Introduction
Buffalo is one of the major meat producing animal in south-east Asia (Kandeepan et al., 2013). The compromised feeding, absence of specific meat breed combined with slaughtering of younger buffalo animals produces low muscle:bone ratio of buffalo carcasses in developing countries like Pakistan (Bilal et al., 2006; Purchas et al., 2002). However in commercial abattoirs, buffalo meat is rapidly chilled overnight and deboned at 24 h postmortem. Rapid chilling would benefit the industry by reducing the evaporative loss and growth of spoilage microbes. These benefits to the meat processor consequently, causing meat quality issues like cold toughening that affect the tenderness and color and decreases the consumer likeliness of buffalo meat (Kuffi et al., 2018; Locker and Hagyard, 1963). Numerous techniques have been used currently to avoid the development of cold toughening and to improve the meat tenderness. In this study, two methods have been tested to avoid this defect in buffalo.
Electrical stimulation (ES) minimized the detrimental effect of rapid chilling and improved the meat quality. ES causes faster depletion of adenosine-triphosphate (ATP), creatine phosphate (CP) and glycogen contents from postmortem muscles (Simmons et al., 2008). Therefore, ES avoids the cold toughening by accelerating the postmortem glycolysis and pH decline in postmortem muscles (Simmons et al., 2008). Furthermore, electrical current causes physical disruption of the myolemma that results in release of calcium ions from sarcoplasmic reticulum. Calcium ions then activates the calpain system that lead to proteolytic breakdown of myofibrillar protein, which increases tenderness of the meat (Mota-Rojas et al., 2012). In addition, many studies have shown the role of ES in improvement of beef color characteristics (McKenna et al., 2003).
Pelvic suspension (PS) technique also known as tenderstrech is the alternative method to avoid muscle shortening by hanging the carcass from obturator foramen of hip bone (Eikelenboom et al., 1998). Traditionally carcass is hanged using achilles suspension (AS) method. However in this method, vertebral column gets less stretch and become curved that causes shortening of muscles fiber and promotes cold toughening (Torrescano et al., 2003). However in PS method, sarcomere length of the muscles fiber is increased that helps to prevent the cold toughening. Many studies have reported the role of PS method in improvement of tenderness and water-holding capacity of meat (Ahnström et al., 2006; Wahlgren et al., 2002). Furthermore, suspension methods had different effect for each muscle type. Ahnström et al. (2012) studied the effect of different suspension methods on meat quality of five beef muscles and reported that tenderness of only two (longissimus dorsi and gluteus medius) muscles was improved by PS of bull carcasses. Moreover, PS increased the sarcomere lengths of semimembranosus, longissimus dorsi, gluteus medius and adductor muscles.
Previous studies examining the ES and suspension method were conducted on cattle and lamb animals (Eikelenboom et al., 1998; Kuffi et al., 2018; Simmons et al., 2008; Toohey et al., 2008). However, the effect of electric stimulation combined with suspension method to prevent the meat quality deterioration during rapid chilling of young buffalo bull is not clear in the literature. Therefore, the objective of current study was to investigate the role of electric stimulation combined with suspension method to prevent the detrimental effect of rapid chilling of young buffalo bulls.
Materials and Methods
A total of 24 water buffalo (Bubalus bubalis) young bulls were selected from Livestock Production and Research Institute Bahadurnagar, Okara, Pakistan, reared under same management conditions and feeding system. Animals were 18 months of age with an average carcass weight of 130 kg (SD=10). All the animals were transported to the University of Veterinary and Animal Sciences, Lahore, Pakistan under same transportation conditions. Animals were kept in lairage facility for one day to minimize the transportation stress. To ensure that meat was processed hygienically, animals were kept off-feed for 12 h before the slaughtering. After recording the live weight, animals were slaughtered in the morning at University commercial slaughter house facility following the Halal slaughtering guidelines described in Pakistan Halal Standards PS3733.
ES (100 V with 60 Hz) was performed using low voltage electrical stimulator (Model BV-80 Low Voltage Beef Stimulator, Jarvis Products Corporation, Middletown, CT, USA) that was connected to the whole carcass for 30 s within 15 min of exsanguination. Twelve of the 24 selected carcasses were electrically stimulated and tagged while rest of twelve were kept un-stimulated. After that all carcasses were bisected, one side of each carcass was hanged with pelvic suspension (PS) method while another side was hanged by AS method in the walk-in chiller operating at 0°C–4°C. After overnight chilling, both halves of stimulated or un-stimulated carcasses were transferred into the deboning hall operating at 10°C–15°C. Longissimus lumborum (LL) muscle of every half-carcass was removed between 12th thoracic and last lumbar vertebra at 24 h postmortem. From posterior end of LL muscles, three 2 cm steaks were removed to measure instrumental color. Then three 1 cm (with 50 g of weight) steaks were cut for moisture loss analysis. After that, three 3 cm thick steaks were separated for measurement of cooking loss and tenderness. All the meat quality attributes were measured in triplicate from both sides of stimulated or un-stimulated carcasses. A brief layout of experimental design was shown in Table 1.
The pH of the meat sample was measured with pH meter having meat penetrating probe (pH 3210 SET2, WTW, Germany) after calibration with buffers of pH 4.00 and 7.00. The pH was recorded between 12th thoracic and the first lumbar vertebra at 0 (within 20 min of exsanguination i.e., right after ES), 1, 3, 5, 7, 11, and 24 h postmortem.
For color measurement, meat samples were placed in food-grade trays such that the muscle fibers had a perpendicular orientation to the exposed surface. The samples were overwrapped with oxygen-permeable film and displayed in horizontal chiller at 0°C–4°C for 1 h of blooming. Then different parameters of color i.e., CIE L*(lightness), a* (redness), b* (yellowness) were recorded using colorimeter (Konica Minolta® CR-410, Osaka, Japan) from three random locations over the samples by avoiding the connective tissue and fat and averaged for statistical analysis. Before measurements, colorimeter was calibrated using the standard white tile CR-A44 at L*=94.93, a*=–0.13, b*=2.55 and C=2.55. The color was measured at 1, 2, 3, 4, 5, 6, and 7 d postmortem.
For cooking loss, meat samples were weighed using portable weighing scale (SF-400, Yongkang ZhezhongTM, Ningbo, China), vacuum packaged (Multivac® Baseline P-100, Geprüfte Scherhert, AGW, Germany) by using bags (SR 150×200, PA/PE 90, Dalziel®, Bellshill, Scotland) and placed in a water bath (WNB45, memmert®, Schwabach, Germany) working at 80°C. Samples were drawn out of the water bath when the core temperature of 72°C was achieved by following the methods of Ijaz et al. (2020). After this samples were placed at room temperature (20°C) for 45 min and then patted dry with a hand towel and reweighed to calculate the cooking loss. The cooking loss was calculated using the following formula:
The cooked meat samples were cut down into cubes of 1 cm×1 cm×6 cm along the direction of muscles fiber using scalpel handle blades. Warner-bratzler shear force (WBSF) values were measured by shearing the cubed under V-Slot blade of Texture Analyzer (TA.XT plus® texture analyzer, Godalming, UK). Before measurement, Texture Analyzer was calibrated with 1 kg weight, at 50 mm distance of return, with 10 mm/s speed of return and an 8 g contact force. The WBSF values were measured in Newton (N/cm2) as the peak force needed to shear the cubes perpendicular to direction of muscle fibers. WBSF values were taken from at least three cubes and averaged to calculate the tenderness of the samples.
Meat moisture loss was measured using suspension technique by following the methods of Kim et al. (2015). Samples were weighed and hung in polystyrene bags in display chiller (ALVO, MD-12, Technosight®, Lahore, Pakistan) for 48 h at 4°C. After this samples were blotted dry using a paper towel and reweighed again to measure the moisture loss. The moisture loss was calculated using the following formula:
Statistical analysis was carried out using Statistical Analysis System (SAS) ver. 9.1 (SAS Institute Inc., Cary, NC, USA). Data were analyzed using MIXED procedure with ES, suspension method and their interactions as fixed effects and animal as random effect. The level of significance was calculated using Duncan’s Multiple Range test and p<0.05 was considered significant. The data were presented as means±SE.
Results
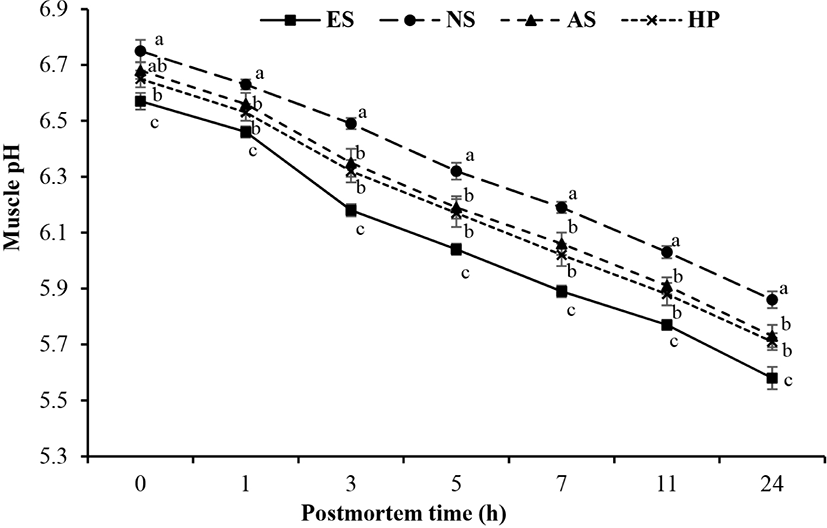
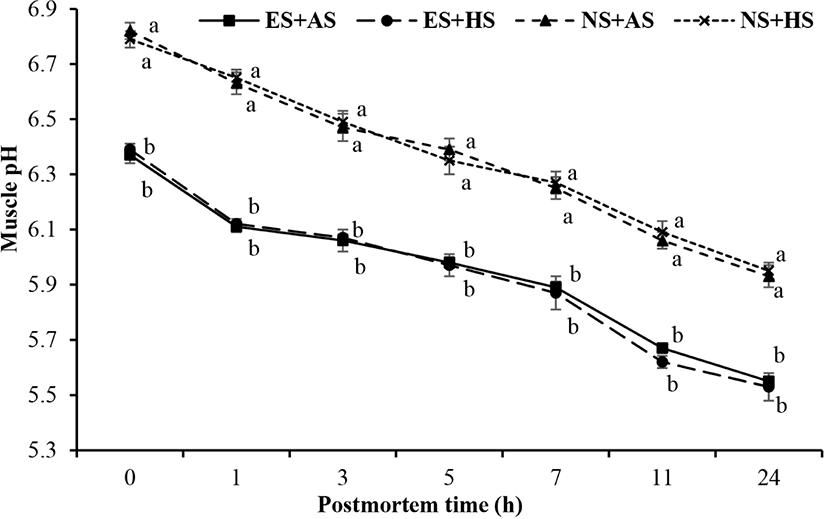
The decline of pH of young buffalo carcasses treated with different electric stimulation and suspension methods is shown in Fig. 1. Results indicated that ES of buffalo calves exhibited rapid pH decline compared to NS carcasses (p<0.05). However, there was no any difference of pH decline between AS and hip suspension (HS) methods (p>0.05). Interaction effects of ES and suspension methods on rate of pH decline are presented in Fig. 2. It showed that rate of pH decline of ES combined with AS (ES+AS) was same with the ES combined with HS (ES+HS), however, higher than that of the non-stimulation combined with achilles (NS+AS) and HS (NS+HS) methods (p<0.05). Overall, results showed that ES had strong effect on rate of pH decline compared to suspension method.
Meat shear force values of ES and suspension methods are shown in Table 2. ES carcasses displayed significantly (p<0.05) lower shear force value compared to the NS carcasses. Meat shear force value of HS method were significantly (p<0.05) lower as compared to AS method. Shear force showed significant interaction (p<0.05) between ES and suspension methods and their interactions are further explored. Interestingly, shear force values of ES together with HS method (ES+HS) were lowest (33.06), however, non-stimulated (NS) along with AS (NS+AS) produced highest (40.86) shear force values (p<0.05).
Meat cooking and moisture losses of ES and suspension methods are shown in Table 2. Results indicated that cooking loss as well as moisture loss were non-significant (p>0.05) between stimulation method and suspension method. Similarly, interactions of stimulation and suspension methods were also non-significant (p>0.05).
Meat color parameters of ES and suspension methods are shown in Table 2. Results revealed that ES significantly (p<0.05) increases the color CIE L* (lightness), a* (redness) and b* (yellowness) values as compared to NS meat. Whereas, color L*, a*, and b* values were similar between achilles and HS methods (p>0.05). The interactions of ES with AS and HS were non-significant, similarly, interactions of non-ES with suspension methods were also similar (p>0.05) for all color parameters (L*, a*, and b*). However, interactions of ES with suspension methods presented significatly (p<0.05) higher L*, a*, and b* values as compared to the interactions of non-ES with the supension methods. It showed that ES has substantial effect on meat color than that of the suspension methods. The results of stimulation and suspension methods and their interaction on 2, 3, 4, 5, 6, and 7 d postmortem were non-significant for color CIE L*, a*, and b* and presented in Table 3.
Discussion
Present study explained that electrically stimulated carcasses showed rapid pH decline as compared to NS carcasses, as a result of this cold shortening of the young buffalo meat can be avoided (Davey et al., 1976). These results were similar with the findings of Cross (1979) and Honikel et al. (1983). This may be due to the fact ES causes faster depletion of ATP, CP, and glycogen from muscles by accelerating the postmortem glycolysis which leads to the rapid pH decline in postmortem muscles fibers (Simmons et al., 2008). When the carcass is electrically stimulated, ATP level is depleted, which is required for the contraction of muscle structure so severe contraction of muscle or cold shortening is avoided, as a result of this tenderness of meat is enhanced (Dutson et al., 1980). On other the hand, rate of pH fall of achilles and HS methods were same. Ahnström et al. (2012) and Hou et al. (2014) explained the same results in their study that suspension methods did not affect the pH value.
WBSF represents the tenderness of meat, higher the WBSF values lower will be th tenderness of meat. ES enhances the tenderness of meat by significantly reducing the shear force values. Similar findings were also found by Aalhus et al. (1994) and Simmons et al. (2008), they noted the lower shear force value of electrically stimulated compared to NS carcasses. Geesink et al. (2006) explained that ES enhances the tenderness of meat by accelerating the postmortem proteolysis. The acceleration in postmortem proteolysis is primarly due to the increased activity of μ- and m-calpain. ES increases intracellular calcium level, which is required for initiating the proteolytic activity of calpain system, especially μ-calpain. Therefore, ES enhances the tenderness of meat by accelerating the degradation of myofibrillar and cytoskeleton structure (titin, nebulin and desmin), which are responsible for structural integrity of myofibril lattice (Soria and Corva, 2004). Furthermore, ES increases the physical disruption of cells and helps the release of lysosomal proteases like proteolytic cathepsins and calpains into the cytosol, which again favor the enhancement of meat tenderness (Dutson et al., 1980). Additionaly, ES leads to rapid pH decline and helps to prevent the determinental effect of cold toughening. On the other hand, HS significantly lowers the shear force value of the carcasses. These findings were also reported in the literature (Bayraktaroglu and Kahraman, 2011; Wahlgren et al., 2002). Ahnström et al. (2012) explained in his study that HS improves the tenderness of meat about 15%–40%. Stretching during HS method results in the reduction of adhesion between myofilaments and decrease connective tissue strength, so shear force value is decreased (Liu et al., 2016).
ES did not show any effect on cooking and moisture losses. These observations were also found in previous studies (Derbyshire et al., 2007; Strydom et al., 2005). ES induced fast pH decline and earlier activation of proteolytic enzymes in postmortem muscles. The fast pH decline accelerated the reduction in net negative ions and lactate ions (CH3CHOO−) act as anionic chaotrope that would weaken the interaction between proteins and water molecules (Fujita et al., 2007; Li et al., 2011). Moreover, the establishment of actomyosin bond during rigor development could decrease the space between myofilaments (Offer and Cousins, 1992). All these processes could favor the decrease of water-holding capacity in the postmortem muscles. In contrast, early activation of proteolytic enzymes could degrade the myofibrillar proteins that could help to increase the space between myofilaments to hold the water in myofibres (Huff-Lonergan et al., 2005). As a result, the overall effect of ES on water-holding capacity of buffalo meat remained negligible. Similarly, HS method had no effect on cooking and moisture losses that was supported by Ahnström et al. (2012) and Strydom et al. (2005). Derbyshire et al. (2007) explained that suspension methods do not affect the meat losses, because the rate of pH fall and proteolysis remained the same in hip and AS methods.
ES increased the color L*, a*, and b* values of the meat as compared to NS meat. Similar findings were also reported by Li et al. (2011) and Toohey et al. (2008). Nazli et al. (2010) revealed that ES leads to rapid acidification and denaturation of myofibrillar proteins, both result in more reflectance of light from the meat surface, which increased the color lightness (L*) of meat. Higher rate of postmortem proteolysis in electrically stimulated meat lead to weakening of ultra-structure of myofibers that adversely affect the actomyosin bond and allows the oxygen to penetrate deeper into the muscles, which produced a thick layer of oxymyoglobin and increased the color redness (a*) value (Toohey et al., 2008). Conversely, meat color was not affected by the suspension methods. Color is primarily depends upon rate of pH decline and protein degradation, which remained same between hip and AS methods (Bayraktaroglu and Kahraman, 2011). In the current study, ES and suspension methods did not affect the color parameters during 2 to 7 d of postmortem storage of buffalo meat. Li et al. (2011) explored the effect of low-voltage ES on color stability of bovine muscles and reported that ES increased the color a* values at 24 h postmortem but it did not affect the color stability, which is in agreement with the current study. On the other hand, Hou et al. (2014) studied the impact of suspension methods and ageing time on meat quality of beef. They reported that color L*, a*, and b* values at 1 day were similar with 7 d postmortem and suspension methods did not show any significant effect on color stability during first 7 d of postmortem storage.
Conclusion
The results of this study showed that ES increased the rate of pH decline, improved the tenderness and color of buffalo meat. Furthermore, HS had no impact on pH, water-holding capacity and color of meat, however, it increased the tenderness. It is recommended that the local meat industry should adopt such post-slaughter technologies i.e., ES in combination with HS to improve the meat quality and to prevent the detrimental effects of postmortem chilling of young buffalo bulls.













