Introduction
Chicken meat is widely consumed in Southeast Asia considering as traditional healthy food (Nagai et al., 1996). Over the past few decades the per capita of poultry meat has been increased by approximately 3 kg based on report of the Korean Ministry of Agriculture and Forestry. Despite of increasing circumstances, the Korean native chickens have not been produced sufficient amount because of the limited productivity with desirable characters of meat.
Some bioactive endogenous compounds such as carnosine (β-alanyl histidine), anserine (1-methyl carnosine) in the skeletal muscle of vertebrate animals was considered as strategy for poultry production (Schmid, 2009). Mammalian skeletal muscles rich with carnosine act as a pH buffer in muscles, antiglycation, antiaging, antioxidation and neurotransmitter functions (Schmid, 2009). The color and flavor of meat depends on oxidative mechanisms (Ouali et al., 2006). Carnosine combination with dietary vitamin E supplementation improves the lipid stability in meat (Morrissey et al., 1998). Carnosine also suppresses some diseases such as diabetes, cataracts, ischemia, and Alzheimer’s diseases (Hipkiss, 2009) and attenuates acidosis as well as loss of force during exercise in human body (Baguet et al., 2010). Carnosine is an activator of several enzymes including calpain II, myofibrillar ATPase and phosphorylase (Iwaniak and Dziuba, 2009). Anserine (1-methylated carnosine) derivate from carnosine perform similar functions as like carnosine (Schmid, 2009).
Reducing sugars are important addition in meat in term of flavor react with amino acids by Maillard reaction during cooking (Mottram and Maarse, 1991). Maillard reaction occurs between an amino compounds (amine, amino acid, peptide, or protein) and a carbonyl compounds (usually a reducing sugar), the thermal degradation of thiamin, the oxidation of lipids, and the interactions between these pathways. The reaction of sugar and cysteine can lead to characteristic meat flavour specially for chicken (Varavinit et al., 2000). This was further confirmed by a research where the quantities of carbohydrates and amino acids, in particular ribose and cysteine, are reduced during heating. The main carbohydrates with flavour-forming potential include ribose, ribose-5-phosphate, glucose and glucose-6-phosphate (Meinert et al., 2009). The addition of small quantities of ribose in chicken meat has been shown to enhance the quantities of key odor compound as well as meaty and roasted flavor (Aliani and Farmer, 2005).
Containing all essential amino acids, fatty acids, vitamins, mainly B complex vitamins and especially B12; minerals, mainly iron and zinc and other bioactive compounds meat and meat products are very familiar as high nutritious foods (WHO, 2003). Free amino acids, nucleic acids, peptides, and minerals are the compounds involved in meat flavor (Yamaguchi, 1991). Dietary cholesterol is strongly associated with coronary heart disease and arteriosclerosis that causes human mortality (Simopoulos, 2004). Recently many attempts has been taken to decrease the cholesterol contents of chicken meat adding dietary supplements like omega-3, garlic, and copper (Chowdhury and Smith, 2002). Chicken meat is a great source of many functional compounds and micronutrients i.e. iron, vitamin B12 as well as zinc, B complex, selenium and phosphorus (Pereira and Vicente, 2013). Chicken meat contains of many minerals (Zn, Fe, and Cu) are essential for life and also may attribute for consumer satisfaction (Ribeiro et al., 2019).
Recently attempts has been taken by Korean government to develop new breed of chicken. This project mostly focused on developing the meat producing chicken breed with high quality traits by using Korean native chickens in order to meet the consumers demands (Park et al., 2012). Three new native chicken strains (i.e., 2A, 2C, and 2D) were anticipated as candidates for selection considering their growth rate and eating qualities. The aim of this study was to investigate the concentration of health promoting compounds such as antioxidant dipeptides, reducing sugar, and micronutrients contents of three new native chicken strains compared with commercial Korean native chicken and broiler to pick up the data which will be helpful for future development of breeding selection strategies for poultry meat production.
Materials and Methods
Totally 200 male chicks from a commercial native chicken (HH) and three newly bred native chicken strains (2A, 2C, and 2D) were reared up to 12 wk at an experimental farm of Harim corporation (Gimje, Korea) under similar conditions and diets. The new native chicken strains were bred by mating combinations from a paternal line (2) and 3 different maternal lines (A, C, and D). Broilers were also reared up to reach at similar slaughter weight with those HH and new native chicken strains. HH and broiler were considered as controls. Native chicken, three newly bred native chicken strains were slaughtered with those broiler with similar live weight, then carcass were vacuum-packed after chilling at 4°C for 24 h, and stored in a freezer at −20°C until analysis. The breast fillets were used for analysis.
The frozen carcasses were thawed in a refrigerator at 4°C for 24 h before analysis in the laboratory. After thawing the right and left muscles were dissected and breast muscles also dissected in following pattern. After separation of samples then should be minced using food mixer. Then from minced meat samples, target supernatant were made in different terms and conditions to perform the respective analysis.
Standards (carnosine, and anserine) were obtained from Sigma-Aldrich (St. Louis, MO). The external and internal standard (IS) used in cholesterol analysis were cholesterol (Tokyo chemical industry, Tokyo, Japan) and 5α-cholestane (Sigma-Aldrich Co., St. Louis, MO, USA). Standard products used for retinol and tocopherol analysis were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Other reagents and solvents were used HPLC grade. Vitamin B12 standards (cyanocobalamin, purity 96.0%–102.0%) were purchased from Sigma-Aldrich (St. Louis, Mo., USA) and sodium acetate trihydrate and acetic acid were purchased from Wako (Osaka, Japan).
The contents of carnosine and anserine were determined using the slightly modification method ascribed by (Mora et al., 2007) where 2.5 g of minced meat samples from each chicken were homogenized with 7.5 mL of 0.01N HCl at 1,130×g for 30 s (T25 basic, IKA GmbH & Co. KG, Germany). After homogenization then the samples were centrifuged at 10,000×g for 30 min at 4°C (1580 R, GYROZEN Co., Ltd). 0.5 mL of centifugated supernatant was taken in 2 mL tube and mixed with 1.5 mL of acetonitrile and performed centrifuge again for 10 min at 10,000×g at 4°C (1580 R, GYROZEN Co., Ltd). Filtrated through 0.2 μm membrane filter the supernatant was injected into HPLC (1580R, GYROZEN Co., Ltd) column.
The reducing sugar contents of each extract were measured by dinitrosalicylic acid method as described by the (The Korean Society of Food Science and Nutrition, 2000). Beta-carotene content was determined by using HPLC (Agilent, Santa Clara, CA, USA) following the method of (Thomas et al., 2001) after extracting with alkali saponification method.
Tocopherols and tocotirenols were determined by mobile phase HPLC after saponification followed by (Food and Drug, 2016). 2 mL of the extract was taken accurately, the solvent was volatilized with nitrogen, and redissolved with 1 mL of n-hexane. The redissolved extract was filtered through a 0.5 μm membrane filter (Advantec, Tokyo, Japan) and analyzed by HPLC (LC-20AD, Shimadzu, Kyoto, Japan). The column was detected using ExT l λ=285 nm and Em λ=325 nm using a Li Chrospher Diol 100 (240×4 mm, 5 μm, Merck, Darmstadt, Germany) using a fluorescence detector (Shimadzu).
Vitamin B12 content was measured by the method of (Mun et al., 2017) equipped with HPLC-DAD analysis through immunoaffinity clean-up techniques where vitamin B12 standard solution is prepared by dissolving 10 mg of cyanocobalamin standard product in 10 mL of water and making stock solution of 1 mg/mL. Then stored it in cold dark place at −18°C, diluted to the range of 0.025–10 μg/mL. The column was incubated with the C18 ACE 3 AQ (3 mm×150 mm, ACE, Scotland, UK) using the HPLC system Agilent 1260 infinity (Agilent, Santa Clara, CA, USA) Gradient conditions. At this time, the flow rate of the mobile phase was 0.25 mL/min, the column oven temperature was 35°C, and the sample injection amount was 100 μL. The separated cyanocobalamin was detected at 361 nm, and the absorbance at 200–600 nm was collected to confirm the peak purity.
Minerals of each chicken breast fillet were analyzed by inductively coupled-plasma atomic emission spectrophotometry with the standard absorbance solutions. The cholesterol contents of minced samples were analyzed by GC (HP 5890 Series II, Hewlett Packard Co., Palo Alto, CA, USA). Analytical column was HP-5 (30 m×0.320 mm, 0.25 μm, Agilent, J & W Scientific, Santa Clara, CA, USA).
The soluble amino acids composition was determined by using a slightly modification of the method which is described by (Huge et al., 2002) where the visible external fat was removed and meat sample three grams from each treatment was mixed with 27 mL of 2% TCA solution. The mixture was homogenized at 13,500 rpm/min for 1 min. The homogenate was then centrifuged at 17,000 g for 15 min (after centrifuged 1 mL of sample was again centrifuged at 17,000 g for 15 min) and filtered through 0.45 μM membrane filter. Instrumental conditions during free amino acid analysis are as follows: S433, auto analyzer, Cation separation column (LCAK07/li), 4.6×150 mm; buffer change (A, pH 2.90; B, pH 4.20; C, pH 8.00); (Lithium citrate buffer solution), buffer flow rate: 0.45 mL/min, ninhydrin flow rate: 0.25 mL/min, Column temp.: 37°C.
Statistical analysis was done by GLM procedure using (SAS, 2003). A significance test between the results was used by Tukey’s multiple test method (p<0.05). The results of the analysis are presented as standard errors of the mean and the least-square means.
Results and Discussion
The carnosine and anserine contents of HH, broiler, 2A, 2C, and 2D from male breast meat are presented in Fig. 1. The concentration of carnosine in HH, broiler, 2A, 2C, and 2D were 511.04 (mg/100 g), 257.94 (mg/100 g), 287.39 (mg/100 g), and 359.31 (mg/100 g) respectively. The carnosine content was highest in HH and lowest in broiler (p<0.05). The concentration of carnosine was more dominant in native chickens than broiler which was an agreement with the previous study stated by (Intarapichet and Maikhunthod, 2005). It was found that the new native chicken strains, 2A, 2C, and 2D contained more carnosine content than broiler (Fig. 1). Comparing of native chicken strains with broiler, these results were consistent with those reported by (Jung et al., 2013) in Korean native chicken. This is due to different composition of muscle fiber type I (slow-twitch oxidative red fiber), IIA (fast-twitch oxidative-glycolytic white fiber), IIB (fast-twitch glycolytic white fiber) in meat (Cornet and Bousset, 1999). The inconsistent of carnosine in different chickens may be due to genotypic trait that could be different among traits such as enzyme and transporters agreed by (Everaert et al., 2013). Higher concentration of carnosine has been found with the higher concentration of white muscle rather than the tissue of red muscle that continue their energy-rich phosphate ester in a condition of anaerobic means (Boldyrev et al., 2004). There for further studies are needed to elucidate the biological significance of carnosine among the native chicken strains. The anserine content of HH, broiler, 2A, 2C, and 2D from male breast meat were 1,525.80 (mg/100 g), 660.38 (mg/100 g), 1,286.34 (mg/100 g), 962.70 (mg/100 g), and 1,059.76 (mg/100 g) respectively. It was found that HH contained significantly (p<0.05) higher anserine content among all groups of chickens. The new native chicken strains, 2A, 2C, and 2D contained higher amount of anserine than broiler. Anserine is the predominating histidine dipeptide in poultry meat, whereas carnosine for beef and pork (Abe, 1995). Anserine (1-methylated carnosine) derivated from carnosine mostly abundant in non-mammalian species, such as poultry agreed with the results conducted by (Abe, 1995). Anserine is produced by catalyzed of carnosine as it is the derivated product of carnosine (Drozak et al., 2013). The effect of meat type on anserine content corresponded with that on carnosine content. Native chickens contained high anserine than broiler that was due to the activity of anserine act as a physico-chemical buffer against proton production by anaerobic glycolysis in different muscle traits (Dunnett and Harris, 1995).
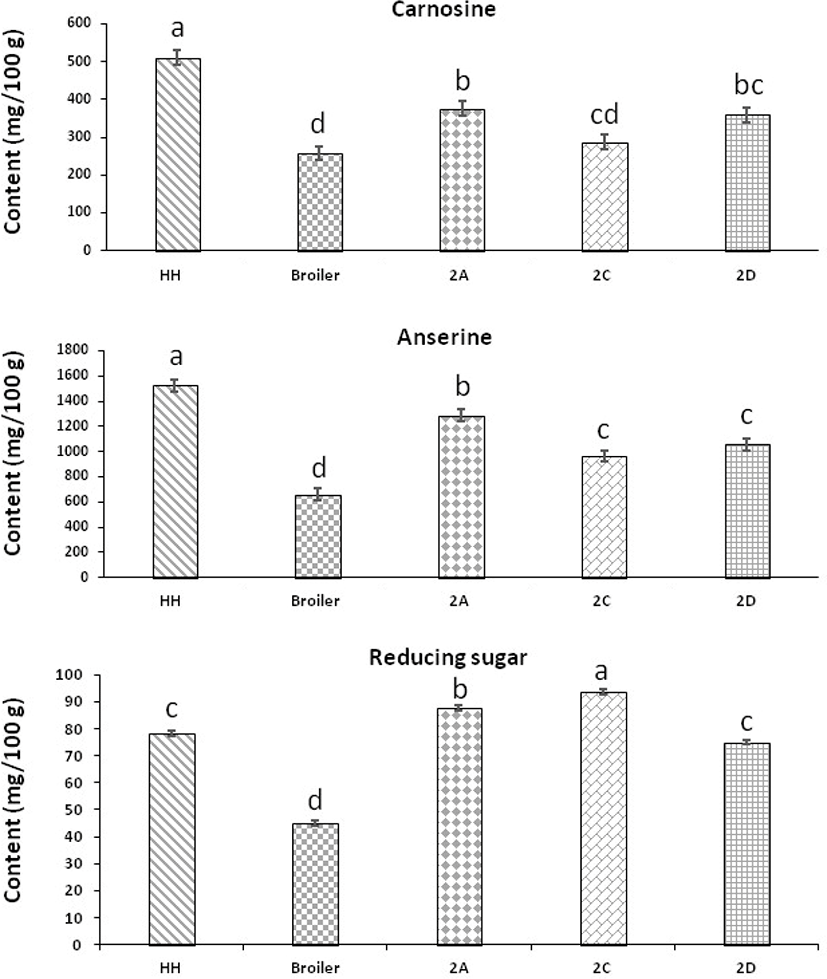
The reducing sugar contents of HH, broiler, 2A, 2C, and 2D from male breast meat are shown in (Fig. 1). The reducing sugar concentrations of HH, broiler, 2A, 2C, and 2D were 78.32 (mg/100 g), 44.87 (mg/100 g), 87.55 (mg/100 g), 93.47 (mg/100 g), and 75.01 (mg/100 g) respectively. Among the groups, it was found that new native chicken strain, 2C contained significantly higher reducing sugar than broiler (p<0.05). And, between native chickens and broiler, the broiler had lower reducing sugar in breast meat than other native chickens. In general, the native chicken contained higher reducing sugar than broiler supported by (Jung et al., 2013). The reducing sugars responsible for some bothy and aroma flavor in chicken meat (Sasaki et al., 2007). (Mottram, 1998) reported that free sugars, sugar phosphates, nucleotide bound sugars, free amino acids, peptides, nucleotides and other nitrogenous components, such as thiamine as the main water-soluble flavor precursors varies according to genetic makeup and muscle structure. The reaction of cysteine and sugar can lead to characteristic meat flavour specially for chicken (Varavinit et al., 2000). During heating of breast meat, ribose which is a reducing sugar in meat associated with ribonucleotides in the muscle produce the flavor (Mottram, 1998).
The vitamins (α-tocopherol, γ-tocopherol α-tocotirenol, Vitamin A, and Vitamin B12) contents of HH, broiler, 2A, 2C, and 2D from male breast fillet are presented in Table 1. The new native chicken strains, 2A, 2C, and 2D contained higher levels of α-tocopherol than HH and broiler but among the groups α-tocopherol content was highest in 2C which was 0.26 (mg/100 g) and showed a significant difference among groups (p<0.05). Vitamin A was higher in 2A among the groups but did not show any significant difference (p>0.05). New native strains, 2C, 2D, and HH showed the lower contents of vitamin A than broiler that agreed with study conducted by (Lee et al., 2015). Cholesterol contents of HH, broiler, 2A, 2C, and 2D were 51.26 (mg/100 g), 63.17 (mg/100 g), 54.43 (mg/100 g), 49.04 (mg/100 g), and 49.51 (mg/100 g) respectively. It is showed that (Table 1) the highest cholesterol content was found in broiler which was 63.17 (mg/100 g) and higher than all native chickens that was agreed by (Jaturasitha et al., 2008). But among new native chicken strains, 2C and 2D implied the lower inflate of cholesterol than HH. It is, however, the cholesterol level varies according to contents of body fat, skin fat, and bone marrow (Demos and Mandigo, 1995).
Mineral contents of HH, broiler, 2A, 2C, and 2D from male breast fillet are given in Table 2. The predominant minerals, phosphorous was significantly higher in broiler than that of all treatment groups. For magnesium, broiler also contained more contents than all groups. But in 2A and 2C had higher concentration of magnesium than HH (Table 2). New native chicken strains, 2A, 2C, and 2D had higher sodium contents than HH. Although P, Mg, and Na were higher in broiler compared with other groups but these minerals have no health effects in human body. Lower calcium content in each treatment indicates the less bone ion meat. The micronutrients investigated in this study were zinc, iron, manganese and copper. 2A had higher zinc contents than HH, 2C, and 2D. Higher iron found in HH compared with all groups. Meat with zinc and iron has a great importance to reduce the incidence of some diseases and being accumulated as a part of beneficial effect on health (Lombardi-Boccia et al., 2005). Copper content was higher in 2A from all treatment groups. The contents of mineral can be influenced by many factors such as species, breeding condition, age, and supplementation with various ingredients (Park and Kim, 2001).
Free amino acid concentrations of HH, broiler, 2A, 2C, and 2D from male breast fillet are depicted in Table 3. In the fraction of essential amino acids, the predominant amino acids were the tryptophan and threonine whereas alanine was superior found in the non-essential fraction, followed by serine while lowest values were reported for arginine among treatments. Umami or savory amino acid, glutamic acid content in 2A was highest among the all groups (p<0.05). Glutamic acid which is one of the most important amino acid in chicken meat regarding of enhancing the flavor of meat either combination with other test-associated compounds or its by self (Kurihara, 1987). Glutamic acid in native chicken was higher than broiler that is agreed with the study conducted by (Ahn and Park, 2002). The flavor related amino acids, valine, isoleucine, leucine, phenylalanine, arginine, and proline were significantly higher in 2A among the groups (p<0.05). Subsequently HH contained more flavor producing amino acid than broiler (Table 3). The Korean native chickens are superior in flavor than broiler supported by (Choe et al., 2010). It is however, tasty amino acids, asparagine, threonine, serine, glutamic acid, glycine, and alanine contents were significantly higher in 2A than HH and broiler (p<0.05). The more functional compound, taurine contents of HH, broiler, 2A, 2C, and 2D were 5.76 (ppm), 4.18 (ppm), 18.66 (ppm), 4.71 (ppm), and 6.18 (ppm) respectively (Table 3), while 2A contained significantly higher taurine content than other groups (p<0.05) which are not association with taste or odor of meat (Batzer et al., 1960). The native chickens compared with broiler had higher taurine content supported by (Choe et al., 2010). Taurine influences many functions like as bile salt formation, stabilization of membrane, calcium homeostasis, and growth moderator (Redmond et al., 1998). And, moreover, broiler had higher cysteine which is sulfur containing amino acid but lower content of methionine than other groups. The amino acid which performs the immune functions in the human body was significantly higher in 2A among the groups (p<0.05).
Conclusion
In general, the new native chicken strains have more carnosine, anserine, and reducing sugar than broiler. Due to higher tocopherol and lower cholesterol values, some native chicken strains may be accepted to the consumers with health benefits aspects. The new native chicken strain have more umami taste compare to broiler or HH. Tasty and flavor related amino acids of new native chicken strains are better than broiler or HH. Comparing with HH or broiler, the new native chicken strains have some distinct superior characters that lead to select in poultry breeding sector. So, this work may be regarded as benchmark for poultry breeder to take into consideration in breeding policy to get more nutritional meat with desired quality.













