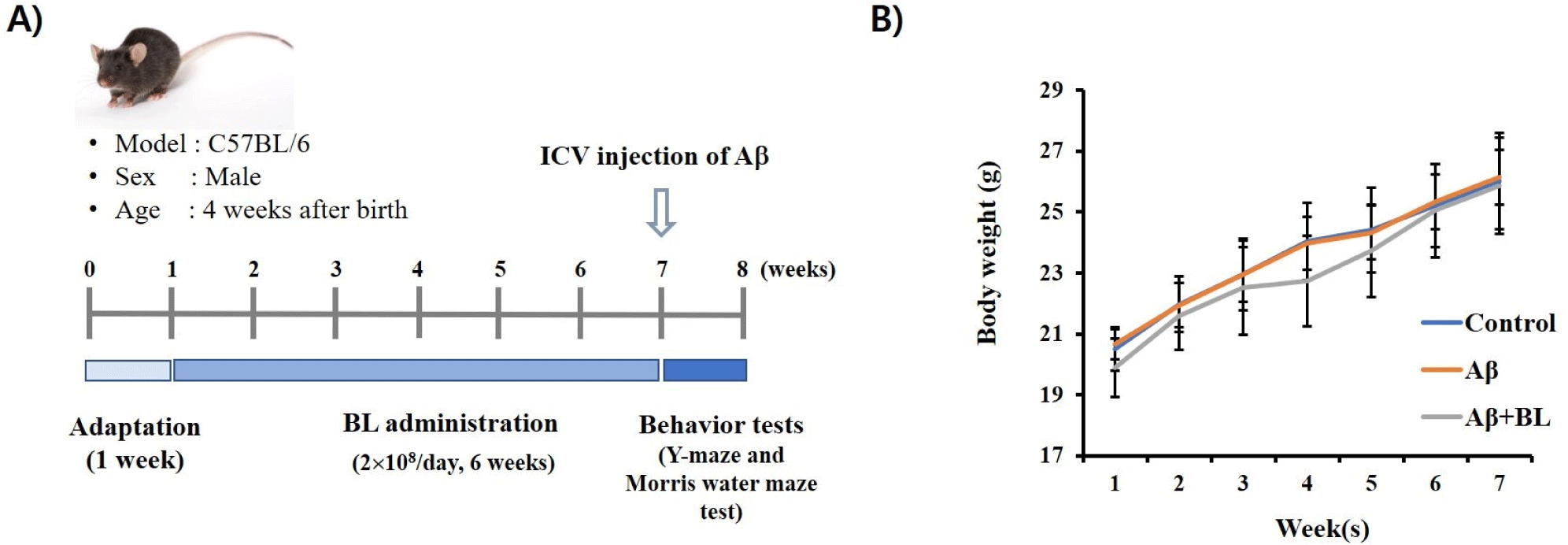
The Y-maze test is a well-known behavioral test used to measure the willingness of mice to explore new environments. Typically, mice prefer to explore new places rather than areas they have visited before. Therefore, when the mouse was placed in the center of the Y-maze, it started to explore the three arms according to its instincts. However, if the mouse brain is damaged enough to impair memory by ICV Aβ injection, the mouse will re-enter the arm of the Y-maze that was previously visited. In our study, although the number of entries was not significantly different among the mouse groups (p>0.05; Fig. 2A), the alteration (%) of the Aβ mouse group (86.64 3.54) in the Y-maze test was significantly (p<0.05) lower than that of the control group (100 3.49). However, it was significantly (p<0.05) rescued by the oral administration of BL to ICV Aβ mice (103.68 3.16; Fig. 2B). These results suggest that the administration of BL effectively ameliorated the Aβ-mediated impairment of short-term memory in mice. Furthermore, imbalanced movement was detected in the Aβ mouse group (142.23 6.10) compared to the control group (100 6.66). However, it significantly (p<0.05) disappeared in the Aβ+BL mouse group (112.43 7.84; Figs. 2C and D). These results suggest that impairments in spatial working memory caused by ICV Aβ injection can be prevented by oral administration of BL.
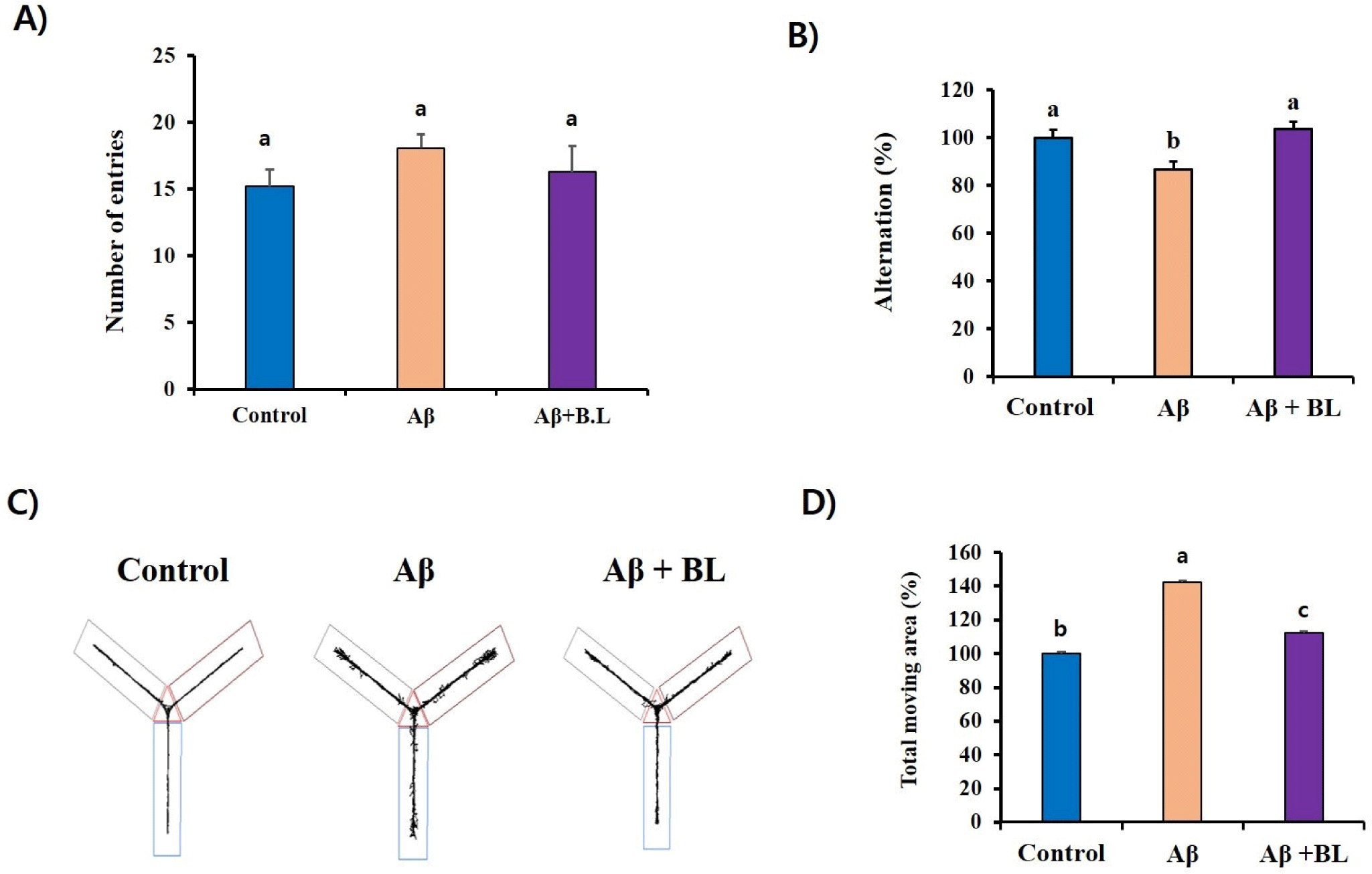
Next, we evaluated the effects of BL on Aβ-induced impairment in long-term memory using the MWM test. The time spent to reach the escape platform (escape latency), which was in the SW zone of the MWM chamber, was calculated for 4 d. As shown in Fig. 3A, the escape latency (s) of the control group gradually decreased, indicating that the control group learned the location of the escape platform. However, the escape latency (s) of the Aβ mouse group was not reduced during the MWM test (4 d) and showed significant (p<0.05) differences compared to that of the control group. Interestingly, the BL mouse group showed similar escape latency (s) as the control group from day-2 of the MWM test, indicating that the administration of BL significantly attenuated the loss in long-term memory induced by ICV Aβ injection. The effects of BL on long-term memory impairment were further evaluated by measuring the time spent in the SW region without an escape platform (probe test). As shown in Figs. 3B and C, the Aβ mouse group spent significantly (p<0.05) less time (7.68 2.65) in the SW region compared to the control group (19.46 2.33), suggesting that the AD-like mouse group had problems with long-term memory. The BL mouse group spent as much time (21.00 4.10) as the control group in the SW region, indicating that oral administration of BL effectively prevented the loss of long-term memory induced by ICV Aβ injection.
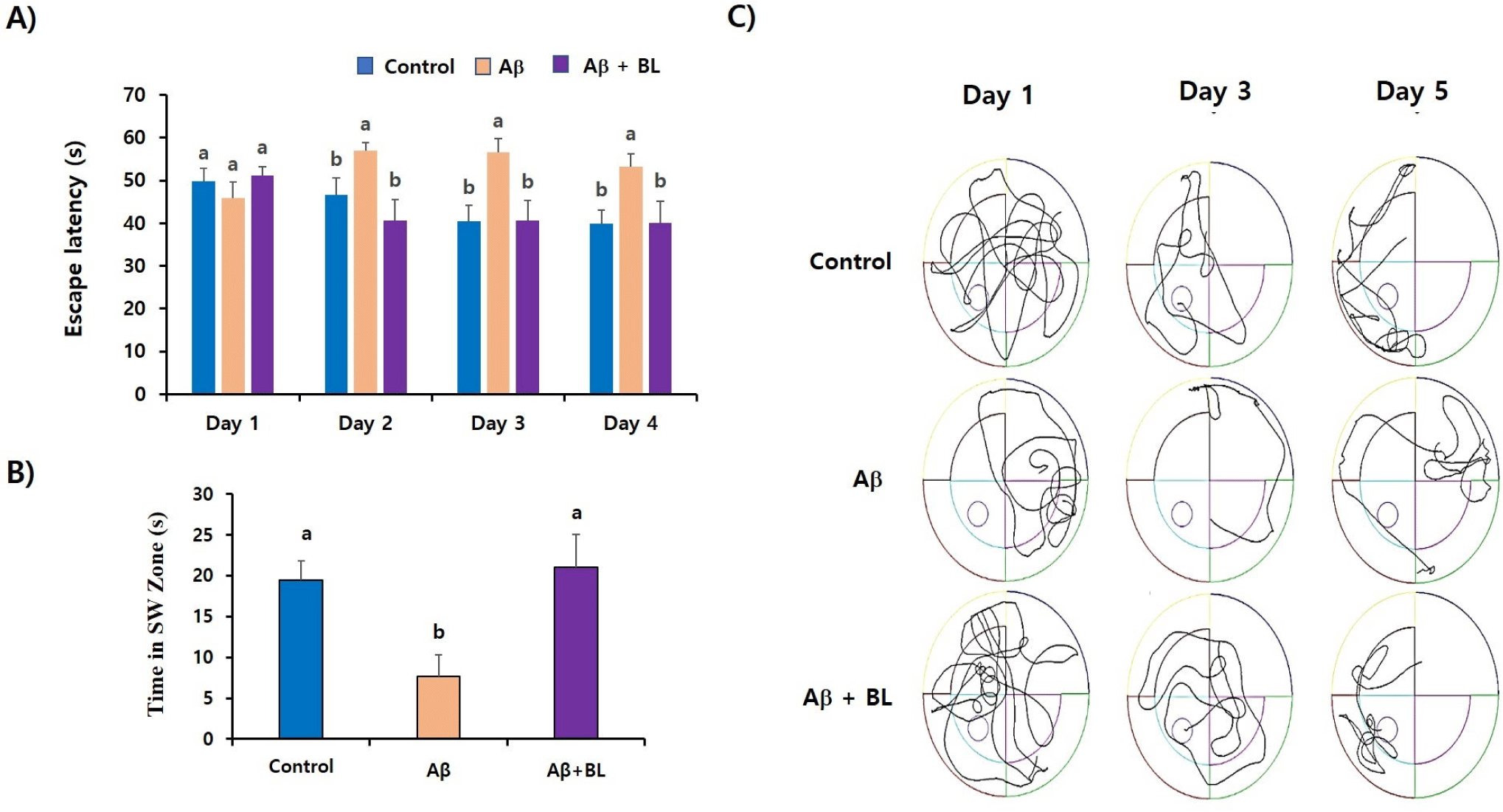
To evaluate the effects of the oral administration of BL on Aβ-induced apoptosis in the mouse brain, we compared the expression of apoptosis-related proteins, such as caspase-9, caspase-3, and cleaved PARP, in the brain tissues of each mouse group using western blotting (Fig. 4A). The relative expression of apoptosis-related proteins in the Aβ mouse group was significantly higher (p<0.05) than that in the control group. However, these increases were significantly (p<0.05) abolished in the brain tissues of BL-fed mice (Fig. 4B). These results strongly suggest that the expression of apoptosis-related proteins that can induce neuronal death can be attenuated by oral administration of BL.
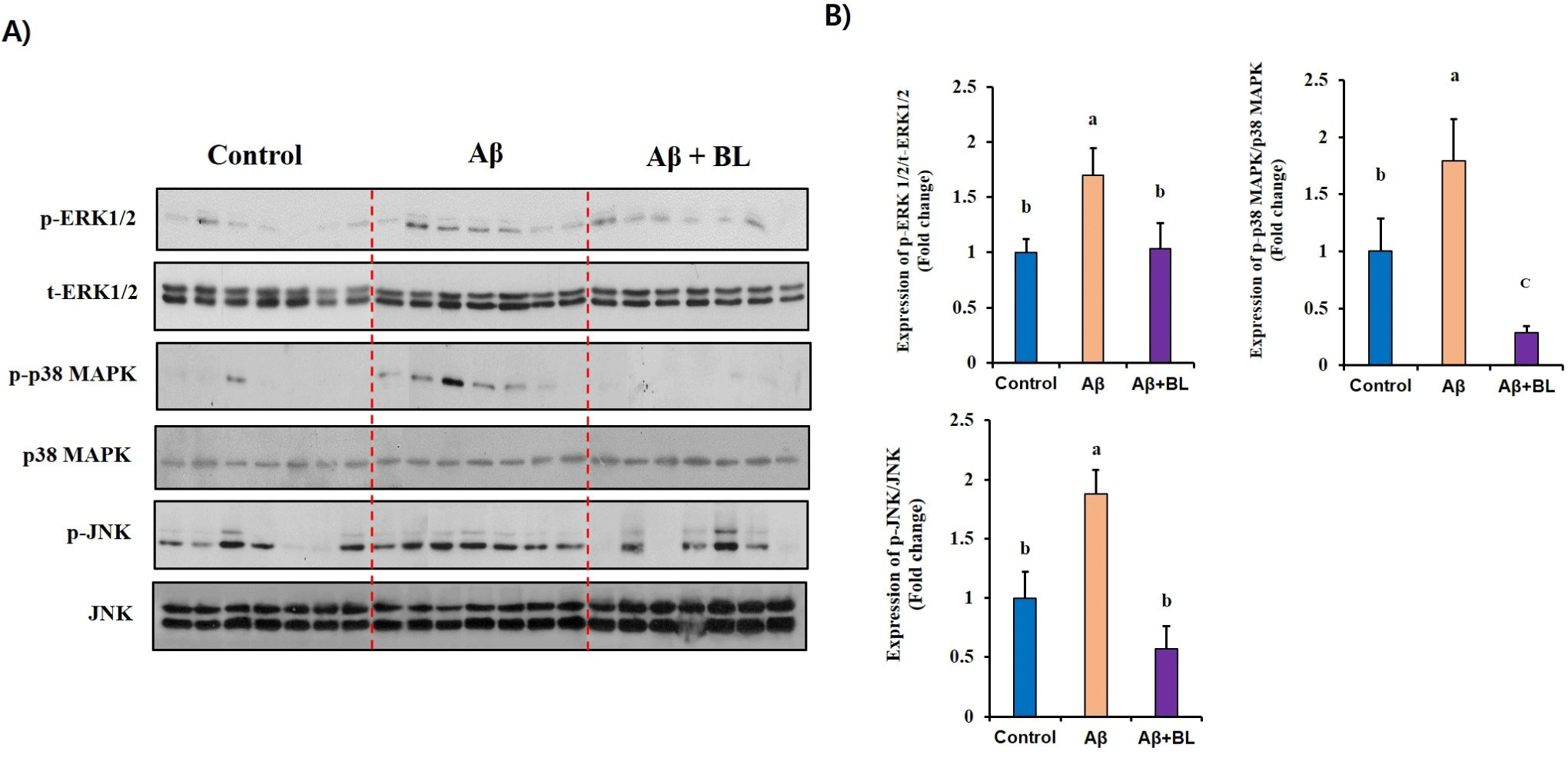
MAPKs have been suggested to be key intracellular regulators of the abnormal expression of AD-related genes. Therefore, to determine whether MAPK activation is involved in the BL-mediated anti-AD effects, we investigated changes in the activation/phosphorylation of MAPKs in the brain tissues of each mouse group. As shown in Fig. 5, the relative expression of MAPKs (p-ERK1/2/ERK1/2, p-p38 MAPK/p38 MAPK, and p-JNK/JNK) was significantly (p<0.05) upregulated in the brain tissue of the Aβ mouse group compared to that in the control group. Interestingly, we found that MAPK activation/ phosphorylation in the brain tissue of the Aβ+BL mouse group was significantly (p<0.05) downregulated compared to that in the Aβ mouse group. These results suggest that the activation/phosphorylation of MAPKs in Aβ-injected mouse brains was ameliorated by the effects of BL in the mouse gut.
Discussion
AD is a progressive neurodegenerative disease in which dementia symptoms such as memory loss and inappropriate language gradually worsen. Neuronal damage to the brain affects memory, thinking, and language (Jack et al., 2009; Reiman et al., 2012; Villemagne et al., 2013). As AD is a progressive disease, suppressing the increase in damaged neurons is thought to be important for attenuating the progression of AD.
Apoptosis in the AD brain is a common physiological process and considered a major cellular cause of cognitive impairment (Behl, 2000; Reiss et al., 2018; Yankner et al., 1989). Caspases are well-known proteinases that play important roles in the final steps of apoptosis. Once the apoptotic signaling pathway is triggered by Aβ, the expression of caspases such as caspases-9 and caspases-3, increase sequentially, resulting in the cleavage of various cellular proteins (Sahoo et al., 2023; Shimohama, 2000). PARP is a polymerase that plays an important role in maintaining cell stability by repairing DNA (Pascal, 2018). In addition, when the cell death pathway is activated, the cleavage of PARP by caspase-3 is often detected in several neurological diseases, such as AD and Parkinson’s disease (Chaitanya et al., 2010; Park et al., 2020). Therefore, the level of cleaved-PARP have is a hallmark of neuronal apoptosis. Therefore, caspase targeting could be a useful strategy for developing efficient anti-AD drugs. Interestingly, we found that administration of BL attenuated the expression of caspase-9 and caspase-3 as well as cleaved PARP in ICV Aβ-injected mouse brains. These results provide direct molecular evidence that increased BL levels in the mouse gut can inhibit the upregulation of apoptosis induced by Aβ accumulation in the mouse brain. Therefore, the data strongly suggest that BL can be developed as an anti-AD agent to prevent Aβ-induced apoptosis in the brain.
MAPKs are central signaling molecules that control a variety of cellular events such as proliferation, differentiation, apoptosis, and neurodegeneration (Kim and Choi, 2010; Sun et al., 2015). Aβ-induced memory impairment as well as neuronal apoptosis were reported to be closely related with abnormal phosphorylation of MAPKs; therefore, several MAPK inhibitors have received much attention as potent anti-AD drugs (Sharma et al., 2021; Tiwari et al., 2019). In the current study, we found abnormal expression of phosphorylated MAPKs in Aβ-administered mouse brains but this was resolved in the brains of BL-administered mice, suggesting that BL in the mouse gut downregulated the Aβ-induced activation/phosphorylation of MAPKs, which may result in the stimulation of the expression of apoptosis-related genes in the mouse brain. This direct evidence of BL-mediated molecular changes in AD-like brain tissue provides high reliability regarding the anti-AD effects of BL.
BL, isolated from human feces, has shown anti-atopic dermatitis and alcohol tolerance activities (Kim et al., 2019; Lim et al., 2021). In addition, it has been reported that the administration of a probiotic mixture containing BL reduced the manifestations of diarrheal disorders in patients with irritable bowel syndrome (Drozdov et al., 2023). However, to the best of our knowledge, this is the first study to show the ameliorating effect of BL on cognitive impairment. To verify this, we used an ICV Aβ-injected mouse model, which is recognized as an acute AD-like animal model (Kwon and Lee, 2022), and focused on whether oral administration of BL could attenuate Aβ-induced short- and long-term memory loss. We found that oral administration of BL to ICV Aβ-injected mice dramatically restored alternation (%) in the Y-maze test and escape latency (s) in the MWM test to the levels of the control group. These results strongly suggest that impairments in brain function induced by Aβ accumulation are attenuated by the action of BL in the mouse gut. Although the actual mediator that connects BL in the gut and brain of ICV Aβ-injected mice could not be elucidated in this study, BL-derived short-chain fatty acids (SCFAs), such as butyrate, propionic acid, and acetate, may be possible candidates for transmitting the beneficial effects of BL in the mouse gut to the brain. SCFAs produced by the gut microbiota are known to play critical roles in many physiological processes such as metabolism, immunological responses, and intestinal integrity (Quagebeur et al., 2023). In addition, studies have shown that SCFAs produced by the gut microbiota move into the bloodstream and finally reach the brain by penetrating the blood-brain barrier (Kekuda et al., 2013; Oldendorf, 1973). SCFAs are endogenous ligands that regulate gene expression by binding to G protein-coupled receptors (Quagebeur et al., 2023) and attenuating the activity of histone deacetylases (Fischer et al., 2007; Schroeder et al., 2007). Therefore, although the effects of BL on the changes in SCFAs in our animal study were not determined, BL-mediated SCFAs may affect gene regulation related to cognitive impairment in the brain tissue of an AD-like mouse model. A detailed analysis of the BL-induced changes in the gut microbiota and SCFAs will be investigated in our next study to verify the actual mediator that controls BL-mediated anti-AD effects.
Conclusion
In the present study, we evaluated the in vivo efficacy of BL against cognitive impairment using an AD-like mouse model. We found that impairments in short- and long-term memory in ICV Aβ-injected mice were inhibited by the oral administration of BL. Furthermore, the expression of apoptosis-related genes and activation of MAPKs, which are able to upregulate the Aβ-induced neuronal apoptosis, could be attenuated by the oral administration of BL. These results suggest that BL could be developed as a potent anti-AD agent to ameliorate Aβ-induced cognitive impairment.













