Introduction
The Korean native black goat (Capra hircus coreanae) is a unique indigenous goat that accounts for approximately 80% of the number of domesticated native goats in Korea (Son, 1999). Indigenous meats produced from native animal species occupy a niche market in the region because of their unique characteristics as valuable genetic resources (Huang et al., 2020). In practice, Korean native black goat is traditionally consumed as the type of soup, marinated meat, boiled meat, and hot water extract with herbal medicine. As goats are less challenging to breed than conventional livestock, such as chicken, cattle, and pigs, the number of Korean native black goat populations has also increased markedly with the recent societal trends of people returning to rural farming in Korea. Owing to the increased production and consumption of Korean native black goats, the practical use of goat by-products generated from slaughterhouses has also been interesting.
Animal by-products account for approximately 20%–30% of the live body weight and primarily include hair, hides (skin), internal organs, and blood (Ockerman and Hansen, 1999). Mammalian skin containing the epidermal and dermal layers of mammals is composed of water and collagenous connective tissues, and bovine hides and porcine skins are commercially valuable materials for producing collagen and gelatin (Gómez-Guillén et al., 2011). According to Sen et al. (2004), the proportion of major goat byproducts to live body weight is as follows: Blood (3.71%), head (5.62%), and skin (9.53%). Thus, although it could be expected that Korean native black goat skin may be a valuable source of collagen and gelatin, little is known about its extraction process and quality characteristics.
Commercial food gelatin is generally produced from bovine hide and porcine skin through acid or alkali pre-treatment, neutralization, hot-water extraction, and drying processes (Gómez-Guillén et al., 2011). The quality of commercial gelatin is determined by its yield (for economic feasibility), rheological and mechanical properties (e.g., gel strength, melting point, and viscosity), emulsifying and foaming properties, and sensory properties, including off-flavor and odor (Abedinia et al., 2020). Moreover, it has been well documented that the physicochemical characteristics of gelatin are primarily affected by the raw material species and extraction conditions (pre-treatment method, extraction temperature and time, drying method, etc.; Gómez-Guillén et al., 2011). In this regard, previous studies have investigated the impacts of various factors, such as alkali treatment (Mad-Ali et al., 2016a), sodium sulfate/hydrogen peroxide pre-treatment (Mad-Ali et al., 2016b), drying method (Mad-Ali et al., 2016c), and extraction conditions (temperature and time; Mad-Ali et al., 2017a), on the quality attributes of goat skin gelatin. Regarding the sensory properties, as well as, our previous study found that the off-flavor intensity of Korean native black goat skin gelatin was similar to that of commercial porcine skin gelatin (Lee et al., 2021).
According to Mad-Ali et al. (2017a), an increase in the extraction temperature and time increased the extraction yield of goat skin gelatin, but decreased its gel strength. Considering that both yield and gel strength are the most crucial qualities of commercial gelatin, it may be necessary to optimize the extraction conditions to maximize the two main factors with opposing variations with temperature and time. In the food industry, response surface methodology (RSM) is widely used as a powerful technique to optimize various factors with more than two parameter changes, which could guarantee the improvement of process efficiency and save cost and time (Yolmeh and Jafari, 2017). In numerous previous studies, RSM has been extensively used to optimize gelatin extraction conditions, mainly temperature and time, of mammalian, poultry, and fish skins (Gómez-Guillén et al., 2011).
With the final purpose of the practical use of Korean native black goat skin as a commercial gelatin source, this study was performed to optimize the extraction temperature and time of gelatin from Korean native black goat skin and to compare the quality characteristics of goat skin gelatin and other commercial gelatin products.
Materials and Methods
RSM was applied to optimize the extraction temperature and time of gelatin obtained from native Korean black goat skin. The effects of temperature (X1: 50°C–70°C) and time (X2: 2–4 h) on two response variables (extraction yield and gel strength) were investigated using a face-centered central composite design (Table 1). The three coded levels (–1, 0, and +1) of temperature and time were considered to set the design of 13 runs. All experiments were conducted in triplicate and the effects of temperature (X1) and time (X2) on the two response variables (Y) were determined using second-order polynomial regression Eq. (1).
where β0 is a constant; β1 and β2 are the coefficients of the linear effects; β3 is the coefficient of the interaction effect between the two independent variables; β4 and β5 are the coefficients of the quadratic effects.
Fresh skin of female Korean native black goats was obtained from a local distributor at 7 days postmortem. The commercial gelatin products used for quality comparison were as follows: Fish skin gelatin I (250 bloom, ES Ingredients, Gunpo, Korea), fish skin gelatin II (250 bloom, QLS16265, Hangzhou Qunli Gelatin Chemical, Hangzhou, China), bovine bone gelatin I (150 bloom, QLS16498, Hangzhou Qunli Gelatin Chemical), bovine hide gelatin I (280 bloom, QLS16269, Hangzhou Qunli Gelatin Chemical), porcine skin gelatin I (280 bloom, QLS16275, Hangzhou Qunli Gelatin Chemical), and porcine skin gelatin II (250 bloom, BL250P, Sammi Industry, Ansan, Korea). All reagents used were of analytical grade and the commercial gelatin products were of food grade.
Gelatin extraction from Korean native black goat skin was prepared through serial processes of alkali pretreatment, bleaching, neutralization, hot-water extraction, and freeze-drying according to the procedure described by Mad-Ali et al. (2016a), Mad-Ali et al. (2016b), Mad-Ali et al. (2017a) with minor modifications. In detail, fresh goat skin was manually cut into approximately 4×4 cm pieces, separated into 13 groups, and randomly assigned to each experiment set by the face-centered composite design mentioned above. The goat skin was soaked in 10 volumes (v/w) of 0.75 M NaOH solution for 48 h with agitation at 6 h intervals. The swelled goat skin was neutralized with running water below pH 7.0 for approximately 12 h. The washed goat skin was reweighed, bleached in a 2 M H2O2 solution for 24 h, and washed with distilled water several times. The hot-water gelatin extraction was conducted in a water bath at the experimentally designed temperatures and times (Table 1), and then the gelatin extract was filtered using a cheesecloth. The filtrate was cooled to room temperature, frozen in a –70°C deep freezer, and freeze-dried using a freeze dryer (80×10–3 Torr pressure, PVTFD10R, Ilshin Lab, Daejeon, Korea). The freeze-dried gelatin was weighed to determine the extraction yield, powdered, vacuum-packaged, and stored in a refrigerator at 4°C until further use.
The extraction yield was determined by calculating the weight difference between the initial fresh goat skin and freeze-dried gelatin powder using the following equation: Extraction yield (%) = [weight of freeze-dried gelatin powder (g) / weight of initial goat skin (g)] × 100.
Gel strength was determined according to the AOAC method 948.21 (AOAC, 1990) described by Rafieian et al. (2015). The gelatin powder was dissolved in distilled water (6.67 %, w/v) at 60°C for 2 h and cooled at 10°C for 18 h. The gelatin gel was cut to a diameter of 33 mm and height of 60 mm. The strength of the gelatin gel was determined using a texture analyzer (CT3, Brookfield Engineering Laboratories, Middleborough, MA, USA) equipped with a 12.7 mm diameter cylindrical probe, and the cross-head speed was 1 mm/s. Six samples per treatment were used, and the maximum force (g) required to penetrate 4 mm of the gelatin gel was averaged to obtain the gel strength as a bloom.
Five grams of gelatin gel sample (6.67%, w/v) were homogenized with four volumes of distilled water for 60 s using a homogenizer (UltraTurrax SK15, Janke & Kunkel, Staufen, Germany; Park et al., 2013). A pH meter (Orion Star A211, Thermo Fisher Scientific, Beverly, MA, USA) was calibrated at room temperature using standard pH buffers (pH 4.01, 7.00, and 10.01).
The gelatin gel sample (6.67%, w/v) was cut into a 2×2×2 cm3 cube size, and the surface color of the gel was measured using a colorimeter (Chroma meter, CR 400; Minolta, Osaka, Japan) with an 8-mm measurement area and an 11-mm illumination area. The illuminant was set as a D65 source, and the observer was a standard 2°. The colorimeter was calibrated using a white calibration tile (CIE L*=+97.83, CIE a*=–0.43, CIE b*=+1.98) according to the manufacturer’s instructions. The CIE L*, CIE a*, and CIE b* were recorded for all six samples.
The turbidity of gelatin powder was determined by the method of See et al. (2010) described by Rafieian et al. (2015). One hundred milligrams of gelatin powder were dissolved in 100 mL of distilled water in a 60°C water bath for 30 min. The absorbance of the gelatin solution was measured at 660 nm using a spectrophotometer (Libra S22, Biochrom, Cambridge, UK). The turbidity of the gelatin powder was expressed as kaolin mg per kilogram of sample.
The emulsion activity index (EAI) and emulsion stability index (ESI) of the gelatin powder were determined using the method described by Duan et al. (2018). Six milliliters of gelatin solution (2%, w/v) were emulsified with 2 mL of commercial soybean oil using a homogenizer (UltraTurrax SK15, Janke & Kunkel). The emulsion (100 μL) was homogenized in 5 mL of a 0.1% sodium dodecyl sulfate solution for 10 s. The absorbance (500 nm) of the homogenate was measured at 0 min (initial phase) and 10 min using a spectrophotometer. The EAI and ESI were calculated using the following Eqs. (2) and (3):
where Ainitial is the absorbance at 500 nm immediately after emulsification and A10 min is the absorbance at 500 nm after standing for 10 min.
The foaming capacity (FC) and foaming stability (FS) of the gelatin powder were determined using the method described by Duan et al. (2018). The gelatin solution (2%, w/v) was placed at 20°C for 20 min and homogenized at 13,000 rpm for 1 min. The volume of the whipped sample was immediately measured in 25 mL cylinders. The whipped sample was incubated at 20°C for 3 min, and the volume was measured again in 25 mL cylinders. The FC and FS were calculated using the following Eqs. (4) and (5):
where V0 is the sample volume before whipping, Vinitial is the sample volume immediately after whipping, V3 min is the sample volume after standing for 3 min.
The experimental design, visualization, and optimization of RSM were performed using the Minitab statistical software. For quality comparison, analysis of variance was performed on the measured variables using the one-way ANOVA procedure of the SPSS program (SPSS, Chicago, IL, USA). Duncan’s multiple range test was used to determine significant differences between the means (p<0.05).
Results and Discussion
In this study, the face-centered central composite design was considered with three coded levels of two independent variables to optimize the extraction temperature and time for gelatin from Korean native black goat skin. A total of 13 runs with five replicates at the center were set (Table 2). The ranges of extraction temperature and time were within 50°C–70°C and 2–4 h, based on previous studies indicating successful gelatin extraction from goat skin (Mad-Ali et al., 2016a; Mad-Ali et al., 2016b; Mad-Ali et al., 2016c; Mad-Ali et al., 2017a).
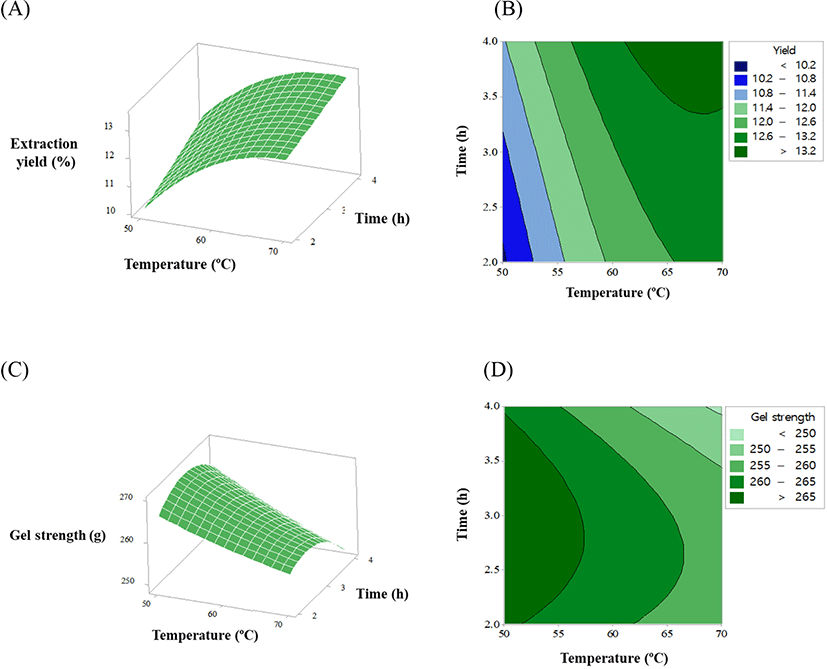
The extraction yield and gel strength of gelatin from native Korean black goat skin at various temperatures and times are shown in Table 1. The observed extraction yield and gel strength of the goat skin gelatin were 10.2%–13.4% (w/w) and 251.8–271.6 g, respectively. The mathematical model equations between the independent (temperature and time) and response variables (extraction yield and gel strength) are listed in Table 2. The result for the lack of fit indicated the proper fitness of both models for two response variables (p>0.05), and the R square of the models for the extraction yield and gel strength was 0.95 and 0.83, respectively. The ANOVA results, indicating the regression coefficients and their significant differences, are shown in Table 3. In the quadratic polynomial models, the linear terms of temperature (p<0.001) and time (p<0.001), and the quadratic term of temperature (p=0.005) had remarkable effects on the extraction yield. The linear term of temperature (p=0.002) and quadratic term of time (p=0.043) greatly affected the gel strength. Similarly, Jakhar et al. (2014) reported that gelatin extraction from blackspotted croaker skin was significantly affected by the linear terms of temperature and time in the optimization of the extraction process. Moreover, many previous studies have indicated that the extraction temperature of gelatin from mammalian and fish skins could be a critical factor affecting extraction yield and gel strength (Alfaro et al., 2014; Cho et al., 2005; Jakhar et al., 2014).
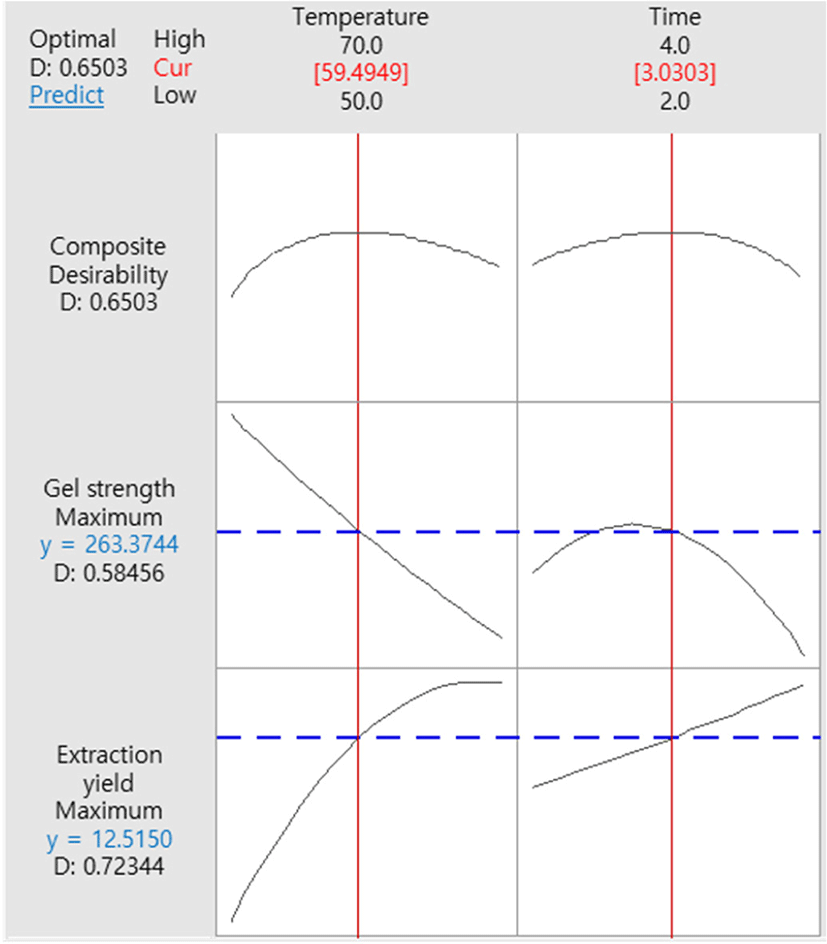
The response surfaces and contour plots showing the effects of the extraction temperature and time on the extraction yield and gel strength of gelatin from Korean native black goat skin are shown in Fig. 1. The optimization plot of the extraction temperature and time using the Minitab software is shown in Fig. 2, in which the optimization objective of both response variables was set to maximum values within 50°C–70°C and 2–4 h, respectively. The optimization plots with the optimized settings (vertical red line) and the optimized values (red numbers) show the effects of the extraction temperature and time on the extraction yield and gel strength of goat skin gelatin. The optimized setting conditions and values were validated using three independent tests (data not shown). Finally, the optimized setting conditions for extracting the temperature and time of the goat skin gelatin were 59.49°C and 3.03 h, and the optimized values of extraction yield and gel strength were 12.52% and 263.37 g, respectively. Similarly, Mad-Ali et al. (2017a) observed that the extracting conditions for the goat skin gelatin with high gel strength was 50°C for 2.5 h. Thus, it could be thought that those setting conditions may be useful to guarantee the industrial productivity of gelatin manufacturing using Korean native black goat skin.
Commercial food gelatin is industrially manufactured from porcine skin (gelatin type A), bovine hide and bones (gelatin type B), and fish. According to Karim and Bhat (2009), porcine skin, bovine hide, and bovine bone account for 98.5% of the gelatin extraction sources worldwide. Along with religious reasons and the value addition of underutilized by-products, previous studies on the diversification of gelatin materials have been extensively conducted (Huang et al., 2019). In the current study, the quality characteristics of gelatin extracted from Korean native black goat skin were compared with those of six commercial gelatin products to ensure the possibility of the industrial utilization of goat skin gelatin (Table 3). One-way ANOVA showed a significant difference in all measured dependent variables.
The pH value of gelatin extracted from Korean native black goat skin was 5.57 (Table 4), which was within the pH range of commercial gelatin products (5.43–5.85). Generally, commercial gelatin has a pH of approximately 5 (Baziwane and He, 2003). The pH of gelatin is a critical factor affecting its techno-functional properties, such as emulsifying capacity and gel strength (Montero and Borderías, 1991). In this regard, the pH value of gelatin is generally formed through neutralization (pH 5.5–7.0) after acidic or alkali pre-treatment, which is a range that minimizes its adverse impacts on the physicochemical and sensory properties of applied food products.
The gelatin extracted from Korean native black goat skin was darker and redder than commercial gelatin products (p<0.05; Table 3). Moreover, the highest turbidity was observed for goat skin gelatin (p<0.05). Commercial gelatin is yellowish and its color is affected by the raw materials and extraction conditions (Alfaro et al., 2015). Previously, Mad-Ali et al. (2016a) reported that the lightness, redness, and yellowness of goat skin gelatin treated with 0.5 and 0.75 M NaOH for 2–4 h were 16.88–18.17, 2.30–2.89, and 7.16–8.56, respectively, and suggested that the pre-treatment condition could be an important factor on the color characteristics of goat skin gelatin. However, previous results showed a lower lightness of goat skin gelatin compared to our results. According to Mad-Ali et al. (2016b), an increase in hydrogen peroxide concentration (0–2 mol/L) during the pre-treatment process could increase the lightness (21.15–28.97) of goat skin gelatin. Thus, in the current study, treatment with hydrogen peroxide as a bleaching reagent could also contribute to the increased lightness of goat skin gelatin. Furthermore, because the turbidity of gelatin may not be important depending on the application practice (Alfaro et al., 2015), the color and turbidity characteristics of the goat skin gelatin, there would be no color and turbidity issues in its practical use in the food industry.
In terms of functional properties, the highest EAI and ESI were observed for gelatin extracted from Korean native black goats (p<0.05), in which the ESI of goat skin gelatin was approximately 2.6 times higher than that of porcine skin gelatin I (p<0.05). However, the FC and FS of goat skin gelatin were lower than those of the commercial gelatin treatments (p<0.05). The emulsifying and foaming capacities of gelatin are associated with the presence of a hydrophobic region in its amino acid sequence (Abedinia et al., 2020). Rasli and Sarbon (2015) found that the high FS of chicken skin gelatin could be related to a high amount of hydrophobic amino acids. Mad-Ali et al. (2016a) noted that the number of hydrophobic amino acids in goat skin gelatin, such as valine, leucine, isoleucine, proline, phenylalanine, methionine, and tryptophan, was comparable to that of bovine gelatin and porcine gelatin. A recent study reported that the proportion of hydrophobic amino acids (34.1%) in goat skin gelatin is higher than that in commercial bovine gelatin (29.2%; Zilhadia et al., 2018). Despite the excellent emulsifying capacity of goat skin gelatin, the opposite result was observed for foaming properties. A similar result was reported by Zilhadia et al. (2018), who noted that goat skin gelatin had lower foaming ability and stability than commercial bovine gelatin. According to Cho et al. (2004), the decline in the foam properties of shark cartilage gelatin might be associated with protein aggregation responsible for protein-water interactions and suggested that the increased protein aggregation could increase turbidity. Our result was also in agreement with previous observations, considering the high turbidity of goat skin gelatin.
The gel strength of gelatin extracted from Korean native black goat skin was 280 g, similar to that of fish skin gelatin II and bovine hide gelatin I (p>0.05). In previous studies, the gel strength of goat skin gelatin was reported as 209–267 g, in which the gel strength was affected by the pre-treatment method, extraction conditions, and drying conditions (Mad-Ali et al., 2016a; Mad-Ali et al., 2016c; Mad-Ali et al., 2017a; Mad-Ali et al., 2017b). The gel strength of commercial gelatin ranges from 50 g to 300 g (Baziwane and He, 2003). Our results also showed that the gel strength of goat skin gelatin is within the normal gel strength range of commercial gelatin.
Conclusion
In this study, the optimal extracting conditions for the gelatin from Korean native black goat skin, to guarantee maximum extraction yield and gel strength, were obtained as 59.49°C and 3.03 h. Based on the results, the entire procedure for gelatin extraction was performed as follows; alkali pre-treatment, bleaching, neutralization, hot-water extraction, and freeze-drying. Gelatin extracted from Korean native black goat skin showed a darker color than commercial gelatins, and excellent emulsifying properties and gel strength were observed. Thus, the results of this study indicate that Korean native black goat skin may be a valuable source for gelatin extraction. Further studies on the application of goat skin gelatin in the food, pharmaceutical, and cosmetic industries are required to improve the practical value of underutilized goat skin.













