Introduction
Nowadays, tendency to consumption of functional dairy products including probiotic, prebiotic and synbiotic products has increased due to their health benefits (Minervini et al., 2017). Consumption of probiotic products is a way to restore intestinal microflora. Addition of probiotic bacteria to milk is interested not only for their beneficial effects but also for their ability to enhance organoleptic quality and wide diversity of product. Yogurt has the potential for carrying probiotic bacteria (Heller, 2001). Destroyed in gastrointestinal tract, the starter bacteria of yogurt (Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus) are not resistant to acid and bile. On the other hand, the growth of probiotics in milk is slow due to its low proteolytic activity. Thus, yogurt bacteria are added to probiotic products in order to reduce fermentation time (Talwalkar and Kailasapathy, 2004). The most common probiotic bacteria used in dairy products consist of species of Lactobacillus and Bifidobacterium. Many studies have investigated the viability of L. acidophilus and B. bifidum in dairy products (Dave and Shah, 1998; Horiuchi et al., 2009; Miller et al., 2003; Tang et al., 2010). Presence of oxygen is harmful for viability of probiotic bacteria especially bifidobacteria due to their anaerobic metabolism. The resistance of bacteria to oxygen pressure depends on the presence of some enzymes and morphological and structural changes on the surface of cells (Ruiz et al., 2011).
Glucose oxidase (GOX) oxidizes β-D glucose to Δ-gluconolactone by means of oxygen molecule, which is consequently auto hydrolyzed to glucuronic acid and hydrogen peroxide (Hecth et al., 1993). Therefore, this enzyme can be used in order to reduce oxidative potential of soluble oxygen due to its negative effect on probiotic bacteria.
Cruz et al. (2012) investigated the effect of GOX on physicochemical and microbial characteristics of yogurt after 1, 15, and 30 days of storage. The samples showed lower increasing of soluble oxygen and lower reduction of B. longum and L. acidophilus. Batista et al. (2015) evaluated the efficiency of probiotic yogurt containing GOX compared to commercially available yogurt in Brazil local market. They reported that the viability of probiotic and lactic acid bacteria increased in samples containing GOX. Moreover, the amounts of diacetyl, acetaldehyde, conjugated linoleic acid, polyunsaturated fatty acids and proteolytic activity increased in these samples.
Many investigations have been conducted to immobilize GOX in different organic and mineral matrices. These studies showed enhanced reusability, recovering, thermal and process stability, and improved storage time (Blandino et al., 2001; Vikartovska et al., 2007). Recent studies have been shown that magnetic nanoparticles are suitable matrices in order to immobilize enzymes. For example, Abbasi et al. (2016) immobilized GOX on modified iron oxide magnetic nanoparticles. They reported that covalent immobilization of GOX on nanoparticles caused little structural and conformational changes.
In this study for the first time GOX was immobilized on magnetic chitosan nanoparticles (MCNP) and used in probiotic drinking yogurt. The effect of immobilized enzyme on the viability of probiotic bacteria and the physicochemical properties of drinking yogurt was assessed.
Materials and Methods
Magnetic Fe3O4 nanoparticles were prepared as described by Ghadi et al. (2015). Magnetic nanoparticles (0.02 g) was dissolved in 50 mL deionized water and then 50 mL of 0.0054 M trisodium citrate solution was added. The obtained solution was sonicated for 20 min. Chitosan (0.3 g) with average molecular weight and degree of deacetylation 85% was dissolved in 100 mL of 1% acetic acid solution and then stirred at 78×g for 25 min at 20°C. The obtained clear solution was sonicated (Amplitude 60, cycle 0.5) and pH was adjusted to 5 by addition of HCl or NaOH; then, it was filtered (0.2 μ mesh). Coating of chitosan on magnetic iron oxide nanoparticles was conducted as described by Ghadi et al. (2015). As described by Liu et al. (2012), enzyme immobilization was conducted with some modifications 480 μL of glutaraldehyde (25%, v/v) was added to 50 mL of double distilled water and 2 mL MCNP solution was added under severe stirring. The surplus of glutaraldehyde was removed 3–7 times using shaker and centrifugation (10,000×g, 20 min) and then put in ice 1 mg GOX (18.2 U/mg) was dissolved in 10 mL phosphate buffer at pH 7.4 and put in ice. The MCNP-glutaraldehyde solution was gradually introduced to the enzyme solution within 1 min. The addition was stirred under constant 78×g.
Fourier transform infrared (FTIR) spectrophotometer (Nicolet Avatar-FTIR-FTIR-ATR, Thermo, USA) was used to characterize the chemical bonds between MCNP and GOX.
The drinking yogurt samples were produced using 1.5% fat milk in Pak Dairy Co. (Tehran, Iran). Skim milk powder (2.5%) was added to the milk. Then, the milk was heated at 85°C for 15 min and cooled to 40°C. DVS (Direct Vat Set) probiotic cultures (B. lactis BB12 and L. acidophilus La5) were inoculated to the milk. Both probiotic bacteria (108 CFU/mL of each bacterium) were simultaneously added to the DVS yogurt starter (YC-X11). The sample was incubated at 40°C until reaching the pH of 4.6. Then it was cooled down until 10°C and the gel was broken by using a laboratory homogenizer (High shear mixer, Novin Abzar Co., Iran). In order to obtain better result, GOX was added during mixing because the last steps of mixing enter the most of the soluble oxygen into the yogurt. Different concentrations (0, 250, 500, 750, and 1,000 mg/kg) of free and immobilized enzyme were added (Table 1). Finally, probiotic dinking yogurt samples were stored at 4°C for 21 d.
| Sample | Free enzyme (mg/kg) | Immobilized enzyme (mg/kg) |
|---|---|---|
| C (Control sample) | 0 | 0 |
| FE250 | 250 | - |
| FE500 | 500 | - |
| IE250 | - | 250 |
| IE500 | - | 500 |
Determination of titratable acidity was performed according to AOAC method (AOAC, 2005). Acetaldehyde and diacetyl were measured by static headspace (HS) method using gas chromatography (GC) (Agilent 6890, USA). An aliquot of 10 g of drinking yogurt and 10 g of anhydrous sodium sulphate were mixed in a 20 mL vial that was hermetically sealed with a polytetrafluoroethylene-coated rubber septum and an aluminum cap. An autosampler (Agilent 7694, USA) was used to equilibrate the sample at 80°C for 60 min to accomplish volatilization of volatile compounds in drinking yogurt. Volatile compounds were separated on an Agilent HP-5 (30 m 0.25mm, 0.25 μm thickness) column. Injector temperature was 250°C, carrier gas helium used at a flow rate of 1 mL min−1, oven temperature program initially held at 35°C for 6 min. Then programmed to 250°C at raising rate of 30°C min−1. Peak identification of aroma compounds was performed with MSD detector (Agilent 5973, USA) (Serra et al., 2009).
Spectrophotometric method using o-Phthaldialdehyde was used for evaluation of proteolytic activity (proteolysis index) (Church et al., 1983).
Syneresis was determined using centrifuge (Hettich-universal 320R, Tuttlingen, Germany) at 1,957×g for 20 min at 4°C (Horiuchi et al., 2009).
Drinking yogurt samples for counts of probiotic bacteria were plated on MRS-bile agar (Merck Co., Germany). Incubation was performed at 37°C for 3 d under both aerobic and anaerobic (using an anaerobic jar) conditions (Sabooni et al., 2018).
Results and Discussion
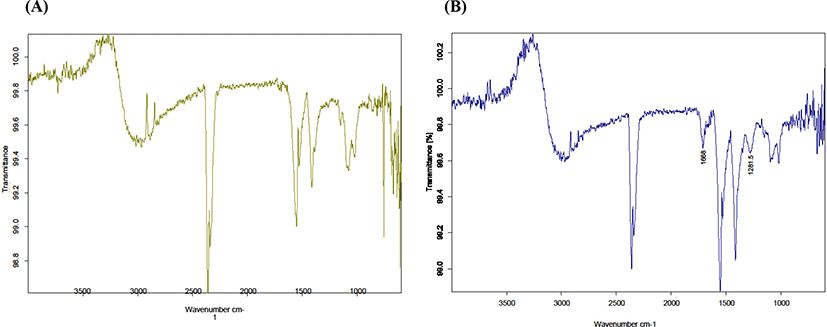
Fig. 1 indicates FTIR for MCNP before and after immobilization. Fig. 1A indicates the prepared MCNP with 0.3 g chitosan before immobilization. The peak around the 500 cm−1 can be related to vibrations of Fe-O that confirms the presence of magnetic nanoparticles. This result is in line with the finding of Moon et al. (1999). The peaks at 1,060 cm−1, and 1,070 cm−1 are related to C-O-C stretching vibrations of chitosan. The peak at 1,355 cm−1 can be resulted from C-O stretching vibration of primary alcoholic group of chitosan. The peak at 1,560 cm−1 is probably related to N-H bending vibration. Fig. 1B shows the FTIR spectrum of GOX-MCNP (0.3 g chitosan) after immobilization. Two new peaks are appeared. The first peak at 1,281.5 cm−1 is related to covalent joint of Schiff’s base between carbonyl group of glutaraldehyde and amine group. The second peak at 1,668 cm−1 is resulted from amide group of GOX. These two peaks confirm the enzyme immobilization.
The acidity, syneresis, proteolysis index, acetaldehyde and diacetyl contents of probiotic drinking yogurt samples during storage are shown in Tables 2, 3, 4, 5, and 6, respectively. According to Table 2, on the first day, the lowest acidity was observed in IE500 and IE250. On the 11th and 21th days, the lowest acidity was related to IE500. The control sample had the highest acidity on the first, 11th and 21th days. Acidity significantly increased (p<0.05) during storage period. Previous studies indicated that increasing acidity of probiotic samples was lower during storage (Kailasapathy, 2006). They also reported that acid production in probiotic samples was lower than non probiotic samples during storage. The reason could be due to the viability and compatibility effect between probiotic bacteria and starter culture and consequently reduced the growth of L. delbrueckii subsp. bulgaricus. So it seems that lower acidity of the samples with immobilized enzymes could be related to higher growth rate of probiotic bacteria and competitive influence of probiotics on the growth of L. delbrueckii subsp. bulgaricus, the main acid producing bacterium in yogurt starter. No et al. (2002) reported that chitosan significantly has inhibitory effect on the growth rate of gram-positive microorganisms such as Staphylococcus aureus, L. delbrueckii subsp. bulgaricus, L. plantarum and L. brevis.
Table 3 shows that on the first day, the lowest syneresis was related to IE500 and FE500. There was no significant difference between syneresis of other samples. On the 11th day, FE500 and IE500 showed the lowest syneresis, which was not significantly different from FE250. The highest syneresis was related to the control sample and IE250 that was not significant compared to other samples except FE500. On the 21th day, FE500 and IE500 showed the lowest syneresis which was not significantly different from other samples except FE250 and control sample (p>0.05). FE250 and control sample showed the highest syneresis which was not significantly different from other samples except FE500 and IE500 (p>0.05). During cold storage, syneresis increase was significant (p<0.05). Reducing water holding capacity (increasing syneresis) at the end of the storage period could be due to the impact of enzymes produced by starters on casein micelles (Sahan et al., 2008). Aryana and McGrew (2007) reported that the syneresis of yogurt increased with increasing acid production which is in agreement with our findings.
According to Table 4, on the first day, the highest proteolysis index was observed in IE500. Other samples had lower proteolysis index than IE500 and there was not a significant difference between these samples in respect to proteolysis index. On the 11th day, the lowest proteolysis index was observed in FE250, IE250 and control sample. IE500 had the highest proteolysis index. On the 21st day, the lowest proteolysis index was related to the control sample. IE500 and FE500 showed the highest proteolysis index. During storage period, proteolysis significantly increased (p<0.05). Probiotics especially Bifidobacterium species, are sensitive to low pH and show different proteolytic activity depending on their species (Shihata and Shah, 2000). Proteolysis in yogurt is mainly performed duo to proteolytic activity of lactic acid bacteria. Hydrolyzation of proteins was done by proteases attached to the cell wall. Milk protein hydrolyzation led to sharp release of amino acids and peptides (Gonzalez-Gonzalez et al., 2011). In the present study, utilization of immobilized GOX caused moderate proteolysis. Cruz et al. (2012) reported that adding medium concentrations of free GOX (250 and 500 mg/kg) led to a moderate proteolytic activity in comparison to high concentrations of this enzyme (750 and 1,000 mg/kg). So, it seems that medium concentration of immobilized enzyme is financially more suitable by respect to moderate proteolytic activity and appropriate pH of the yogurt. In the other words, higher concentration of GOX is not necessary because of insufficient amount of substrate (glucose).
According to Table 5, on the first day, FE500 had the highest content of acetaldehyde. On the 11th day, the highest content of acetaldehyde was observed in IE250 and FE500. On the first and 11th days, the control sample showed the lowest content of acetaldehyde. On the 21st day, the lowest content of acetaldehyde was related to the control sample and FE250. FE500 had the highest content of acetaldehyde. During storage period, acetaldehyde content significantly decreased (p<0.05). Acetaldehyde is the most component that mainly responsible for taste and flavor of the yogurt. On the first day of storage, comparison of test samples with the control sample showed suitable content of acetaldehyde which is responsible for the flavor of the yogurt. Acetaldehyde (23–41 mg/L) leads to appropriate flavor in yogurt and less than 10 mg/L of this component causes low flavor score for the samples (Tamime and Deeth, 1980). Decreasing pH reduces acetaldehyde due to oxidation of acetaldehyde to acetate (Tamime and Robinson, 2007). Probiotic bacteria do not produce flavor components. Probiotic fermented dairy products usually have weak flavor due to low activity of threonine aldolase which catalyzes acetaldehyde production from threonine substrate (Gardini et al., 1999). Reduction of acetaldehyde at the end of the storage period could be resulted from the presence of alcohol dehydrogenase which hydrolyzes acetaldehyde to ethanol. L. acidophilus produces this enzyme (Marshall and Cole, 1983). Some researchers observed that ethanol content increased during storage because acetaldehyde hydrolysis to ethanol by alcohol dehydrogenase (Varga, 2006). Martin et al. (2011) showed that oxidoreduction potential influenced the flavor compounds production which is in agreement with our findings which showed that addition of GOX had no negative effect on flavor compounds.
As represented in Table 6, on the first and 21th days, the lowest content of diacetyl was belonged to the control sample. The test samples had higher content of diacetyl than the control sample and there was not a significant difference between test samples in respect to diacetyl content. On the 11th day, the lowest content of diacetyl was observed in the control sample that was not significant compared to FE250 and IE250. IE500 and FE500 contained the highest content of diacetyl which was not significantly different from IE250 and FE250. During storage, diacetyl content significantly increased in the samples (p<0.05). Diacetyl or 2,3-butanedione is another aromatic component in yogurt. The specific citrate-utilizing lactic acid bacteria can produce 2,3-butanedione by fermentation of citrate to pyruvate in milk (Vedamuthu, 2007). Some researchers reported that diacetyl level in yogurt increased during refrigerated storage and it could be related to glucose content, the main precursor of this flavor compound (Venica et al., 2018; Wolf et al., 2015).
According to Table 7, on the first and 21th days, the lowest number of L. acidophilus was related to the control sample that was not significant compared to FE250. On the 11th day, the lowest number of L. acidophilus was recorded in the control sample. IE500 had the highest count of this bacterium on the first, 11th and 21th days. During storage period, the number of L. acidophilus significantly decreased (p<0.05). The number of B. lactis in the samples was shown in Table 8. On the first day, FE500 had the highest count of this bacterium. On the 11th and 21th days, IE500 and FE500 had the highest count. On the first, 11th and 21th days, the control sample showed the lowest number of B. lactis. During storage period, the number of B. lactis significantly decreased (p<0.05). Oxygen presence is detrimental for metabolic activity and viability of probiotic bacteria due to their anaerobic metabolism. The resistance of these bacteria against oxygen depends on their ability to alter morphological properties, change surface cell component and produce some enzymes (Ruiz et al., 2011). B. lactis changes hydrophobicity of surface and increases protein content in the presence of oxygen (Shakirova et al., 2010). Aerobic condition decreased the production of some exopolysaccharides in some species of Bifidobacterium such as B. longum (Ninomiya et al., 2009) which could be considered as a negative effect because exopolysaccharides could increase viscosity and create a suitable texture in fermented products (Salazar et al., 2009). Moreover, in order to diminish the oxygen pressure in probiotic yogurt, utilization of nitrogen gas during production and fermentation at 37°C was recommended (Horiuchi et al., 2009). Addition of some components such as ascorbic acid (Dave and Shah, 1997; Dave and Shah, 1998), utilization of laminated polystyrene as a high inhibitor against gas diffusion (Miller et al., 2003) and encapsulation (Talwalkar and Kailasapathy, 2004) are some examples for reducing the negative effect of oxygen presence in yogurt. Our results showed that addition of GOX positively affected the oxygen reduction in probiotic drinking yogurt which is in agreement with another study (Cruz et al., 2012). Addition of GOX is a convenient and biotechnological method that can be accepted by food technologists who have had negative opinion about using chemical materials (Behrens et al., 2010; Cruz et al., 2012; Shim et al., 2011). It seems that higher survival of probiotic bacteria in the samples with immobilized enzyme (IE250 and IE500), compare to addition of free enzymes, is related to higher activity of enzymes in pH of the yogurt. Moreover, chitosan, as a prebiotic compound, can increase the viability of Lactobacillus and Bifidobacterium species (Tang et al., 2010).
Conclusion
The results of this study indicated that addition of GOX immobilized on MCNP in drinking yogurt decreased negative effect of oxygen more effectively than the control sample or samples with free enzyme. It consequently provided a more desirable condition for probiotic bacteria that are anaerobic or microaerophile. Moreover, the use of immobilized enzyme caused moderate proteolysis, appropriate acidity and enough contents of flavor compounds such as acetaldehyde and diacetyl in probiotic drinking yogurt. Since recovery of the immobilized enzyme from the complex medium by a foreign magnetic field is convenient and fast, this method (addition of immobilized enzyme) can be applied in probiotic drinking yogurt. In addition, this method is safe, natural and financially feasible.













