Introduction
Pink color defect is a quality issue that causes the uncooked pink appearance observed in fully cooked, uncured poultry meat products (Suman and Joseph, 2014). Although not a safety issue, it is recognized as a quality issue which is economically detrimental to meat processors (Friesen and Marcy, 2000). Many factors are responsible for the pink color of cooked meat, including nitrite or nitrate contamination, types and states of meat pigments, use of non-meat ingredients, cooking temperature, storage conditions, and irradiation treatment (Ahn and Maurer, 1987; Ahn and Maurer, 1989c; Claus and Jeong, 2018; Claus et al., 1994; Cornforth et al., 1998; Helmke and Froning, 1971; Howe et al., 1982; Mugler et al., 1970; Nam and Ahn, 2002a; Nam and Ahn, 2002b; Nash et al., 1985). Due to the numerous factors involved and the sporadic occurrence of pink color defect, previous studies attempted to eliminate or reduce the pink color defect using non-meat ingredients (Dobson and Cornforth, 1992; Kieffer et al., 2000; Sammel and Claus, 2003a; Sammel and Claus, 2003b; Sammel and Claus, 2006; Sammel and Claus, 2007; Schwarz et al., 1999; Slesinski et al., 2000a; Slesinski et al., 2000b). These studies indicated that some non-meat ingredients were effective in reducing the pink color in the cooked products which resulted from the addition of pink color generating ligands (nitrite or nicotinamide) to the meat system. Slesinski et al. (2000a) reported that nonfat dry milk reduced CIE a* values in cooked turkey breasts containing 10 ppm sodium nitrite, while dairy proteins reduced CIE a* values in nicotinamide-treated samples. Sammel and Claus (2003a) found that 2% to 3% citric acid and 1.0% sodium citrate reduced the pink color developed by sodium nitrite and nicotinamide in ground turkey rolls, but unaffected the pink color of intact turkey breasts. However, these studies did not mimic the practical processing conditions that generated the naturally occurring pink color without pink generating ligands. Therefore, Claus and Jeong (2018) studied which conditions produced the most intensive pink color by simulating industrial processing conditions via salt addition and storage to generate pink color naturally.
Phosphates, along with sodium chloride, are widely applied in the meat industry to increase the capacity of meat proteins for binding and retaining water (Petracci et al., 2013; Sebranek, 2009). Phosphates affect the behavior and denaturation of meat pigments such as myoglobin, hemoglobin, and cytochrome c during cooking. Ahn and Maurer (1989c) reported that phosphate increased the heat stability of myoglobin and decreased that of cytochrome c by increasing pH, whereas sodium chloride increased the heat stability of myoglobin and hemoglobin significantly. Thus, addition time of sodium chloride and phosphate to ground chicken breast may affect the naturally occurring pink color in cooked products. Cooking may influence hemochrome formation and reducing conditions, contributing to the pink color of cooked meat. Cornforth et al. (1986) found that nicotinamide denatured globin hemochromes caused the pink defect in cooked turkey rolls. They also reported that reducing conditions promoted hemochrome formation, while oxidizing conditions prevented it. Jeong and Claus (2010) determined the ability to eliminate pink color associated with the storage of presalted ground turkey with and without phosphate using pink inhibiting ligands at different cooking rates. They found that a slow cooking rate reduced CIE a* values of cooked turkey breast in absence of phosphate compared to a fast cooking rate, but the opposite result was observed in the presence of phosphate. It is hypothesized that both the addition of NaCl and phosphate and time of addition will affect the color and pigment characteristics related to the pink color defect at different cooking rates. Therefore, this study investigated the occurrence of pink color without pink generating ligands in cooked chicken breast, due to timing of NaCl and phosphate addition and different cooking rates.
Materials and Methods
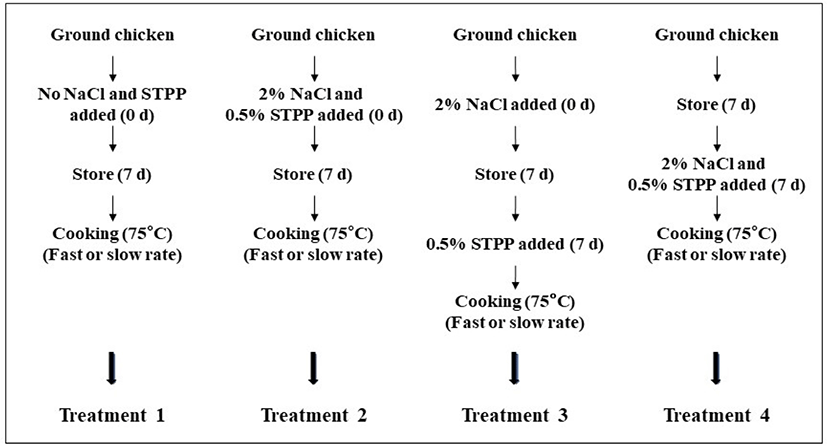
Fresh, skinless, and boneless chicken breasts (1 d postmortem) were obtained from a local processor (Kwangsung Food, Yangsan, Korea). Raw material was shipped in an insulated cooler and refrigerated (2°C–3°C) until used. Three separate replications of ground chicken breast were received and used immediately after arrival in this study. A total of 15 kg of raw chicken trimmings were used for each replication and ground using a chopper with a 0.3 cm plate (TC-22 elegant plus, Tre Spade, Valperga, Italy). The ground meat was randomly separated into four individual bathes (3 kg each) depending on the addition timing of NaCl and sodium tripolyphosphate (STPP) which included (Fig. 1): treatment 1, no NaCl and STPP added and stored for 7 d; treatment 2, NaCl+STPP added on 0 d and stored for 7 d; treatment 3, NaCl added on 0 d and STPP added on 7 d; and treatment 4, stored for 7 d and NaCl+STPP added. Except for treatment 1, at the time of salt addition (d 0, treatments 2 and 3; d 7, treatments 3 and 4), the ground meat was mixed with 2% NaCl and/or 0.5% STPP of meat weight basis using a mixer (5K5SS, Whirlpool, St. Joseph, MI, USA) for 5 min before being individually vacuum-packaged into polyethylene/nylon bags using a vacuum packaging machine (M6-TM, Leepack, Incheon, Korea) and stored under refrigeration (2°C–3°C) prior to being remixed and stuffed (Fig. 1). After storage for 7 d, ground meat was remixed using a mixer (5K5SS, Whirlpool) for 5 min and then stuffed into conical centrifuge tubes (50 g each). These tubes were centrifuged at 2,000×g for 10 min (FELTA5, Hanil Science, Gimpo, Korea) to remove air pockets. The tubes from each batch were further separated into two groups (fast cooking rate or slow cooking rate). The fast cooking (5.67°C/min) was achieved by loading the tubes into a preheated 90°C water bath (CB60L, Dongwon Scientific Machinery, Busan, Korea) and cooked to an internal endpoint temperature of 75°C. The slow cooking (2.16°C/min) was achieved by loading the tubes into a 50°C water bath and then immediately setting the water bath to 90°C. The temperature was monitored by randomly placing four thermocouples attached to a 4-channel digital thermometer (Tes-1384, Ketech Scientific Instrument, Kaohsiung, Taiwan) in the center of extra samples throughout the water bath. After cooking, samples were immediately cooled on ice for 20 min and stored at 2°C–3°C overnight in the dark until further analysis. Experiments were replicated three times.
A colorimeter (CR-400, 8 mm aperture, illuminant C; Konica Minolta, Osaka, Japan) calibrated with a white plate (L* 94.90, a* −0.39, b* 3.88) was used for determination of the CIE L*, a*, and b* values and measured freshly cut surfaces of each cooked sample following immediately cutting.
Stuffed ground chicken meat samples were weighed prior to cooking to determine raw sample weights. Cooked weights were also measured to determine cooking yields. Cooking yield was calculated as: [cooked sample weight/raw sample weight]×100. The sample (5 g) was homogenized in 25 mL of distilled water and pH values were measured with a pH electrode attached to a pH meter (Accumet AB50, Thermo Fisher Scientific, Singapore). ORP was measured for cooked chicken products following the method of Cornforth et al. (1986) and John et al. (2005) with slight modifications.
Myoglobin (Mb) was extracted from both uncooked and cooked chicken breasts using a procedure of Warriss (1979) and Trout (1989). The extracted supernatants were further clarified by filtration using Whatman No. 1 filter paper. The absorbance (A) of the filtrate was subsequently determined at 525, 572, and 700 nm (Krzywicki, 1979) using a UV/VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The total Mb content and PMD were calculated using the following formulas (Trout, 1989): Mb (mg/g)=(A525–A700)×2.303×dilution factor; PMD=[1–(Mb concentration after heating/Mb concentration before heating)]×100. To obtain the percentage reflectance, the absorbance data on the filtrate from 400 to 700 nm were converted to percentage reflectance using the equation described by Stewart et al. (1965). Nitrosyl hemochrome (rNIT) was estimated using the percent reflectance ratio, %R650 nm/%R570 nm (AMSA, 1991). Nicotinamide hemochrome (rNIC) was estimated by the percent reflectance ratio of %R537 nm/%R553 nm (Schwarz et al., 1998).
The experimental design was split plot with four different treatments (addition timing of NaCl and STPP) representing the whole plot factor and two cooking rates (fast cooking and slow cooking) representing the split plot factor. The main effects for addition timing of NaCl and STPP and cooking rate, and their interactions were analyzed using Proc Mixed procedure of SAS 9.4 software (SAS, 2013). When significance (p<0.05) was found in the models, means was separated by pairwise comparisons using the PDIFF option. The experiment was replicated three times.
Results and Discussion
The significance of main effects, addition time of NaCl and STPP (T) and cooking rate (C), and their interaction (T×C) on color and pigment properties of cooked ground chicken breasts is presented (Table 1).
2) Dependent variables: CIE L* (lightness), CIE a* (redness), CIE b* (yellowness), rNIT (reflectance estimator of nitrosyl hemochrome, %R650nm/%R570nm), rNIC (reflectance estimator of nicotinamide hemochrome, %R537nm/%R553nm, higher ratio more), ORP (oxidation-reduction potential), Myoglobin (amount of undenatured myoglobin), and PMD (percentage myoglobin denaturation).
The effects of addition time of NaCl and STPP and cooking rate on color, rNIT, and rNIC of cooked ground chicken breasts are shown (Table 2). The CIE L* values of cooked ground chicken breasts were affected by the addition time of NaCl and STPP (T, p<0.0001) and cooking rate (C, p<0.0001; Table 1). Regardless of addition time of NaCl and STPP, treatment 2, 3, and 4 had lower CIE L* values (p<0.05) compared to treatment 1 (Table 2). Sammel and Claus (2007) found that adding 0.25% or 0.5% STPP to meat reduced lightness, compared to cooked ground turkey without STPP. This is similar to our finding. The increase in lightness in treatment 1 may be due to relatively lower pH, which causes light-scattering effects in meat (Nam and Ahn, 2002b). Fast cooking rates resulted in higher CIE L* values than slow cooking rates. Both treatment (T) and cooking rate (C) affected CIE a* values (p<0.05) in cooked ground chicken breasts (Table 1). CIE a* values (redness) of ground chicken products were 3.49, 3.23, 3.34, and 3.62 for treatment 1, 2, 3, and 4, respectively (Table 2). Reported CIE a* values ranged from 3.59 to 4.25 for cooked ground chicken breasts without the addition of pink generating ligands (Bae et al., 2020). Bae et al. (2020) found that adding a combination of NaCl and STPP to ground chicken breasts lowered CIE a* value than adding only NaCl to meat (CIE a* 3.86 vs 4.18). Addition of NaCl and STPP on 0 d and storing for 7 d (treatment 2) lowered pink color in cooked chicken (p<0.05), reducing redness (lower CIE a* values) compared to that of treatment 1 (7.4% reduction) and treatment 4 (10.8% reduction). Reduction of CIE a* values was likely as STPP was added to treatment 2 earlier than in other treatments during pre-salting, resulting in it acting as an iron biding agent, which bound heme iron of myoglobin, thus competitively inhibiting pink generating ligands from binding myoglobin (Sammel et al., 2006; Sammel and Claus, 2003a; Sofos, 1986). Cooked product showed the most (p<0.05) redness (highest CIE a* values) in ground chicken stored for 7 d and treated with NaCl and STPP (treatment 4). Fast cooking of ground chicken meat reduced redness more effectively than slow cooking (p<0.05). Jeong and Claus (2010) reported that CIE a* values of cooked turkey breasts following 6 d of pre-salting in the presence of STPP was significantly lower with a fast cooking rate compared to a slow cooking rate, whereas the opposite was true in the absence of STPP. CIE b* values of cooked ground chicken breasts were influenced by the treatments (T, p<0.0001; Table 1). Treatment without NaCl and STPP (treatment 1) showed higher (p<0.05) yellowness than treatments 2, 3, and 4 (Table 2). Similar results were found by Ahn and Maurer (1989a), where the highest yellowness of oven-roasted turkey breast was due to a combination of 0% NaCl and 0.5% STPP, while the lowest was via 2% NaCl and 0.5% STPP. Treatment 3 and 4 had lower CIE b* values compared to treatment 2 (p<0.05), but were similar to each other (Table 2). It was likely due to the addition timing of STPP, wherein STPP was added to ground chicken on 7 d and immediately cooked (treatments 3 and 4) although the addition time of NaCl differed. However, cooking rate (C) did not affect CIE b* value in cooked ground chicken (p>0.05).
1) Dependent variables: CIE L* (lightness), CIE a* (redness), CIE b* (yellowness), rNIT (reflectance estimator of nitrosyl hemochrome, (%R650nm/%R570nm, higher ratio more), and rNIC (reflectance estimator of nicotinamide hemochrome, %R537nm/%R553nm, higher ratio more).
2) Treatments: (1) no salt added, stored for 7 d before being cooked; (2) NaCl and STPP added on 0 d and stored for 7 d before being cooked; (3) NaCl added on 0 d, stored for 7 d, STPP added on 7 d, and then cooked; (4) stored for 7 d before NaCl and STPP added on 7 d, and then cooked.
Nitrosyl hemochrome is a heat-stable pigment of cured meat induced by nitrite reacting with meat myoglobin upon cooking. Nitrite is one of the causes of pink color in cooked products (Holownia et al., 2003), a small amount of nitrite is naturally present in chicken (0.07 ppm) and turkey (0.7 ppm) breasts (Ahn and Maurer, 1987; Claus and Jeong, 2018). In this study, the rNIT ratio, reflectance estimator of nitrosyl hemochrome, was influenced by treatment (T) (p<0.05; Tables 1 and 2). Treatments 3 and 4 had higher rNIT ratios than treatment 1 (p<0.05), but did not differ from that of treatment 2. Generally, acidic conditions are favorable for formation of NO from NO2 in meat (Ahn and Maurer, 1989a), promoting nitrosyl hemochrome formation in cooked products. The higher rNIT ratio in products treated with NaCl and STPP may be due to an increase in pH resulting from STPP addition, because higher pH is favorable for hemochrome formation (Sammel and Claus, 2007; Trout, 1989). Bae et al. (2020) reported that cooked chicken breasts with 2% NaCl and 0.5% STPP had significantly higher rNIT ratios than those with NaCl alone. However, cooking rate had no effect on the rNIT ratio in cooked ground chicken breasts (p>0.05; Table 2).
The treatments (T) had no effect on the rNIC ratio, the reflectance estimator of nicotinamide hemochrome (p>0.05; Tables 1 and 2). The rNIC ratios of cooked ground chicken were similar for both cooking rates (p>0.05). Reportedly, nicotinamide hemochrome is a pigment potentially involved in the pinking defect of cooked, uncured turkey (Schwarz et al., 1998). Claus and Jeong (2018) reproduced a pink color defect (natural pink) without adding a pink-generating ligand in fully cooked ground turkey, by adjusting addition timing of salt and fresh meat storage before cooking. They suggested that the presence of salt and storage of turkey meat promoted conditions associated with reduced nicotinamide-denatured globin hemochrome formation. This discrepancy between our results and those of others may be due to inherent levels of nicotinamide in meat, because nicotinamide is naturally present in turkey meat at a higher level than in other meats (Schwarz et al., 1997).
The effects of addition time of NaCl and STPP and cooking rate on cooking yield, pH values, ORP, myoglobin content, and PMD of cooked ground chicken breasts are presented (Table 3). Cooking yield of ground chicken breasts was affected by treatments (T, p<0.0001) and cooking rates (C, p<0.05; Tables 1 and 3). Regardless of addition time, treatments with NaCl and STPP (treatments 2, 3, and 4) resulted in greater cooking yields compared to treatment 1 without NaCl and STPP (Table 3). This was due to NaCl and STPP functions (Petracci et al., 2013; Sebranek, 2009). Ground meat cooked at fast cooking rates resulted in greater cooking yields than at slow cooking rates. This higher cooking yield may be caused by shorter cooking time (Bigner-George and Berry, 2000).
1) Dependent variables: ORP (oxidation-reduction potential), Myoglobin (amount of undenatured myoglobin), and PMD (percentage myoglobin denaturation).
2) Treatments: (1) no salt added, stored for 7 d before being cooked; (2) NaCl and STPP added on 0 d and stored for 7 d before being cooked; (3) NaCl added on 0 d, stored for 7 d, STPP added on 7 d, and then cooked; (4) stored for 7 d before NaCl and STPP added on 7 d, and then cooked.
Treatments (T) affected the pH of cooked ground chicken breasts (p<0.0001; Table 1). The pH values in treatment 1 were lower than those in treatments 2, 3, and 4 (p<0.05), but were not different from each other (Table 3), regardless of addition timing of NaCl and STPP. The higher pH in samples with NaCl and STPP may be due to the basic pH of STPP (Petracci et al., 2013). In the present study, cooking rates had no effect on the pH of cooked ground chicken breasts (p>0.05).
The ORP values of cooked ground chicken breasts were affected by the treatment (T, p<0.05; Tables 1 and 3). Treatment 2, 3, and 4 had more negative ORP values (more reducing condition) than treatment 1 (p<0.05), while there were no differences between ORPs of cooked ground chicken products, regardless of addition timing of NaCl and STPP (p>0.05). The ORP is affected by processing ingredients such as salt and phosphate (Holownia et al., 2003). Ahn and Maurer (1989b) reported that ORP was reduced by adding salt and phosphate, which would agree with our result. ORP values are pH dependent, thus affecting the formation of reduced globin hemochrome, which promotes the pink color of meat products (Cornforth et al., 1986; Trout, 1989). Increased pH due to STPP addition may result in generating more reducing conditions (Antonini and Brunori, 1971). In the current study, these effects were expected from treatments 2, 3, and 4 containing STPP due to their higher pH values. In addition, vacuum packaging may cause an additional decrease in the oxidation-reduction potential (ORP) of meat (Nam and Ahn, 2002a; Nam and Ahn, 2002b). Therefore, the more negative ORP vales of STPP added products (treatment 2, 3, and 4) may be related to increased pH caused by the addition of STPP and vacuum packaging during processing prior to cooking. However, no differences were found between ORP values of cooked samples subjected to fast cooking and slow cooking (p>0.05; Table 3).
Treatment (T) and cooking rate (C) did not affect myoglobin content of cooked ground chicken breasts (p>0.05; Tables 1 and 3). However, treatment (T) affected percentage myoglobin denaturation (PMD) in cooked ground chicken breasts (p<0.05; Tables 1 and 3). Treatment 1 had a higher PMD than treatments 2, 3, and 4 (Table 3). Regardless of addition time of NaCl and STPP, no differences (p>0.05) in PMD were observed across treatment 2 to 4. Similarly, Holownia (2004) reported that the presence of STPP with NaCl reduced the percentage of denatured myoglobin in cooked chicken breasts. In this study, products with NaCl and STPP having less PMD may be due to the phosphate effect on myoglobin denaturation rather than the salt effect, and may be explained via heat stability of meat pigments caused by processing ingredients. Ahn and Maurer (1989c) found that although salt addition destabilized myoglobin, STPP addition greatly increased the heat stability of myoglobin in meat pigments. They speculated that when both salt and phosphate were added, the stabilizing effects of phosphate on myoglobin became greater than the destabilizing effects of salt on myoglobin. PMD in cooked samples between both cooking rates was not different (p>0.05; Table 3).
Conclusion
Treatment conditions (addition time of NaCl and STPP) exerted an effect on the development of pink color in cooked ground chicken breasts. The most intense red color in cooked products was found when ground meat was stored for 7 d before adding NaCl and STPP (treatment 4). On the other hand, adding NaCl and STPP to ground chicken breasts and storing for 7 d before cooking (treatment 2) reduced the pink color in cooked products. Furthermore, cooking at faster rates than at slower rates resulted in lower CIE a* value of chicken products. Therefore, addition of NaCl and STPP to meat, followed by storing and cooking at a fast rate inhibited the sporadically developing pink defect in cooked chicken products more effectively.













