Introduction
Helicobacter pylori is a gram-negative bacterium that approximately has 50% global human infection rate (Suerbaum and Michetti, 2002). H. pylori easily survive in the gut’s acidic environment. Thus, it can easily cause various gastrointestinal diseases such as gastritis, peptic ulcers, and stomach cancer (Danesh, 1999). The development of gastric cancer is known to be caused by inflammatory responses produced in gastric epithelial cells against chronic H. pylori infection (Wen and Moss, 2009). The primary causes of inflammatory responses in H. pylori infections are the cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA), which are associated with the progression to gastric cancer (Suriani et al., 2008). CagA is delivered into gastric epithelial cells through a type IV secretion system, and once inside the cell, it can activate the NF-kB pathway, leading to the production of pro-inflammatory cytokines (Jones et al., 2010). VacA induces the production of reactive oxygen species in gastric epithelial cells, which can also result in the production of pro-inflammatory cytokines (Jones et al., 2010). Due to the carcinogenic potential of H. pylori, the World Health Organization (WHO) and International Agency for Research on Cancer (IARC) classified H. pylori as a Group I carcinogen in 1994 (Lochhead and El-Omar, 2007).
Currently, commonly used eradication treatment for H. pylori infection is combination therapy with antibiotics such as ampicillin, amoxicillin, and metronidazole, along with acid-reducing medications (Mahony et al., 1992). However, antibiotic treatments have many health risks. Indeed, 22,292 patients infected with H. pylori who received these treatments, 22% experienced side effects such as a metallic taste, diarrhea, nausea, and vomiting (Nyssen et al., 2021). Therefore, the development and evaluation of safer alternatives to antibiotics are necessary to help patients who are sensitive or allergic to prescription antibiotics (Hafeez et al., 2021).
A possible solution to the aforementioned problem is the utilization of bacteriocin, antibacterial peptides, produced by bacteria (Todorov et al., 2019). Nisin, the most well-known bacteriocin, is an antibacterial peptide produced by generally recognized as safe (GRAS) registered Lactococcus lactis strains and it has been approved as a food preservative through Food and Agriculture Organization (FAO) and WHO (Chalón et al., 2012). Thus, nisin is widely used as a natural food bio-preservative due to the antibacterial effect against a wide range of clinical and food-borne pathogens (Amiri et al., 2021). Recent studies have also suggested that nisin has anti-inflammatory effects by inhibiting the production of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 β) in rat uterine (Jia et al., 2019). Also, nisin helped wound healing process by down regulating pro-inflammatory cytokines in human keratinocytes (Mouritzen et al., 2019).
However, fermentative production of nisin is complicated and requires additional purification step which increases overall cost of production. This makes nisin relatively more expensive to produce as compared to other antibacterial substances (Elshaghabee et al., 2016). Recently, ongoing research is exploring new ways to use nisin at a lower cost. Previous studies have sought to find substances that can enhance the antibacterial effect of nisin, which is possible to reduce the amount of nisin to help save costs (Kirazli and Tunca, 2022). For example, combination of nisin with essential oils, such as oregano, thyme, or rosemary, have been found to have a synergistic effect, resulting in a reduction in the required dosage of nisin (Bajpai et al., 2012).
Lactic acid (LA), produced by lactic acid bacteria (LAB), is known to effective against a broad range of bacteria to increase the permeability of bacterial membrane (Alakomi et al., 2000). In previous study, nisin and LA can work synergistically to increase the overall antibacterial activity of Escherichia coli in red meat (De Martinez et al., 2002). Also, the combination of nisin and other substances that can enhance the antibacterial effect of nisin can allow for the use of lower concentrations of both agents, which can help to reduce costs (Field et al., 2017).
Yogurt, a type of fermented milk, is a widely consumed fermented dairy product that is popular around the world. During the fermentation process, LAB strains produce LA, which transforms milk into yogurt by coagulation (Jeong et al., 2018). Trials adding nisin to yogurt have not been attempted due to concerns that it may inactivate LAB in yogurt. However, several evidence showed the positive health effects of inactivated LAB, called parabiotics (Kazemi et al., 2021). Parabiotics have shown to have beneficial health effects such as supporting the immune system, improving gut health by supporting the growth of beneficial bacteria, reducing inflammation, and improving digestion (Kazemi et al., 2021). Therefore, exploring the potential health benefits of incorporating nisin into post-fermented yogurt would be a valuable research endeavor.
Consequently, our study aimed to investigate the antibacterial effects of yogurt, which naturally contains LA, and explore the feasibility of supplementing it with nisin. This investigation will assess the potential for incorporating nisin into post-fermented yogurt. We also assessed the antibacterial effect of nisin and LA, as well as their impact on H. pylori bacterial toxins, bacterial membrane damage and inflammatory cytokines in human gastric (AGS) cells infected with H. pylori.
Materials and Methods
A commercial nisin standard (Sigma-Aldrich Chemical, St. Louis, Mo, USA) was used in the present work. A stock nisin solution was prepared by commercial nisin standard into 10 mL of sterile 0.02 N HCl, centrifuging at 5,000×g for 15 min and sterilizing by filtration through 0.22 μm filters. A stock nisin solution was stored at 4°C until use.
H. pylori ATCC 43504 strains (obtained from American Type Culture Collection, Manassas, VA, USA) was used as the test organism. H. pylori was grown in Brucella agar (MB Cell, Seoul, Korea) supplemented with 5% fetal bovine serum (FBS; Welgene, Gyeongsan, Korea). A single colony from brucella agar was inoculated in 10 mL of brucella broth supplemented with 5% FBS and incubated at 37°C with shaking under microaerobic conditions (5%–8% carbon dioxide) until the cell density reached 1×109 CFU/mL.
Minimum inhibitory concentration (MIC) values of nisin, LA, and ampicillin against H. pylori was determined using broth microdilution method on 96-well plates. Briefly, each well was inoculated with 100 μL of H. pylori adjusted to 1×106 CFU/mL. Serial 2-fold dilutions of nisin, LA, and ampicillin were treated in each well at final concentrations of 0.01 to 40 μg/mL, 0.04% to 5%, and 0.01 to 50 μg/mL, respectively. The final total volume of each well was 200 μL, and the final bacterial number was adjusted to 5×105 CFU/mL. The MIC value was defined as the lowest concentration of nisin and LA alone or in combination with nisin and LA that showed visible growth inhibition after 24 h incubating at 37°C under micro-aerobe condition.
A checkerboard assay was carried out to evaluate the synergistic effects of nisin and LA (Zhao et al., 2023). Briefly, the rows on the x-axis of the 96-well plate contained 2-fold dilution of nisin, and the columns on the y-axis contained 2-fold dilution of LA. The final concentration of H. pylori was adjusted to 5×105 CFU/mL for each well and then the plates were incubated at 37°C under micro-aerobe condition. The synergistic effects of each combination were determined by the fractional inhibitory concentration index (FICI; Shi et al., 2017). The FICI was calculated as following formula: FICI= ΣFIC=FIC (nisin)+FIC (LA), where FIC (nisin)=MIC of nisin in combination/MIC of nisin alone, and FIC (LA)=MIC of LA in combination/MIC of LA alone. The interpretations of ΣFIC values were as follows: Synergy, if ΣFIC≤0.5; no interaction, if ΣFIC>0.5 to <2, and antagonism, if ΣFIC≥2.
Yogurt was produced using 12% (w/v) of non-fat milk (Seoul Dairy, Seoul, Korea). The milk was pasteurized at 85°C for 30 min and then cooled to 24°C. Next, a starter culture (2%, v/v) was added to the milk. The starter culture was prepared by incubating a mixture of 100 mL of non-fat milk (12%, w/v) and 0.34 g of starter culture powder (Samik Dairy and Food, Seoul, Korea) for 5 h. The starter culture contained mixed strains of Lactobacillus acidophilus (35%), Bifidobacterium longum (30%), and Streptococcus thermophilus (35%). The inoculated milk samples were then placed in an incubator at 42°C until they reached a pH of 4.5–4.6. The supernatants from the yogurt were separated by centrifuging samples (10 g) twice at 4,000×g for 10 min at 4°C. Subsequently, the supernatants were further centrifuged at 10,000×g for 10 min at 4°C. They were then filtered through 0.45-μm syringe filter (Advantec, Tokyo, Japan) and stored at –80°C until use.
To determine the amount of LA in the yogurt, the titratable acidity was measured. At the end of the fermentation process, 10 g of yogurt was mixed with 10 mL of distilled water and titrated to a pH of 8.3 using 0.1 N NaOH. The TA was calculated using the following formula:
Here, 0.009 is the conversion factor for LA, which is used to convert the volume of NaOH using into an equivalent amount of LA.
To evaluate the antibacterial effect, the agar well diffusion assay was used for both the yogurt supplemented with nisin and the combination of nisin and LA. A 100 μL aliquot of a 1×108 CFU/mL H. pylori suspension was spread over brucella agar with 5% FBS, and then nisin (0, 1.25, 2.5, or 5 μg/mL) or nisin-supplemented yogurt supernatant (0, 1.25, 2.5, or 5 μg/mL) were added to each well on the agar. To further evaluate the effects of nisin, LA and their combination, and ampicillin (positive control), each was added to individual wells in the agar with different concentrations. The clear inhibition zone diameter (mm) was measured after 24 h of incubation at 37°C under micro-aerobe conditions.
Lactate dehydrogenase (LDH) assay was carried out by investigating the cytotoxicity of H. pylori (Sohn et al., 2020). In brief, AGS cells grown in 96-well plate were treated with different concentration (106, 107, 108, and 109 CFU/mL) of H. pylori and incubated at 37°C for 24 h. 45 min before the end of incubation, cells were treated with lysis buffer for a positive control. After that, the medium was transferred to a 1.7 mL tube. Centrifugation was done to collect the supernatants. Supernatants 50 μL were transferred to a new 96-well plate and 50 μL of the Cytotox 96® reagent were added. The 96-well plate was incubated at 25°C for 30 min in the dark. The optical value was measured at 490 nm with spectrophotometer, and the percentage of LDH was calculated as following formula:
AGS cells were maintained in RPMI 1640 medium (Welgene) containing 10% FBS, 1% penicillin /streptomycin (v/v) at 37°C in humidified atmosphere containing 5% CO2. For the experiment, H. pylori was centrifuged and washed twice with phosphate-buffered saline (PBS). Before cell treatment, H. pylori was resuspended in antibiotic-free RPMI 1640 medium and diluted to the desired concentration.
The cell viability was evaluated by the trypan blue dye exclusion assay (Kim et al., 2021). AGS cells were grown in 6-well plates and treated with different concentration of nisin, LA, their combination, or ampicillin, and incubated at 37°C for 24 h. Viable cell number were counted using a hemocytometer (Hausser Scientific, Horsham, PA, USA) under an optical microscope.
The levels of mRNA expression for bacterial toxins (i.e., VacA and CagA) and pro-inflammatory cytokines (IL-6, IL-8, IL-1β, and TNF-α) in AGS cells were evaluated by RT-PCR. For quantification of mRNA expression of H. pylori toxin factors, 100 μL of H. pylori culture was inoculated in 10 mL of brucella broth containing 5% FBS with different concentration of nisin, LA, its combination and ampicillin for 3 h. Then, the bacterial pellet was obtained through centrifugation at 8,000×g for 5 min at 4°C. TRIzol reagent (Life Technologies, Eugene, OR, USA) 500 μL was used to extract RNA and TOPscript RT DryMIX kit (Enzynomics, Daejeon, Korea) was used for cDNA synthesis. The reference gene 16S rRNA expression levels were used to quantify the relative mRNA expression levels. To quantify mRNA expression of pro-inflammatory cytokines, AGS cells were grown in a 6-well plate and treated with 108 CFU/mL of H. pylori in antibiotic-free RPMI medium for 6 h. Afterward, the bacteria-containing medium was withdrawn and rinsed thrice with PBS. Next, the cells were treated with nisin, LA, its combination or ampicillin for 3 h. After incubation, the medium was withdrawn and rinsed thrice with PBS. TRIZOL 500 μL was used to extract RNA, and TOPscript RT DryMIX kit was used for cDNA synthesis. The reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels were used to quantify the relative mRNA expression levels. The PCR conditions were as follows: 95°C for 15 min (denaturation) and 80 cycles of amplification at 95°C for 20 s, followed by respective annealing temperature and finally held at 4°C. The primer sequences used in current study are shown in Table 1.
DNA and protein leakage was determined by measuring the absorbance of culture supernatants using spectrophotometer (Kirazli and Tunca, 2022). In brief, nisin (5 μg/mL), LA (0.6%), their combination, or ampicillin (6.25 μg/mL) were added to the 108 CFU/mL of H. pylori suspensions in 1×PBS. The suspensions were incubated at 37°C with shaking under microaerobic conditions for 3 h. Then, 0.22 μm microporous membrane was used to remove the bacteria. The leakage of DNA and protein was measured using Epoch microplate spectrophotometry at the absorbances of 260 and 280 nm, respectively.
The morphology changes of H. pylori were observed by field emission scanning electron microscope (FE-SEM; SU-8010, Hitachi, Tokyo, Japan). H. pylori suspensions were treated with the antibacterial substances at 37°C for 3 h under micro-aerobe condition (5%–8% carbon dioxide). After incubation, the cells were collected and washed thrice with PBS and were fixed with 2.5% glutaraldehyde overnight at 4°C. After washing with PBS, the cells were dehydrated with 30%, 50%, 70%, 90% ethanol solutions subsequently. After freeze drying, the cells were fixed on the FE-SEM support and sputtered with platinum under vacuum. Then, the morphologies were observed by FE-SEM.
Data are presented as mean±SEM. All statistical analyses were performed with SPSS-PASW Ver. 18.0 (SPSS, Chicago, IL, USA). Analysis of variance (ANOVA) was analyzed using one-way ANOVA with Dunnett’s test post hoc test. Differences were considered statistically significant when p-values were <0.05.
Results
The MIC of nisin, LA, and ampicillin alone or in combination against H. pylori was shown in Table 2. The MIC of nisin against H. pylori was determined to be 10 μg/mL and the MIC of LA and ampicillin was determined to be 0.6% and 0.195 μg/mL, respectively. The combined effect of nisin and LA was calculated using checkerboard test (Table 2). The synergistic effect of nisin and LA was observed against H. pylori. After combining nisin and LA, MIC of nisin was 1.25 μg/mL (8 times lower than the MIC when used alone) and MIC of LA was 0.15% (4 times lower than the MIC when used alone). As shown in Table 2, the combined FICI value of nisin and LA was determined to 0.375, indicating synergism (FICI<0.5). The results suggest that combination of nisin and LA could effectively inhibit H. pylori growth and lower the concentration of nisin and LA.
| Microorganism | MIC Individual | MIC Combination | FICI | Result | |||
|---|---|---|---|---|---|---|---|
| Nisin (μg/mL) | Lactic acid (%) | Ampicillin (μg/mL) | Nisin (μg/mL) | Lactic acid (%) | |||
| H. pylori ATCC 43502 | 10 | 0.6 | 0.195 | 1.25 | 0.15 | 0.375 | Synergism |
The yogurt contained 0.61±0.01% LA based on titratable acidity. Agar well diffusion method was performed to determine the combined antibacterial effect of nisin (1.25, 2.5, and 5 μg/mL) and yogurt supernatant (Fig. 1A). The combination of yogurt supernatant with 5 μg/mL of nisin exhibited the highest inhibitory activity against H. pylori, with an inhibition zone diameter of 14.33±0.33 mm. This inhibition zone was significantly greater than that of nisin alone as well as than that of yogurt supernatant alone (Fig. 1A). In fact, nisin alone did not exhibit any inhibition against H. pylori at all concentration. To further investigate the antibacterial effects of nisin, LA, nisin-LA combination, an agar well diffusion assay was conducted (Fig. 1B). Different concentrations of nisin (1.25, 2.5, and 5 μg/mL) were combined with 0.6% LA, and their antibacterial activities were compared with those of nisin and LA alone, as well as with ampicillin. The combination of nisin (5 μg/mL) with LA (0.6%) showed the highest antibacterial activity against H. pylori, with an inhibition zone diameter of 22.33±0.33 mm, which was significantly greater than that of the same concentrations of nisin and LA treated alone, and comparable to that of ampicillin (22.67±0.67 mm).
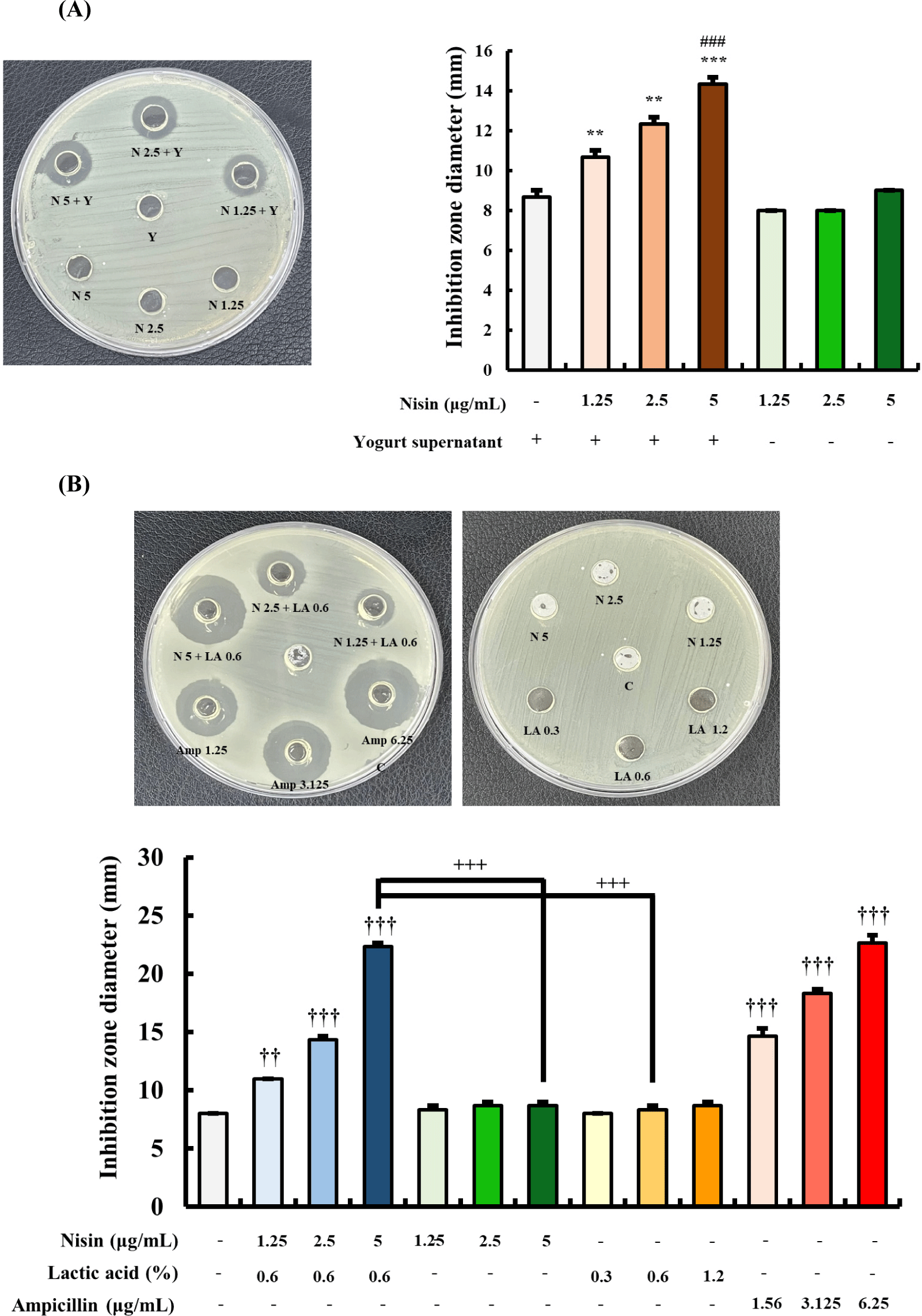
Trypan blue dye exclusion assay was used to evaluate the effect of nisin, LA, their combination, or ampicillin of AGS cell viability (Fig. 2A). Treatment of cells with nisin (1.25, 2.5, 5 μg/mL) and ampicillin (3.125, 6.25 μg/mL) for 24 h did not show any significant changes in cell viability compared to the control (p>0.05). Similarly, treatment with LA (0.3%, 0.6%) and combination of nisin and LA group did not significantly affect cell viability (p>0.05). However, treatment with 1.2% of LA for 24 h resulted in a significant decrease in cell viability (p<0.001) compared to the control. Based on these results, further in vitro studies were performed using 5 μg/mL nisin, 0.6% LA, and 6.25 μg/mL ampicillin. AGS cells treated with a concentration of 109 CFU/mL of H. pylori for 24 h showed significantly higher LDH release than control (Fig. 2B). Therefore, a concentration of 108 CFU/mL of H. pylori was used for further in vitro studies.
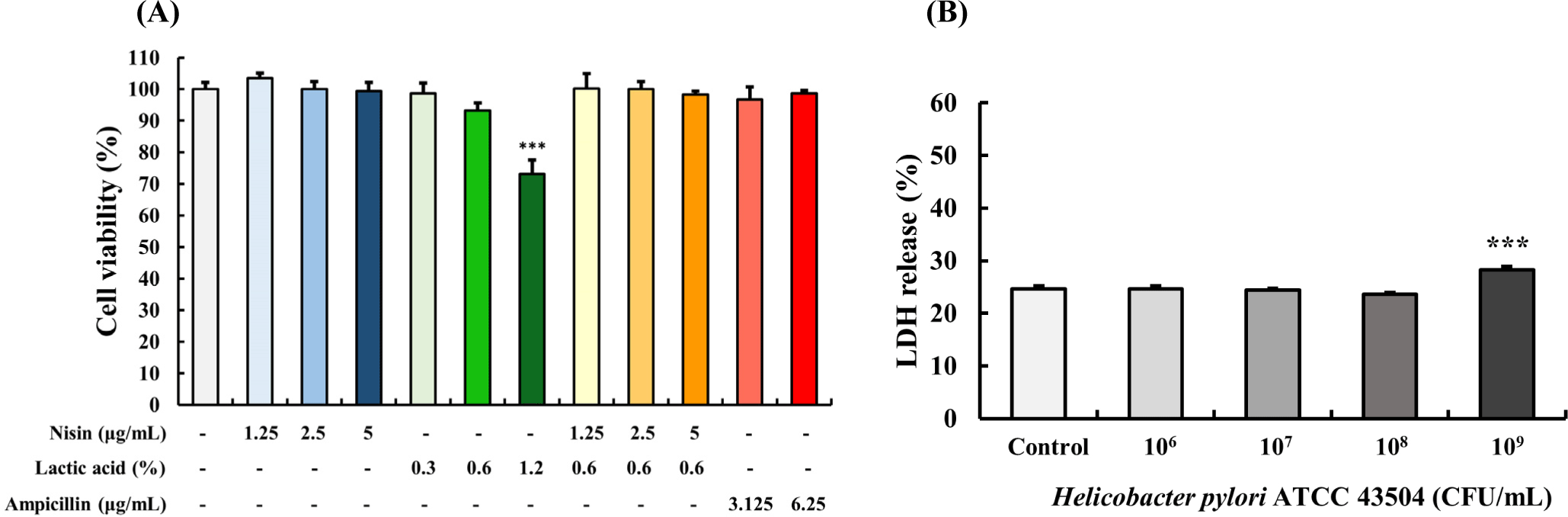
The results of the mRNA levels of bacterial toxins (i.e., CagA and VacA) in H. pylori are presented in Fig. 3. Treatment of H. pylori with 5 μg/mL of nisin resulted in a decrease in mRNA expression levels of CagA (Fig. 3A) and VacA (Fig. 3B), while LA or ampicillin alone did not affect mRNA levels (p>0.05). However, when nisin and LA were used in combination, the mRNA level of CagA decreased significantly, compared to the control (p<0.001). Similarly, the mRNA level of VacA decreased significantly compared to the control (p<0.05). These results suggest that the combination of nisin and LA effectively reduce bacterial toxins in H. pylori.
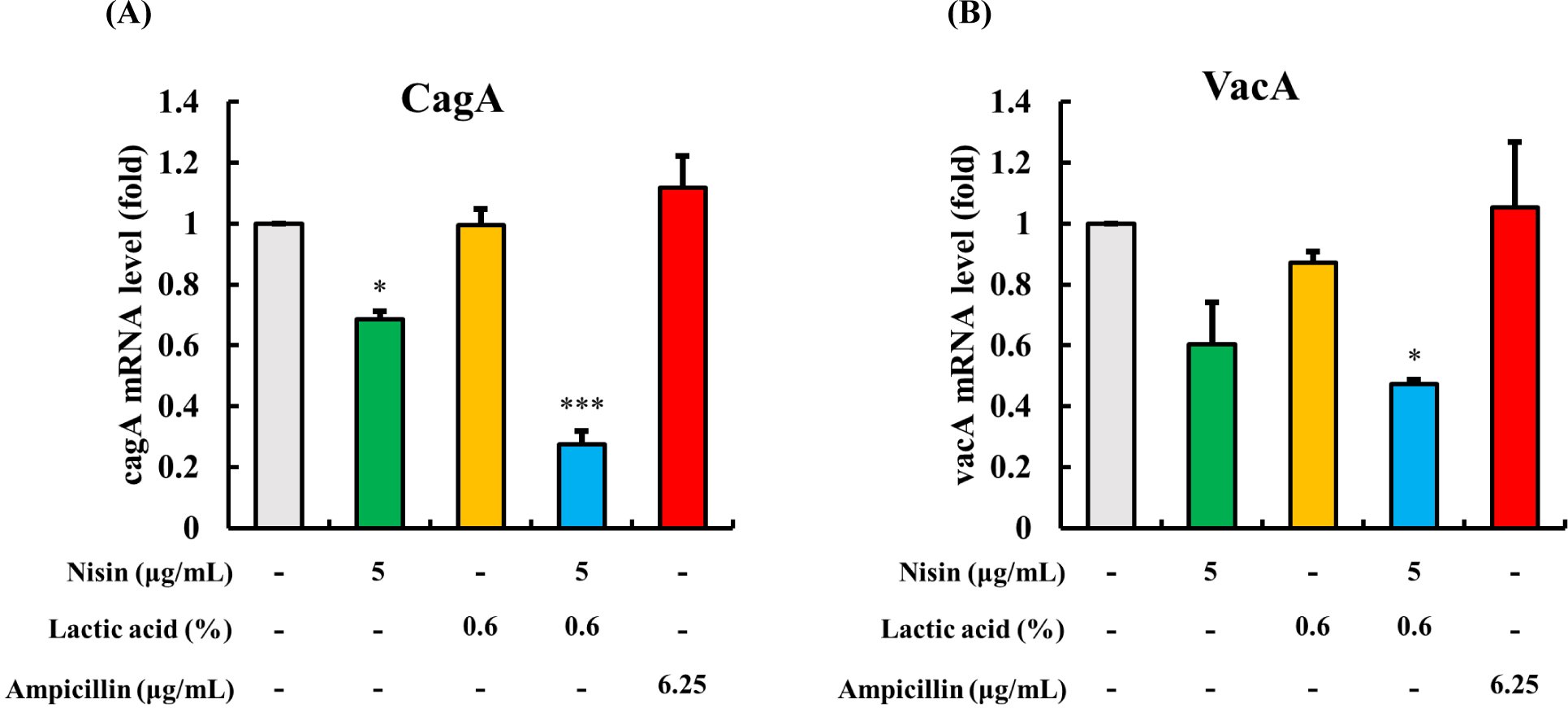
The mRNA expression levels of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-8, and IL-6, increased in AGS cells upon addition of 108 CFU/mL of H. pylori (Figs. 4A, B, C, and D). Treatment of AGS cells with nisin alone resulted in a decrease in the mRNA expression of these cytokines, while LA alone didn’t show a significant decrease in their mRNA expressions. However, when nisin and LA were used in combination, a significant reduction in the mRNA levels of the pro-inflammatory cytokines was observed, with levels similar to the control without H. pylori infection (p>0.05) for all pro-inflammatory cytokines. In contrast, the ampicillin-treated group showed no such effects. These results suggest that the combination of nisin and LA may have a synergistic effect in downregulating the inflammatory in H. pylori infected AGS cells.
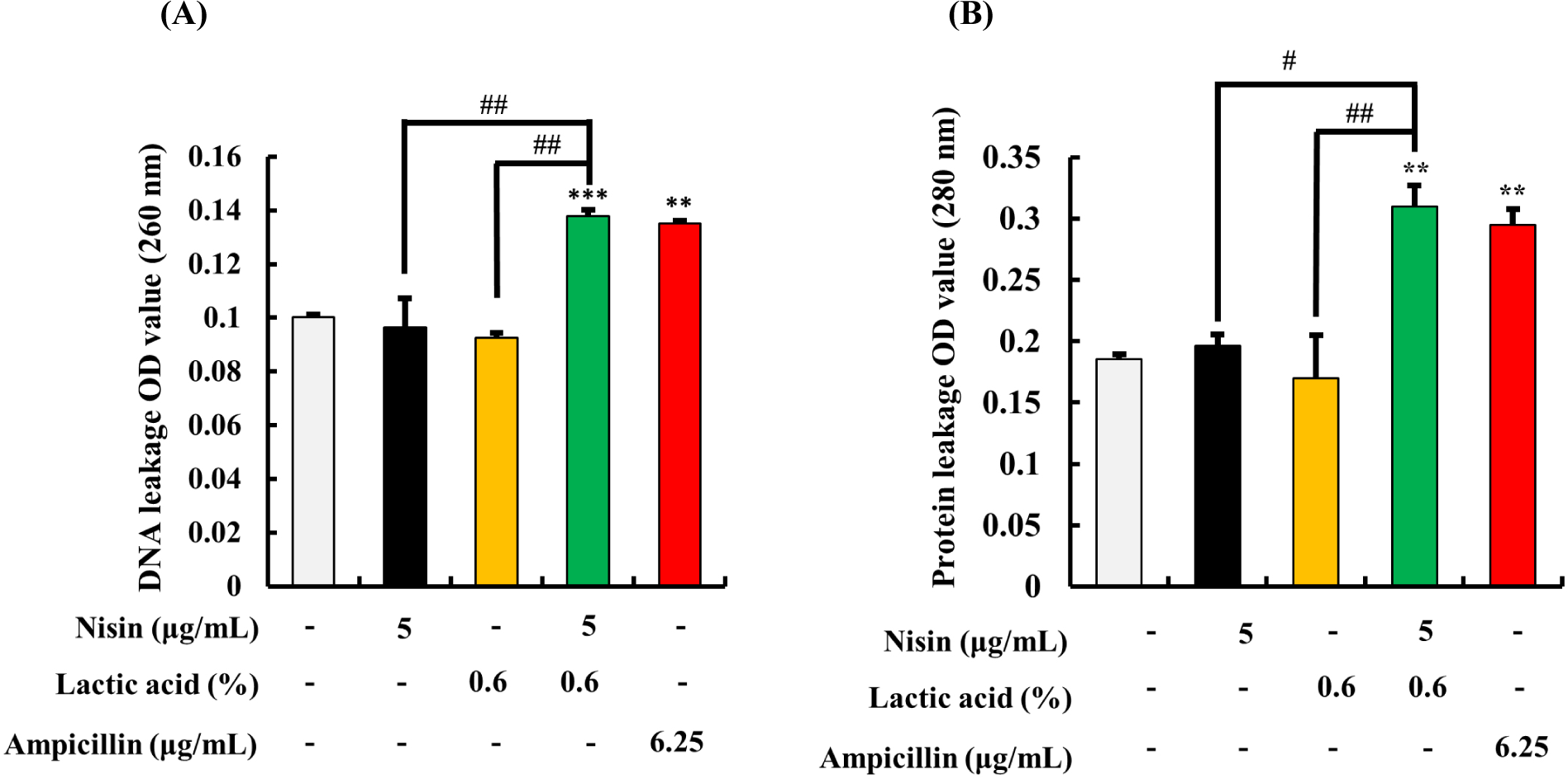
The effect of nisin and LA on the leakage of the DNA and protein from H. pylori is shown in Fig. 5. The results showed that the highest levels of DNA and protein leakage were observed in samples treated with 5 μg/mL nisin and 0.6% of LA in combination (Fig. 5A and B). Ampicillin showed similar degree of DNA and protein leakage like nisin-LA combination. However, nisin or LA alone at the same concentration did not show any significant differences in DNA and protein leakage, compared to the control (p>0.05).
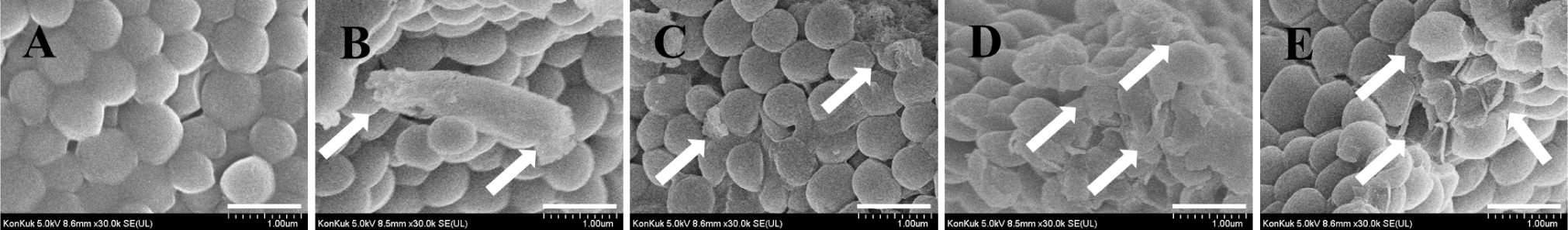
FE-SEM was used to visualize the morphology of H. pylori which was treated with nisin, LA, its combination or ampicillin (Fig. 6). The control group showed intact, smooth, and homogeneous bacterial surfaces (Fig. 6A). Treatment of H. pylori with LA alone caused slight deformation to the bacterial surface (Fig. 6B). Nisin alone resulted in slight membrane damage, with some areas of the bacterial surface appearing rough and uneven (Fig. 6C). However, the nisin and LA combination caused more extensive damage to the bacterial membrane, with significant deformation, and in some cases, visible fragments of the bacteria were observed (Fig. 6D). Ampicillin also resulted in membrane damage with strongly ruptured surfaces (Fig. 6E).
Discussion
The complete eradication of H. pylori is a challenging task due to its persistent nature in the gut. Antibiotic therapy is the primary approach for H. pylori eradication. However, it may cause dysbiosis in the gut microbiota and modulate the immune system, resulting in unwanted effects like metallic taste, diarrhea, nausea, or vomiting during the treatment process (Çalışkan et al., 2022). Consequently, researchers are seeking safe antibiotic alternatives that are devoid of such side effects (Seal et al., 2013). In this regard, nisin is not only safe but also has a broad antibacterial effect on bacteria (Norouzi et al., 2018). However, due to its complex purifying process and limited supply, nisin is relatively expensive compared to other antibacterial substances (Zhao et al., 2022). Thus, there are several ongoing research regarding development and improvement of nisin production by LAB (Özel et al., 2018).
LA is a metabolite of LAB and is known to be a safe substance. It has been extensively studied for its ability as a permeabilizer to disrupt the outer membrane of bacteria by causing changes in the membrane lipid composition (Gyawali et al., 2011). Yogurt or fermented milk is a fermented dairy product, which possess plenty of LA due to the metabolites of LAB during fermentation. Although yogurt has a plenty of LA, there is no data regarding incorporation of nisin to yogurt due to concerns that nisin could inactivate LAB in fermented food. Although nisin can inactivate LAB in yogurt, recent findings suggest that the inactivated bacteria, known as parabiotics, can still confer beneficial effects in human (Kazemi et al., 2021). Some studies have found that parabiotics have a significant impact on serotonin secretion in the gut (Hara et al., 2018). Additionally, several strains of Lactobacillus parabiotics have demonstrated anti-inflammatory and anti-oxidative effects in in vitro and in vivo experimental models (Chung et al., 2019; Jang et al., 2018). Thus, both live probiotics and inactivated parabiotics can have beneficial effects on human health. Therefore, investigating the potential health benefits of incorporating nisin into post-fermented yogurt would be a valuable research endeavor.
While previous experiments have demonstrated the inhibitory effects of LAB and bacteriocins against H. pylori (El-Adawi et al., 2013; Kim et al., 2003), our study focused on the combination effect of nisin and LA for the growth inhibition of H. pylori. To investigate the possibility of incorporating nisin into yogurt, we evaluated the synergistic antibacterial effect of nisin and LA using checkerboard assay and agar well diffusion assay. MIC of nisin (μg/mL), LA (%) and ampicillin (μg/mL) against H. pylori was 10, 0.6, 0.195, respectively. Previous studies indicated that the MIC of nisin was 0.39–25 μg/mL, and ampicillin was 0.015–0.25 μg/mL and it was similar to our data (Neshani et al., 2019; Weiss et al., 1998). The FICI of combined nisin and LA was 0.375, indicating that nisin and LA have synergistic effect.
The LA content in the yogurt was measured to be 0.6%. When the yogurt supernatant and nisin (5 μg/mL) were combined, the growth of H. pylori was inhibited as observed in the agar well diffusion assay. Among the concentrations of nisin tested (ranging from 1.25 to 5 μg/mL), 5 μg/mL of nisin exhibited better H. pylori growth inhibition effects than the MIC value of nisin (10 μg/mL). When nisin is combined with LA, 5 μg/mL of nisin demonstrated the most pronounced H. pylori growth inhibition effects. Based on these data, only 5 μg/mL of nisin was selected.
Nisin and LA are GRAS substances because they have been used in various foods for many years (Müller‐Auffermann et al., 2015). However, to use these two substances together, the safety of their combined effect must be evaluated. As H. pylori is a bacterium that lives in the stomach, we conducted a safety evaluation using the trypan blue dye exclusion assay on AGS cells. Also, since it takes approximately 3 hours for food to be digested in the stomach, the material processing time was set to 3 hours. Live cells exclude trypan blue dye due to intact cell membrane, and blue-stained dye indicates cell death due to cell membrane damage. In previous studies, high concentrations of nisin were reported to possess anticancer properties by inducing apoptosis (Ahmadi et al., 2017). In our study, cell death was not observed in low concentrations of nisin (1.25 to 5 μg/mL), even when used in combination with 0.6% LA. Cell death was only observed in AGS cells treated with 1.2% LA. Based on these results, we selected the concentration of 5 μg/mL of nisin, 0.6% of LA, and 6.25 μg/mL of ampicillin for further in vitro assays.
To determine the optimal concentration of H. pylori to be treated in the cell, an LDH assay was conducted. Cell membrane damage results in LDH release in the culture medium, making it an indicator of damaged cells (Fotakis and Timbrell, 2006). It has been suggested that VacA, which is secreted by H. pylori, regulates pro-apoptotic MMP-9 expression of cells, resulting in apoptosis (Gonciarz et al., 2019). Accordingly, the LDH release increased when AGS cells were treated with 109 CFU/mL of H. pylori. Therefore, the cell density of 108 CFU/mL of H. pylori was selected for further cell culture studies.
H. pylori is known to induce inflammation by modulating pro-inflammatory cytokine production. In previous study, H. pylori treated AGS cells showed an increased mRNA level of pro-inflammatory cytokines (Lamb and Chen, 2013). H. pylori bacterial toxin CagA and VacA are major contributors to the increase in pro-inflammatory cytokines. CagA is transported onto gastric epithelial cells via a type IV secretion system, where it activates the NF-κB pathway, leading to the production of pro-inflammatory cytokines (Jones et al., 2010). Meanwhile, VacA stimulated the production of reactive oxygen species in gastric epithelial cells, which can lead to the production of pro-inflammatory cytokines (Jones et al., 2010). We investigated the effect of nisin and LA on H. pylori infected AGS cells, with a focus on its impact on the mRNA expression levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8 and TNF-α) and bacterial toxins (CagA and VacA). Our findings demonstrate that the combination of nisin and LA was effective in downregulating the mRNA expression levels of pro-inflammatory cytokines by reducing the mRNA expression levels of bacterial toxins. The bacterial toxins have been shown to play a role in the development of pathogen-associated diseases by leading to inflammation and tissue damage. Previous studies have shown that nisin can downregulate the transcriptional levels of Staphylococcus aureus toxin genes, leading to the reduction of bacterium adhesion and modulation of pro-inflammatory and anti-inflammatory cytokines (Jia et al., 2019; Zhao et al., 2016). Additionally, exposure to nisin could damage the cellular membrane and triggers DNA condensation of S. aureus (Jensen et al., 2020). Our data showed that the combination of nisin and LA is more effective in downregulating the gene expressions of bacterial toxins compared to using them alone. This may be attributed to nisin-LA combination ability to damage the bacterial membrane of H. pylori, allowing nisin to enter the inside the cellular membrane and trigger DNA condensation. The combination of nisin and LA led to the leakage of cytoplasmic contents such as DNA and proteins, indicating membrane damage. Nisin and LA used separately did not show such antibacterial effects. These findings were supported by the results of FE-SEM, which showed nisin alone did not cause significant damage to the bacterial membrane, while the combination of nisin and LA resulted in a notable level of damage.
Incorporating nisin into post-fermented yogurt exhibited antibacterial effect against H. pylori. Combination of nisin and LA was found to have a synergistic antibacterial effect against H. pylori, compared to using them alone. This combination was also effective in downregulating gene expressions of bacterial toxins and inflammatory cytokines in human gastric cells. Based on our data, it is suggested that incorporating yogurt, which naturally contains LA, with nisin could provide a safe approach to inhibit H. pylori infections. This combination shows promise as a potential alternative therapy for H. pylori eradication. Further studies, including animal studies, are necessary to validate our findings and assess the potential of these combinations in clinical settings.













