Introduction
Korean native black goats (KNBG; Capra hircus coreanae) are an indigenous breed in Korea, ~80% of which are predominantly black (Son, 1999). KNBG have been raised as domestic livestock in Korea for more than 2,000 years (Kim et al., 2014). However, with its perceived health benefits in the elderly, children, and pregnant persons (Kim et al., 2014; Kim et al., 2019; Son, 1999), consumption of KNBG meat has been increasing (Kim et al., 2019). In Korea, there are 14,664 farms which have raised 542,744 heads of crossbreed black goat (MAFRA, 2019). Although the production size of KNBG is significantly lower than that of crossbreed black goat, research on the production of high-quality meat from KNBG is crucial to meet the increasing demands for KNBG meat.
KNBG farming has transitioned from multiple farming systems into one intensive farming system (Kim et al., 2014), making standardized treatment more attainable. Diet is a major factor influencing meat production and quality (Marinova et al., 2001). In particular, adjusting energy levels can improve meat production and quality. Concentrate feeding is an effective dietary method to breed goats on a large scale. However, large-scale concentrate feeding may hinder the growth of goats by increasing feed costs and the risk of developing metabolic diseases (Jung et al., 2008). Hence, alfalfa hay has been used as a forage source in finishing diets to supply crude protein to ruminants (Hwang et al., 2018). The mixture of pasture and concentrates can stabilize the ruminating environment and improve feed intake, and nutrient utilization efficiency has been attracting attention recently (Lee et al., 2021b). Lee et al. (2019) have reported that feeding a high forage or a high concentrate diet (8:2 or 2:8) altered ruminal fermentation and the bacterial community structure in KNBG; however a limitation on the meat quality of KNBG existed. Generally high-concentrate feeding increases the producibility and palatability (texture and flavour) of meat (French et al., 2000); thus, concentrate abundance and grass quality have increased (Kim et al., 2012). Moreover, fodders high in concentrate can increase the fat content of meat (Steen and Kilpatrick, 2000). Meanwhile, certain studies have reported that meat from grass-fed animals has better antioxidant activity compared to that from concentrate-fed animals (Descalzo et al., 2007; Gatellier et al., 2004; Yang et al., 2002). However, few studies have investigated the effect of concentrate and grass-feeding on the physicochemical characteristics of KNBG meat (Hwang et al., 2018; Kim et al., 2014).
Meat is a significant source of bioactive compounds or nutraceuticals, such as minerals, fatty acids, vitamins, and peptides (Pogorzelska-Nowicka et al., 2018). Specifically, consumption of KNBG can provide various compounds, including carnosine, coenzyme Q10, anserine, and L-carnitine. However, storage of KNBG meat in cold rooms in markets or extended refrigeration can cause degradation of meat quality and loss of bioactive contents. Indeed, we have previously found that the abundance of certain bioactive compounds and antioxidant activity decreases in beef during storage (Kim and Jang, 2021), whereas antioxidants in meat reportedly improve meat shelf-life and quality (Velasco and Williams, 2011).
Therefore, in the current study, we aimed to evaluate the effect of feeding KNBG with alfalfa and concentrate at different ratios on loin meat quality, bioactive compound abundance, and antioxidant activity during cold storage.
Materials and Methods
Ten KNBG (48.6±1.4 kg body weight; 4.8±1.2 years old, castrated male) were randomly divided into two groups and were fed with the experimental diet for 90 days of finishing period. The high alfalfa (KHA) group (n=5) was fed alfalfa and concentrate daily at an 8:2 ratio; the low alfalfa (KLA) group (n=5) was fed alfalfa and concentrate daily at a 2:8 ratio. Animals had free access to water, and experimental feed was provided ad libitum twice daily. The alfalfa composition comprised 7.42% moisture, 15.0% crude protein, 1.49% crude fat, 6.59% crude ash, 56.1% neutral detergent fibre, 44.2% acid detergent fiber, and 2.16 Mcal/kg metabolizable energy. The conventional concentrate (EE0SL0132, TS Rainbow Feed, Seoul, Korea) comprised 8.52% moisture, 18.8% crude protein, 3.80% crude fat, 6.75% crude ash, 23.0% acid detergent fibre, 9.03% neutral detergent fiber, and 2.77 Mcal/kg metabolizable energy. The detailed ingredients and the chemical composition of the experimental diets are shown in Table 1. The KNBG were slaughtered after 90 days on the experimental diet. After slaughtering, the carcasses were chilled for 24 h at 2°C, and loin muscles (longissimus dorsi) were sampled for analysis. The animal care and use protocols were followed under approval of the Institutional Animal Care and Use Committee of the NIAS, RDA, Korea (NIAS-2019-1545).
Samples were cut into 1.5 cm thick slices, placed on a polystyrene tray with low-density polyethylene (LDPE) film, and stored in an aerobic environment at 4±2°C, which reflects the common storage condition at home. Samples were selected on day 1, 5, 10, and 15 and evaluated in terms of quality, bioactive compounds, and antioxidant activities.
Proximate composition was determined using methods established by the Association of Official Agricultural Chemists (AOAC, 1997). The moisture content was calculated using weight difference after drying at 105°C for 12 h. The Kjeldahl method was employed to analyse crude protein content, and the ether extraction method was used to determine crude fat content. The crude ash content was determined based on the weight difference after burning at 550°C in a furnace.
The pH was determined using a homogenate prepared with 10 g of KNBG loin meat sample and 90 mL of distilled water using a pH meter (Orion 230 A; Thermo Fisher Scientific, Waltham, MA, USA).
To analyze meat color, the Commission Internationale de l’Eclairage (CIE) lightness (L*), redness (a*), and yellowness (b*) of KNBG was defined 10 min after removal of the LDPE film using a CR-400 Minolta colorimeter (Minolta, Osaka, Japan) with a C illuminant and 8-mm aperture size.
Ten grams of meat sample was homogenized with 90 mL of saline. One milliliter of the homogenate was placed on Petrifilms to quantify the total aerobic bacteria and E. coli/coliform (3M Microbiology, St. Paul, MN, USA). The Petrifilms were aerobically incubated for 48 h at 37°C, and typical colonies were counted according to the manufacturer’s protocol.
VBN content in meat samples was determined using the micro-diffusion method (Kim et al., 2020). A 10-g meat sample was mixed with 50 mL distilled water for 30 min and passed through a filter paper. Next, one milliliter of the filtrate and 1 mL of saturated K2CO3 were loaded to the outer cell of a Conway dish. To the inner cell, 0.01 N H2SO4 was loaded. After 1 h incubation at 25°C, 20 μL of Brunswick reagent was mixed with and titrated with 0.01 NaOH. The VBN content was presented as mg/100 g of meat.
TBARS was estimated using the methods described by Kim et al. (2020). Briefly, 5 g of meat was added to 50 μL of tert-butyl-4-hydroxyanisole and homogenized with 15 mL of distilled water. One milliliter of the meat homogenate was mixed with 2 mL of 20 mM thiobarbituric acid (in 15% trichloroacetic acid). After boiling at 90°C for 15 min and subsequent centrifugation at 2,000×g for 10 min, the absorbance of the supernatant was measured at 531 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The TBARS content was expressed as mg malondialdehyde (MDA) per kg of meat.
The method described by Kim and Jang (2021) was used to estimate fatty acid composition. Two grams of meat sample was homogenized with Folch reagent (15 mL) with 0.3% butylated hydroxyanisole (40 μL) and then filtered. The bottom layer was collected by shaking vigorously with 0.88% KCl and dried under nitrogen gas. Fatty acid methyl ester derivatives were generated by adding 13% boron trifluoride in methanol by boiling at 90°C for 1 h and used for gas chromatography analysis (6890N; Agilent Technologies, Santa Clara, CA, USA) with a capillary column (100 m×0.25 mm id×0.20 μm film thickness; CP7489, Agilent Technologies) and a flame ionization detector. The temperatures of the injector and detector were 260°C and 280°C, respectively. The initial temperature of the oven was 150°C, which was increased to 200°C at a rate of 7°C/min, held constant at 200°C for 20 min, increased to 250°C at a rate of 3°C/min, and held constant at 250°C for 5 min. The carrier gas was helium, and its flow rate was 1 mL/min. The sample was injected at 1 μL with a splitting ratio of 1:100. Each fatty acid was identified using a standard (PUFA No. 2-Animal Source; Supelco, Bellefonte, PA, USA).
CoQ10 quantitation was performed using a method described by Kim and Jang (2021) with slight modifications to the protocol of No et al. (2011). Briefly, 10 g meat samples were homogenized with 90 mL of ethanol, shaken using a magnetic stirrer for 1 h, and adjusted to 100 mL with ethanol. The supernatant was filtered and used for liquid chromatography (Agilent 1260 Infinity, Agilent Technologies) equipped with a C18 column (ZORBAX Eclipse XDB-C18, 4.6×150 mm, 3.5 μm, Agilent Technologies). The column was isocratically eluted at 1.5 mL/min at 40°C with mobile phase solution (methaol:ethanol mixture=40:60, v/v), and CoQ10 was detected at 275 nm. The CoQ10 content was calculated using a standard curve and expressed as mg per 100 g of meat.
L-Carnitine content was estimated using a method reported by Kim et al. (2019) with a slight modification of the protocol described by Shimada et al. (2004). Briefly, 5 g of samples were homogenized with 0.3 M perchloric acid (PCA) and filtered using a glass microfiber filter. The supernatant was collected after neutralizing it with 1.2 M K2CO3. The standard or the supernatant sample (50 μL) was then reacted with 50 μL of a working solution (0.55 mM acetyl-CoA, 0.93 mM 5,5′-dithiobis-(2-nitrobenzoic acid), 3.05 mM ethylenediaminetetraacetic acid, and 610 mM Tris–HCl; pH 7.5) in a 96-well microplate. After incubation at 37°C for 10 min, the absorbance of the reactant was measured at 415 nm using a spectrophotometer as a blank control. The final absorbance was measured by adding 25 μL of 0.5 U carnitine acetyltransferase (EC 2.3.1.7; Sigma-Aldrich, St. Louis, MO, USA) and incubating the mixture at 37°C for 30 min. The absorbance difference between blank and final was calculated, and L-carnitine content was expressed as μM per gram of meat compared to the standard.
Creatine, creatinine, carnosine, and anserine were estimated using the method reported by Kim et al. (2019). Briefly, meat samples (2.5 g) were homogenized with 0.01 N HCl (7.5 mL) and filtered. The filtrate (250 μL) was mixed with 750 μL of acetonitrile at 4°C for 20 min to remove the protein. The supernatant was filtered after centrifugation at 10,000×g for 10 min and assessed via liquid chromatography (1260 Infinity; Agilent Technologies), equipped with an HILIC column (4.6×150 mm×3 μm; Waters, Milford, MA, USA). The column temperature was 35°C, and mobile phase B was flowed at 1.4 mL/min using a linear gradient method increasing the flow rate from 0%–100% for 13 min. Mobile phase A and B were 0.65 mM ammonium acetate in water/acetonitrile (pH 5.5; 25:75, v/v) and 4.55 mM ammonium acetate in water/acetonitrile (pH 5.5; 70:30, v/v), respectively. Creatine, carnosine, and anserine were detected at 214 nm, while creatinine was detected at 236 nm and expressed as mg per 100 g of meat.
Four grams of meat was homogenized with 20 mL of distilled water and passed through filter paper No. 4. Subsequently, 4 mL of chloroform was added to the filtrate and vortexed. The upper layer was collected and lyophilized at –20°C before analysis.
A colorimetric method was used to evaluate DPPH radical scavenging activity of the samples (Kim and Jang, 2021). The prepared meat sample was reacted with 0.2 mM DPPH solution (100 μL) and incubated for 30 min in the dark at 25°C; absorbance at 517 nm was obtained. The final DPPH radical scavenging activity was expressed as μmol Trolox equivalents (TE)/g dry matter.
To assess ABTS radical scavenging activity, the protocol set out by Kim et al. (2019) was followed. The ABTS+ radical solution was diluted with distilled water to an absorbance of 0.700±0.002 at 735 nm at 30°C. A 50-μL sample was reacted with 950 μL of the ABTS+ radical solution at 30°C for 30 min, and absorbance was measured at 735 nm. The results were expressed as μmol TE/g dry matter.
The FRAP assay was carried out according to the method described by Kim et al. (2019). Each sample (25 μL) was incubated with the FRAP working solution (175 μL) at 37°C for 30 min in the dark, and absorbance was determined at 590 nm. The results were expressed as μmol TE/g dry matter.
To identify the difference in bioactive compounds and antioxidant activity, multivariate statistical analysis was performed using principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA). Analyses were performed with log-transformed and auto-scaled data using Metaboanalyst 5.0 (https://www.metaboanalyst.ca/) according to Lee et al. (2021a). The validity of the PLS-DA model was verified using correlation coefficients (R2) and cross-validation correlation coefficients (Q2).
The SAS software v.9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis. Means among treatment groups were compared by one-way analysis of variance (ANOVA) with a general linear model. Significant differences among means were defined using Tukey’s test at p<0.05. All data were expressed as mean values and SEM.
Results and Discussion
Significant differences were not observed in the proximate composition of loin meat obtained from the two experimental groups (Table 2). Moreover, the moisture, crude protein, and crude ash composition were similar to those in other previous studies (Kim et al., 2019; Sebsibe, 2008). According to Kim et al. (2019), black goat loin contains 75.0% moisture, 21.6% crude protein, and 1.41% ash. In another study investigating different goat breeds, the meats were composed of 67.0%–75.2% moisture, 18.9%–24.8% crude protein, and 0.95%–1.19% crude ash (Sebsibe, 2008). However, crude fat content of black goat meat ranged from 1.48% to 12.6% (Kim et al., 2019; Sebsibe, 2008). Previous studies reported that increased supplementation of concentrate decreases moisture content, while increasing fat content (Kim et al., 2014). However, in this study, no significant differences occurred in the fat contents of loin from the KHA and KLA groups. These findings agree with those of Hwang et al. (2018), who reported that KNBG loin meat fat content is not significantly affected by alfalfa and concentrate supplementation.
| Treatment | Proximate composition (%) | |||
|---|---|---|---|---|
| Moisture | Crude protein | Crude fat | Crude ash | |
| KHA | 73.60 | 20.32 | 5.21 | 1.08 |
| KLA | 74.72 | 20.77 | 5.62 | 1.02 |
| SEM | 0.547 | 0.230 | 0.206 | 0.023 |
The initial pH of loin meat from the KHA and KLA groups was 6.00 and 5.92, respectively (Table 3). During storage, the pH of both groups increased (p<0.05). Generally, the pH of meat increases due to degradation of proteins and growth of spoilage microorganisms, which causes formation and accumulation of amines and ammonia (Kim and Jang, 2021). However, no significant differences were detected in these parameters between the two groups. The lightness (CIE L*) of loin from both groups decreased after day 5 (p<0.05), while redness (CIE a*) and yellowness (CIE b*) significantly decreased during the storage of meat from both groups. No significant differences were detected between groups in the color of KNBG loin meat during storage except for the lightness at day 10. In a study by Realini et al. (2004), a darker meat color of pasture-fed steers was reported compared to that of concentrate-fed steers. However, meat color varies with breed and is not dependent on diet (Kadim et al., 2004). Moreover, feeding alfalfa and concentrate does not reportedly impact KNBG meat color (Hwang et al., 2018), which agrees with our findings. Thus, altering the ratio of alfalfa and concentrate in KNBG feed for 90 days does not impact KNBG meat pH or color.
The total abundance of aerobic bacteria in fresh loin (day 1) from both groups was 2.35 (KHA) and 2.17 (KLA) Log CFU/g, respectively, and increased during storage (p<0.05; Table 4). With the exception of day 1, no significant differences were observed in total aerobic bacteria of KNBG loin meat between groups. On day 15, total aerobic bacteria in loin from the KHA and KLA groups was 6.77 and 7.16 Log CFU/g, respectively. The International Commission on Microbiological Specifications for Foods has recommended the number of total aerobic bacteria to be <7 Log CFU/g (ICMSF, 1986). However, in 2018, the Ministry of Food and Drug Safety (MFDS) in Korea revised the guideline for fresh meat (beef, pork, and chicken) distributed in meat packing centers and meat shops from 7 Log CFU/g (1×107 CFU/g) to 6.70 Log CFU/g (5×106 CFU/g) (MFDS, 2018). Although few studies have monitored total aerobic bacteria in fresh KNBG loin, total aerobic bacteria in Hanwoo beef loin (grade 1) increased from 2.30 Log CFU/g to 6.87 Log CFU/g after 12 days of storage (Sujiwo et al., 2019), which is similar to our results.
Moreover, E. coli and coliform were not detected until day 10, with coliform abundance on day 15 found to be only 0.20–0.51 Log CFU/g. Thus, feeding different ratios of alfalfa and concentrate to finishing KNBG for 90 days did not impact microorganism levels in loin meat; however, meat from both groups may be spoiled by day 15 in aerobic storage at 4°C.
Initial VBN contents of loin meat from both groups were 6.68–6.82 mg/100 g and increased during storage (p<0.05; Table 5). On day 5 and 10, the VBN value in loin meat from the KLA group was higher than that from the KHA group (p<0.05). However, on day 15, VBN values from both groups were not significantly different. It was reported that feeding antioxidants, such as vitamin E, to animals can lower VBN values in meat (Kang et al., 2012). In this study, feeding different ratios of alfalfa and concentrate to finishing KNBG for 90 days did not impact the initial VBN value in loin meat; however, the interaction between feeding ratio and cold storage may have affected the VBN contents of loin meat from both groups on day 5 and 10. According to the Food Code in Korea, meat with a VBN value>20 mg/100 g is considered spoiled (MFDS, 2020).
In this study, VBN values of loin meat from KHA and KLA groups were 19.87 and 18.80 mg/100 g, respectively. Although this could be considered fresh according to the criteria of spoiled meat in Korea, it is necessary to also consider the microorganism abundance. Therefore, we propose that loin meat from the KHA and KLA remains safe for consumption until 10 days of storage, under the conditions described herein.
The initial TBARS value for loin meat from both groups was 0.17 mg MDA/kg and increased during storage to 1.03–1.04 mg MDA/kg on day 15 (p<0.05). However, we observed no significant differences in TBARS values between the two groups. The TBARS value indicates the degree of lipid oxidation, which can be measured based on the intensity of the red color generated by the reaction between malondialdehyde and thiobarbituric acid. According to Filgueras et al. (2010), the extent of lipid oxidative processes in meat is dependent on the balance between muscle antioxidant molecules such as vitamin E and antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase) and lipidic substances sensible to peroxidation (i.e. polyunsaturated fatty acids). In this study, the higher level of antioxidant substances in loin meat such as L-carnitine, carnosine, and anserine which are relatively abundant in lean meat may be responsible for the surprisingly lower anti-lipid oxidation effect. Rancidity of meat, as determined based on lipid oxidation, can lead to an unpleasant flavour. In particular, Prommachart et al. (2020) reported that the threshold of TBARS values, as assessed by panelists, for oxidized flavor varied greatly from 0.6–2.0 mg MDA/kg meat. In our study, TBARS values of loin from both groups reached 1 mg MDA/kg meat on day 15 of storage. A previous study had reported that meat from grass-fed bulls exhibited significantly higher oxidative stability than that from concentrate-fed bulls (Nuernberg et al., 2005). This was postulated to be due to high concentrations of vitamin E in the muscles of grass-fed bulls (Nuernberg et al., 2005). However, in this study, feeding different ratios of alfalfa and concentrate to finishing KNBG for 90 days did not impact lipid oxidation of loin meat during storage.
The predominate fatty acids detected in loin meat from the KHA and KLA groups were palmitic acid, linoleic acid, oleic acid, arachidonic acid, and stearic acid (Table 6). These results agree with those of other studies on goat meat (Hwang et al., 2018; Kim et al., 2014; Kim et al., 2019). Loin meat from the KLA group had higher oleic acid and monounsaturated fatty acids (MUFAs) compared to that from the KHA group during storage (p<0.05). Meanwhile, the meat from the KHA group had higher polyunsaturated fatty acids composition, including arachidonic acid, linolenic acid, and linoleic acid, compared to that from the KLA group (p<0.05). The levels of saturated fatty acids (SFAs) in loin meat from the KHA and KLA groups were 38.72%–42.05% and 39.85%–41.59% during storage, respectively. Similarly, a previous study reported that the composition of SFAs and unsaturated fatty acids (UFAs) in goat meat was 40%–50% and 50%–60%, respectively (Marino et al., 2006). Moreover, according to Marino et al. (2006), the pasture-fed meat contains lower MUFA, higher PUFA, and similar SFA levels compared with concentrate-fed meat. Increasing concentrate in the fodder could alter the microbiota composition in the rumen, which occurs in meat with higher MUFA content (Mateescu et al., 2012). Moreover, the oleic acid and MUFA contents in meat can serve as positive factors for organoleptic properties (Kim and Jang, 2021). In particular, oleic acid is related to fat softness due to the lower melting point of oleic acid, which contributes to the umami taste of beef (Jung et al., 2016). Indeed, the oleic acid composition in goat meat reportedly increases from 43.51% to 48.90%, with an increase in diet concentrate levels (Kim et al., 2014). Therefore, the higher oleic acid content and MUFA in the KLA group than that in the KLA group may have impacted the palatability traits of loin meat.
Meat is a good source of bioactive compounds, including vitamins, minerals, CoQ10, L-carnitine, creatinine, creatine, carnosine, and anserine, all of which exert beneficial effects on human health (Pogorzelska-Nowicka et al., 2018). However, few studies have evaluated the effect of feeding systems and storage on the bioactive compounds in goat meat. Hence, we have evaluated bioactive compound (CoQ10, L-carnitine, creatinine, creatine, carnosine, and anserine) content in loin meat from the KHA and KLA groups during storage (Table 7).
CoQ10 is a fat-soluble vitamin-like compound that can be supplied externally or produced endogenously (Ercan and El, 2011). CoQ10 content in meat samples ranges from 1.38 to 19.2 mg/100 g depending on the animal breed and cut (Kubo et al., 2008). Our results revealed the CoQ10 levels in the loin meat of the KHA group to be 1.30–1.70 mg/100 g, which was significantly higher than that in the KLA group on day 1 and 5 (p<0.05). During storage for 15 days, the CoQ10 levels in the loin meat of both groups did not change. Similarly, Purchas and Busboom (2005) reported that high-pasture-fed New Zealand cattle had higher CoQ10 content than high-concentrate-fed US cattle. CoQ10 can be endogenously synthesized by a process including the synthesis of the benzoquinone ring from tyrosine as a precursor (Bank et al., 2011). Although we did not perform a complete amino acid composition analysis for alfalfa and concentrate, alfalfa contains 3.3%–4.17% tyrosine (Brito et al., 2014; Giner‐Chavez et al., 1997; Kaldy et al., 1980), while concentrate for goat feed has 2.87% tyrosine (Zhang et al., 2020). We, therefore, postulated that the high tyrosine content in alfalfa might be related to CoQ10 synthesis in the loin meat of the KHA group.
L-carnitine contributes to energy production in mitochondria by shuttling long-chain fatty acids and is abundant in red meat (Kim et al., 2019). L-carnitine contents in loin meat from the KHA and the KLA groups were 2.80 and 3.29 μmol/g, respectively, with loin meat from the KLA group having a higher L-carnitine content than that from the KHA group on day 1, 5, and 10 (p<0.05). During storage, L-carnitine content increased on day 5 (p<0.05) and continuously increased until day 15. These results agree with those of Kim and Jang (2021), who reported higher L-carnitine levels in KNBG loin meat compared to that in crossbred black goats (1.37 μmol/g) (Kim et al., 2019), which were similar to those reported in beef round meat (2.64 μmol/g) (Kim and Jang, 2021). L-carnitine is endogenously synthesized in organs by using lysine and methionine as precursors (Sarica et al., 2007). According to previous research, alfalfa contains 6.1%–6.6% lysine and 1.8%–1.9% methionine (Brito et al., 2014; Kaldy et al., 1980). Meanwhile, concentrate in goat feed contains 5.63% and 1.09%, respectively (Zhang et al., 2020). We, therefore, postulated that the high lysine and methionine content in alfalfa may have contributed to the synthesis of L-carnitine in the loin meat from the KHA group.
Creatine and creatinine have important roles in energy metabolism, with creatine non-enzymatically converted to creatinine via dehydration and formation of a ring structure in muscles (Kim and Jang, 2021). In this study, the levels of creatine and creatinine in loin meat from the KHA and KLA groups were 171.39–192.63 and 1.67–3.96 mg/100 g, respectively, which was similar to previous results in black goat loin meat (187.87 mg/100 g creatine and 3.13 mg/100 g creatinine) (Kim et al., 2019). Creatinine content in loin meat from the KLA group was higher than that from the KHA group (p<0.05). However, creatine content did not differ significantly between groups. During storage, creatinine content increased in the meat from both groups, while creatine content decreased (p<0.05). Creatine is enzymatically synthesized from glycine and arginine in the kidneys and liver (Kreider et al., 2017). According to previous studies, concentrate contains significantly higher glycine and arginine contents at 4.9% and 7.6%, respectively (Zhang et al., 2020) compared to alfalfa at 3.7% and 5.3%, respectively (Giner‐Chavez et al., 1997). We, therefore, postulated that the high glycine and arginine content in concentrate may have contributed to creatine synthesis in the loin meat from the KLA group, which was then non-enzymatically transformed into creatinine.
Carnosine exhibits antioxidant and anti-glycation activities via chelating metal ions and scavenging reactive oxygen species (Mateescu et al., 2012). Carnosine content in the loin meat from the KLA group was 54.12 mg/100 g on day 1, which was higher than that in the KHA group (p<0.05). During storage, the carnosine content in meat from the KLA group decreased on day 15 (p<0.05), while that in the KHA group remained unchanged. Moreover, the content of anserine, the methylated form of carnosine, in meat from the KHA and the KLA groups was 52.98–54.99 mg/100 g on day 1, with no significant differences between the two groups during storage. Meanwhile, the anserine content decreased in the meat from the KLA group on day 15 (p<0.05), while that from the KHA group did not change. In comparison, carnosine content has been reported as 462 mg/100 g in pork loin (Kubo et al., 2008), 372 mg/100 g in beef (Mateescu et al., 2012), 65.25 mg/100 g in black goat loin (Kim et al., 2019), and 63.16 mg/100 g in chicken breast (Kim et al., 2019). Meanwhile, the anserine content in pork loin is 10.76 mg/100 g (Mora et al., 2007), 67 mg/100 g in beef (Mateescu et al., 2012), 81.93 mg/100 g in black goat loin (Kim et al., 2019), and 92.60 mg/100 g in chicken breast (Kim et al., 2020).
As carnosine (β-alanyl-l-histidine) and anserine (β-alanyl-l-methyl-l-histidine) are histidine-dipeptides, carnosine is synthesized from β-alanine and L-histidine (Qi et al., 2018). Increased histidine: lysine ratios (0.64) and histidine contents in diet increased the carnosine and anserine contents in chicken muscle and blood (Lackner et al., 2021). Although goats (ruminant) have a different amino acid metabolism than chickens (monogastric) (Teleni, 1993; Vernon, 1980), we postulated that the high histidine: lysine ratio in the concentrate (0.51; Zhang et al., 2020) helps improve the histidine-dipeptide contents in loin meat from the KLA group, compared to that in alfalfa (0.36; Kaldy et al., 1980). However, further analysis is required to evaluate the effect of histidine supplementation on carnosine and anserine contents, as well as the underlying metabolic mechanism in goat meat.
With an increase in storage time, antioxidant activities (DPPH and ABTS radical scavenging activities, FRAP activity) of loin meat from both groups decreased (p<0.05; Table 8), similar to the results of Kim and Jang (2021). Previously, the DPPH, FRAP, and ABTS activities of boiled pork were determined to be 10.58–13.65, 3.66–5.31, and 26.60–39.43 μmol TE/g dry matter, respectively (Gil et al., 2016), while the ABTS and FRAP activities of black goat loin were obtained as 12.90 and 15.92 μmol TE/g dry matter, respectively (Kim et al., 2019). These results imply that loin meat from the KLA group had higher ABTS radical scavenging activity than boiled pork and black goat meat, similar to beef loin.
In our study, loin from the KLA group had higher ABTS radical scavenging and FRAP activities compared to that from the KHA group on days 1 and 5 (p<0.05). In particular, loin meat from the KLA group had 1.5 times higher FRAP activity than that from the KHA group, throughout storage (p<0.05). It was reported that beef from pasture-fed cows in Argentina has a higher level of FRAP than that from grain-fed cows; however, ABTS+ radical scavenging activity did not significantly differ between the cows (Wu et al., 2008). However, vitamin E content and superoxide dismutase activity in loin meat from pasture-fed steers were higher than in those fed a mixed diet composed of silage, cattle-cake, and cereals mixture; however, glutathione peroxidase and OH radical scavenging activities were higher in loin meat from steers fed a mixed diet (Gatellier et al., 2004). Generally, grass and pasture contain many phytochemicals with high antioxidant activity (Gatellier et al., 2004). However, in this study, loin from the KLA group showed higher ABTS radical scavenging and FRAP activities than that from the KHA group. This activity was highly correlated with high carnosine, L-carnitine, and creatinine contents in the loin meat from the KLA group. Moreover, this phenomenon can be explained by the fact that concentrate diet is rich in polyphenols, such as phytic acid and pro-anthocyanidins, which can also trap OH radicals (Yang et al., 2002).
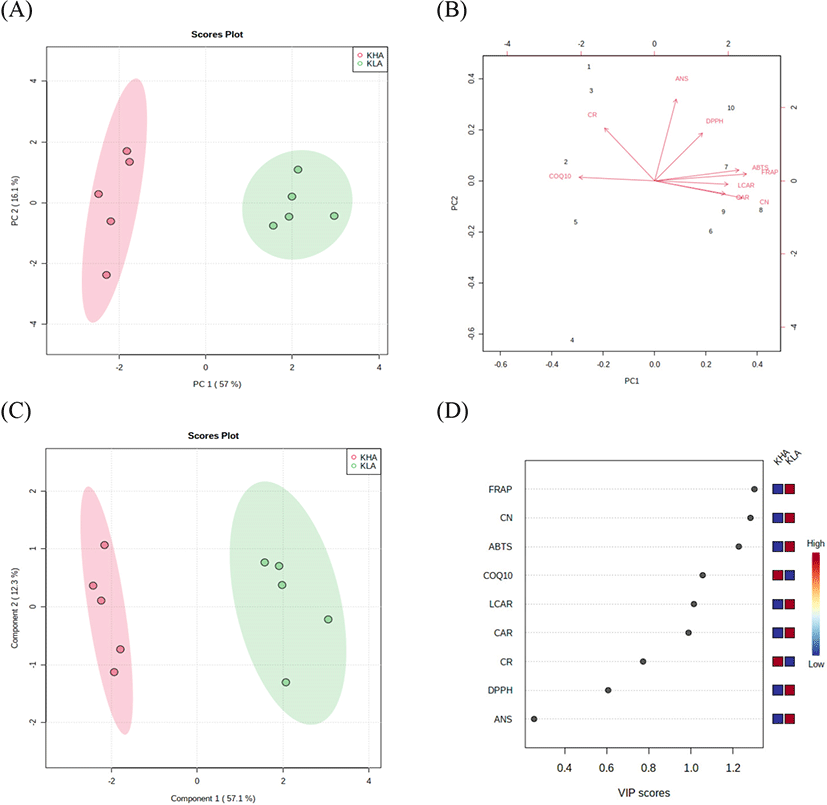
To elucidate the relationship between bioactive compounds and antioxidant activities in the KHA and the KLA groups, PCA and PLS-DA analyses were performed. The PCA plot showed a clear separation between the KHA and the KLA groups. Two principal components (PC1=57%, PC2=16.1%) accounted for 73.1% of the total variation (Fig. 1A). The KLA group was highly correlated with anserine, L-carnitine, carnosine, and creatinine contents and antioxidant activities (Fig. 1B). The PLS-DA score plot also showed a clear separation between the KHA and the KLA groups with a correlation coefficient (R2) of 0.97, an accuracy of 1.0, and a cross-validation correlation coefficient (Q2) of 0.92 (Fig. 1C). FRAP, creatinine, ABTS radical scavenging activity, L-carnitine, and carnosine were evaluated with high VIP scores in PLS-DA and showed high abundance in the KLA group (Fig. 1D). These results supported the correlation between high creatinine, L-carnitine, and carnosine contents and FRAP and ABTS radical scavenging activity in the loin meat from the KLA group. Research on the relationship between bioactive compounds and antioxidant activities in goat meat is associated with a limitation. However, in our previous study, bioactive compounds (carnosine and anserine) and the antioxidant activity of beef loin and round showed a significant positive correlation (0.572≤r≤0.931), which is consistent with our present study results.
Conclusion
In this study, we found that loin from the KLA group had high L-carnitine, creatinine, and carnosine content, suggesting that supplementation with high alfalfa for 90 days to finishing KNBG does not induce an increase in bioactive compounds within loin meat. Rather, supplementation with alfalfa and concentrate at a ratio of 2:8 increased the bioactive compounds in loin meat. We postulate that the various nutrients and certain amino acids contained in concentrates might contribute to the metabolism of bioactive compounds in KNBG; however, further research is needed to evaluate the quality and metabolites of bioactive compounds in KNBG meat and determine whether it is affected by the different dietary ratios of forage and concentrate.













