Introduction
Chinese smoke-cured bacon (Larou) is a famous Chinese traditional smoked-cured meat product with rich aroma, mellow taste, rich nutrition, and unique flavor, and has the history of thousands of years. In its processing procedure, pickled meat after baking or sunlight exposure generates a unique cured flavor. Chinese smoke-cured bacon (Larou) is generally processed only in the cold twelfth lunar month, which is named La Yue in Chinese, and thus named Larou in China. Larou is well known worldwide for its unique flavor, simple processing procedure, and convenient storage and transportation. Chinese annual Larou production is more than 2 million tons (Zhang et al., 2014). Smoke-cured product Larou has the obviously longer shelf life than other meat products, but Larou also has some problems of corruption and deterioration caused by microbial growth and fat oxidation (Asefa et al., 2010). Preserved meat is prone to be contaminated by microorganisms (Choi and Yoon, 2013; Cho et al., 2016; Stadnik et al., 2015). Larou has a high resistance to bacteria (Zhou et al., 2010). There are various microorganisms on the surface of Larou. In the processing and storage period, the surface microflora will change, thus damaging the preservation of meat. Larou are prone to be spoiled during storage and the shelf life may be shortened. These problems of smoke-cured products not only cause huge economic losses and serious environmental pollution, but also endanger the health and life of consumers (Zhou et al., 2003).
To inhibit microorganisms, many techniques have been applied to process and preserve smoke-cured products (Zhou et al., 2010). The technology of edible coating based on sodium alginate and chitosan is one of the potential methods for controlling meat spoilage (Hamedi, 2017). In the technology, with water and alcohol as solvents, edible materials as the substrate are dipped or sprayed on food surface to form the coating, which can provide physical protection, insulate air and water, reduce microbial contamination, improve the sensory quality of foods, and extend the shelf life of foods (Hmc et al., 2012). Intermolecular and intramolecular hydrogen bonds play a significant role in the formation of polysaccharide coating. Antimicrobial agents are added into one or more edible materials to form an edible antimicrobial coating and then slowly released antimicrobial components can inhibit or prevent microbial growth (Ntzimani et al., 2010; Siragusa et al., 1999). Sodium alginate can form coating (Tangwongchai et al., 2000), retain biocompatibility and the moisture (Georgantelis et al., 2007). Wang (2008) adopted different bactericides in beef sterilization, then coated beef with sodium alginate composite and achieved the good preservation effect. Chitosan is non-toxic, biodegradable, and biocompatible, and shows the better coating formation effect and the higher oxidation resistance. Park and Zhao (2004) indicated that the combination of 0.5–1% chitosan and 6% glycerol achieved the good beef preservation effect. Sodium alginate/chitosan is an excellent sustained-release drug delivery system with some advantages such as conveniently available raw materials, simple preparation process, and low cost, and has been extensively studied (Cardoso et al., 2016). Lysozyme is a non-toxic and harmless antibacterial protein (Zhao, 2010). As a natural preservative, lysozyme is safe and may be conveniently used (Hong et al., 2009). Takahashi (2011) treated chopped tuna and salmon roe products with lysozyme during the low-temperature storage period and effectively inhibited the growth of Listeria monocytogenes with 2,000 ppm lysozyme. Lysozyme is a kind of anti-microbial drug with good biocompatibility and related resistance phenomenon has not been reported (Bukharin and Valyshev, 2006). The preservation effect of natural preservatives has become increasingly prominent.
Can lysozyme, sodium alginate, and chitosan film be used in Larou? In order to ensure the freshness of meat products and prolong the shelf life of smoke-cured product (Larou), the natural coating solution of lysozyme, sodium alginate, and chitosan was adopted in the preservation of sliced Larou in the study. With the concentrations of lysozyme, sodium alginate, and chitosan as the factors and the sterilization rate and water activity of sliced Larou as response values of the preservation conditions, according to the Box-Behnken test design, we experimentally optimized the natural preservation conditions of sliced Larou with the coating solution. Through response surface analysis and storage experiments, we determined the optimum preservation conditions of sliced Larou for extending the shelf life and improving the quality. The study provides a novel natural coating solution of Chinese smoke-cured bacon (Larou) for extending its shelf life.
Materials and Methods
Larou was from Guizhou Wufufang Food Co., Ltd., China. With the main raw material of fresh (frozen) pork meat as and other auxiliary materials, by pickling (10°C, 24 h, dry salting), drying (20 h, 52±2°C), smoking, and cooling, non-RTE meat products (Larou) were obtained. Larou was sliced into pieces with the size of 5 cm×5 cm×3 mm and the weight of 10.00–12.00 g and preserved at 25°C.
Sodium alginate was dissolved in distilled water to prepare 5 sodium alginate solutions with different concentrations (0.5, 1.0, 1.5, 2.0, and 2.5%) and then 2% glycerol was added as the plasticizer. Chitosan was added into 1% acetic acid solution to prepare 5 chitosan solutions with different concentrations (0.5, 1.0, 1.5, 2.0, and 2.5%). Sodium alginate solution and chitosan solution was fully mixed with an electric mixer in a water bath at 60°C under the mixing condition and then cooled to form a composite solution for use. Lysozyme was dissolved in distilled water at different concentrations (0.02, 0.04, 0.06, 0.08, and 0.10%). Lysozyme solution was added into the composite sodium alginate-chitosan coating solution. The solution was heated in a water bath until it became transparent and uniform. Then the prepared solution was cooled to room environment temperature (25°C) for use.
In the experiment, the natural coating solution composed of lysozyme, sodium alginate, and chitosan was used to treat sliced Larou. After dipping, draining, vacuum packaging, final Larou products were stored. During the storage period (25°C), physical and chemical indexes of Larou were determined regularly.
In single factor test, lysozyme concentration, sodium alginate concentration, and chitosan concentration were selected as influencing factors. When two factors were selected as the fixed factors, the other influencing factors were tested (Bukharin and Valyshev, 2006). Each factor was tested in parallel for three times. The single factor experiment design is shown in Table 1. Single factor test was conducted to respectively explore the effects of the three influencing factors on the sterilization rate and water activity of sliced Larou.
Based on the results of single factor test, the optimum conditions of all the factors were selected. According to the Box-Behnken response surface (Ahn et al., 2017; Yu et al., 2016) design (Table 2), the effects of lysozyme concentration, sodium alginate concentration, and chitosan concentration on the sterilization rate of sliced Larou were investigated.
| Level | A Lysozyme (%) |
B Sodium alginate (%) |
C Chitosan (%) |
|---|---|---|---|
| –1 | 0.06 | 1.0 | 1.0 |
| 0 | 0.08 | 1.5 | 1.5 |
| 1 | 0.10 | 2.0 | 2.0 |
According to the results of single factor test, 3 factors [the concentration of lysozyme (A), the concentration of sodium alginate (B), the concentration of chitosan (C)] were selected as independent variables. The testing factors and testing levels were selected based on the results of single factor test. Response surface methodology (RSM) was used to test the sterilization rate and water activity of sliced Larou. According to the design principle of Box-Behnken test (Bover-Cid et al., 2012), the optimum conditions for the sterilization rate and the water activity of the natural coating solution were predicted with testing data in Design-Expert 8.0.6 software.
Colonies were counted according to China National Food Safety Standard (GB/T 4789.2-2010): Aerobic Plate Counting (Cai et al., 2015).
Sliced Larou sample (5 g) was put on the sample tray of water activity tester (HD-3A, Wuxi Huake Instrument Co., Ltd., China). The lean part was selected as the sample. The moisture content in the sample was measured and each sample was measured in parallel for three times.
The effects of various factors on the germicidal efficacy of preserved sliced Larou were analyzed based on the experimental results. The sterilization rate is calculated as (Cai et al., 2015):
where Y is sterilization rate; S is the total number of colonies of sliced Larou (CFU/g); S0 is the total number of bacterial colonies in sliced Larou treated with the natural coating solution (CFU/g).The oxidation value was measured in accordance with the analysis method for hygienic standards of edible vegetable oils (GB/T5009.37-2003, China). extractting the fat of sliced Larou by rotary evaporator (RE-2000A, Ya Rong Biochemical Instrument Factory, China). Each sample was measured 3 times to obtain the average value.
Preserved sliced Larou samples (0.6–0.8 g) were evenly placed on the sample plate of moisture analyzer (MB45, OHAUS, USA), and the lean parts of the samples were selected as much as possible. The moisture content of the sample was determined according to the operating procedures of the instrument.
pH measurements were conducted with a pH meter (Testo 205, Testo, Germany) and the pH electrode was calibrated with different buffer solutions (pH 4.00 and 7.00). Each sample was measured 3 times to obtain the average value.
Through the sensory scoring test, the color, odor, and status indicators were obtained (Zhou et al., 2010). The sensory model score (scoring standards of sliced Laro) are shown in Table 3. In the scoring test, 10 food professionals were selected to score. They received a the model of sensory evaluation related to descriptive profile of sensory attributes (color, odor, and status of dry-cured meat) prior to testing so that each panelist could thoroughly discuss and clarify each attribute to be evaluated. All testing was carried out under controlled conditions (in special room with controlled temperature, free from noise and odor with adequate lightening). Two samples (untreated Larou and treated Larou) were tested by 10 experts within one month (5, 10, 15, 20, 25, and 30 d), respectively.
NMR relaxation properties were measured on the MicrNMR magnetic resonance imaging analyzer (Mortensen et al., 2005). Sliced samples were measured on the MicrNMR magnetic resonance imaging analyzer PQ001 (Niumag MicroMR, China). The proton resonance frequency was set as 23 MHz and the measurement temperature was 32 .00°C. T2 was measured with CPMG (Carr-Purcell-Meiboom-Gill) sequences. The parameters were respectively set as follows. r value (the pulse duration between 90° and 180°) was 150 μs. Repeated sampling times was 8. Repeated interval TR was 1,800 ms. Exponentially decaying nuclear magnetic signals were adopted. The sample (1.0 g) was put into the sample tube (diameter=12 mm), which was then sealed with a sealing coating to prevent moisture evaporation. Then the sample tube was placed in the nuclear tube (diameter=15 mm) and put into the analyzer. Each test was repeated for at least three times. The CPMG sequence was used to detect the transverse relaxation of the sample. The relaxation signal is:
where Pi is the signal strength of the ith component; T2i is the transverse relaxation time of the ith component in the sample. The total signal size is the sum of the sizes of signals produced by all components. The CPMG exponential decay curve was inversed by MultiExplnv Analysis software. The synthetic iterative algorithm was adopted in the software. Based on the discrete and continuous T2 spectra, corresponding measurement data were obtained.In this paper, all the data were used to establish the database in Microsoft Excel 2013. Data analysis, analysis of variance, and significance analysis (p<0.05) were performed in SPSS 13.0 software (SPSS Inc., USA). Origin 8.0 software (Originlab Company, USA) was used for data collation and mapping.
Results and Discussion
Fig. 1 shows the effects of lysozyme concentration on sterilization rate and water activity of sliced Larou. With the increase in the lysozyme concentration, the sterilization rate gradually increased. When the concentration of lysozyme was between 0.06 and 0.10%, the sterilization rate was not significantly different (p>0.05). When the concentration of lysozyme was 0.02%, the sterilization rate of sliced Larou products was 96.73% (p<0.05), which was lower than that obtained under 0.10% lysozyme. Lysozyme showed the excellent inhibition effect on bacteria and fungi. When the lysozyme concentration was between 0.02 and 0.10%, its effect of water activity was not significant because sodium alginate and chitosan had good coating-forming properties and could reduce water loss of foods (Dong et al., 2006). Therefore, the concentrations of lysozyme were respectively set as 0.06, 0.08, and 0.10% in the optimization experiment.
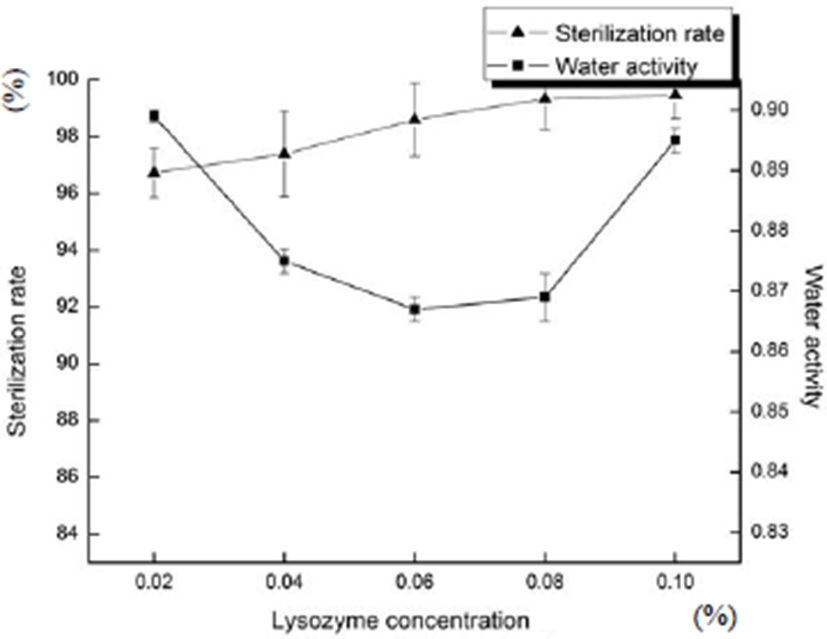
Fig. 2 shows the effects of the concentration of sodium alginate on sterilization rate and water activity of sliced Larou. With the increase in the concentration of sodium alginate, the sterilization rate gradually increased (p<0.05), but the water activity decreased because sodium alginate membrane with antibacterial substance inhibited microbial pollution and showed the good coating formation effect (Cai et al., 2015), and free water decreased. However, with the increase in sodium alginate concentration, because the coating is too thick (Table 3), it decreased the sensory properties of sliced Larou. When the concentration of sodium alginate reached 2.5%, the coating was not uniform and the dosage was large. When the concentration of sodium alginate was between 1.0 to 2.0%, the viscosity of the coating liquid was proper (the coating liquid is neither thin nor thick) and the sterilization rate and water activity of sliced Larou were good. In conclusion, the appropriate concentration range of sodium alginate was selected as 1.0, 1.5, and 2.0%.
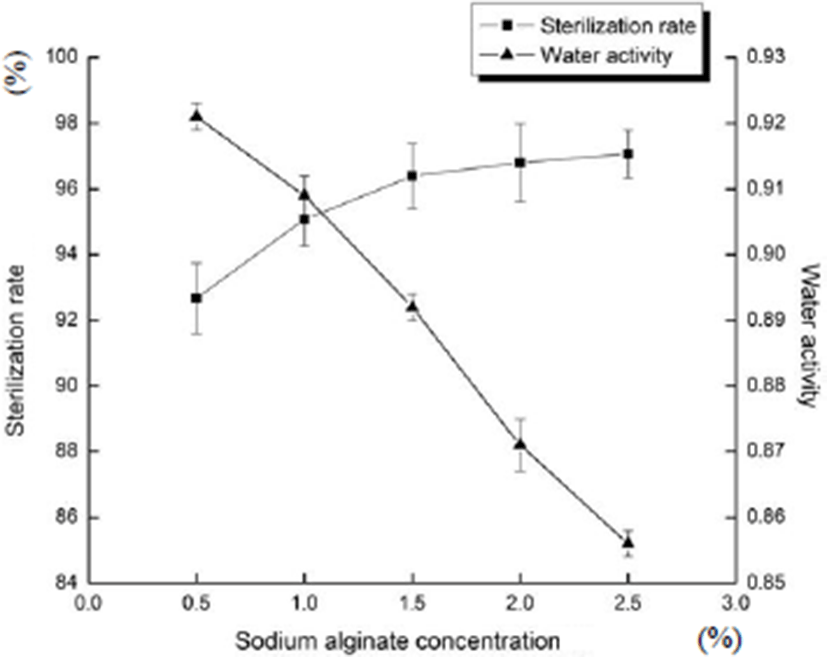
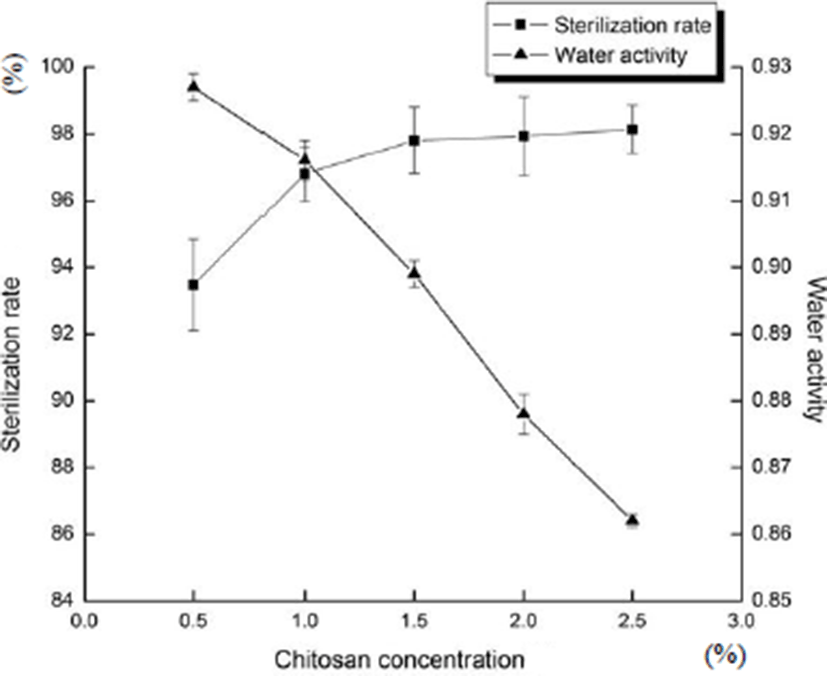
Fig. 3 shows the effects of chitosan concentration on sterilization rate and water activity of sliced Larou. With the increase in the concentration of chitosan, the water activity of sliced Larou decreased gradually (p<0.05), but the sterilization rate of sliced Larou increased gradually and finally tended to be stable (p>0.05). The result was consistent with the conclusions by Kim (2014). When the concentration of chitosan was more than 2.0%, the sensory properties of the coating were decreased due to the viscosity of the coating and the uneven thickness of the composite coating. Chitosan is soluble in acetic acid and insoluble in water, the higher concentration of chitosan leads to the lower solubility in water. So, the concentrations of chitosan in the composite coating were selected as 1.0–2.0%.
The concentration of lysozyme, the concentration of sodium alginate, the concentration of chitosan were selected as the test factors and the sterilization rate and water activity of sliced Larou were treated as response values (Y1, Y2). Response surface experimental results of the sterilization rate and water activity of sliced Larou are shown in Table 4.
Establishment of response surface regression model. In Design-Expert 8.0.6 software, the regression analysis of response surface data was performed. The regression equations of two factors are provided as follows:
where Y1 is the sterilization rate of sliced Larou treated with the natural coating method; Y2 is the water activity of sliced Larou treated with the natural coating method; A, B, and C are respectively the lysozyme concentration, sodium alginate concentration, and chitosan concentration. The positive coefficient indicates that corresponding factor is beneficial to the sterilization rate and water activity. Compared with sodium alginate concentration and chitosan concentration, lysozyme concentration showed the more significant effect on the sterilization rate and water activity of sliced Larou. In the experimental range, the regression equation can be used to predict and interpret the experimental results.Table 5 shows the model (F=17.73, p=0.0005). The two values indicate that the model is significant (El-Sersy et al., 2010) and the test method is reliable. In the missing items, p=0.091>0.05, indicating that the missing items are not significant. That is to say, the pseudo factor does not exist in the regression equation and the goodness of fit of the regression equation is good. Factor A is extremely significant (p<0.0001) and the interactive item BC (p=0.0029<0.01) shows the significant difference in the sterilization effect of sliced Larou. A2 (p=0.0006<0.01) and B2 (p=0.0004<0.01) shows the significant difference in the sterilization effect, but C2 (p>0.05) shows no significant difference in the sterilization effect of sliced Larou. To sum up, the effects of 3 factors are decreased according to the following sequence: A (the concentration of lysozyme)>B (the concentration of sodium alginate)>C (the concentration of chitosan). This phenomenon may be interpreted as follows. Lysozyme can directly affect the coating activity of bacteria and fungi and inhibit the growth of microorganisms (Düring et al., 1999). Sodium alginate and chitosan show the coating formation property, and the antibacterial effect of chitosan is not better than that of lysozyme. The correction coefficient (r2) of the model is 0.9580, indicating that the model can explain 95.80% of the variation of the response value. The r2 is about 1, indicating that the response surface model fits well with the experimental data (Sin et al., 2006). Therefore, A and BC are the two major factors of the sterilization rate of sliced Larou.
Variance analysis models have 2 important indicators: F- and p-values (Li et al., 2010). In the model (Table 6), F=12.51 and p=0.0015 indicate that the model is extremely significant. For the missing items, p=0.6073>0.05, indicating that the missing items are not significant. That is to say, the pseudo factor does not exist in the regression equation and the goodness of fit of the regression equation is good. Factor A shows the significant difference (p=0.0197<0.05) and Factor B shows the significant differences (p=0.0332<0.05). The interactive item BC shows the significant difference in water activity of sliced Larou (p=0.0413<0.05). A2 (p=0.002<0.01) and B2 (p=0.0002<0.01) shows the significant difference in water activity of sliced Larou. In summary, the effects of influencing factors were decreased according to the following sequence: A (lysozyme concentration)>B (sodium alginate concentration)>C (chitosan concentration). The changing trend may be interpreted as follows. Sodium alginate and chitosan have good coating-forming effects and stable properties (Dong et al., 2006) and the effect of sodium alginate and chitosan is greater than that of lysozyme. The correction coefficient (r2) of the model is 0.9415, indicating that the model can explain 94.15% of the variation of the response value. Therefore, A, B, and BC were the important influencing factors of the water activity of sliced Larou.
In the three-dimensional response surface plot and two-dimensional contour line, the two dependent variables of sliced Larou (sterilization rate and water activity) vary with independent variables (the concentration of lysozyme, the concentration of sodium alginate, and the concentration of chitosan). The response surface provides a way to measure the relationship between any response value (sliced Larou sterilization rate or water activity) and any two independent variables. In the predicted values, maximum/minimum values occur at the peak of the response surface and the minimum point occur in 2D contour (Li et al., 2015). At shown in Fig. 4 and Fig. 5, when the concentration of lysozyme is 0.08%, for the two factors (B and C), 1.53% sodium alginate and 1.49% chitosan correspond to the highest peak in 3D response surface plot, the sterilization rate of 99.63%. When the concentration of sodium alginate is more than 1.53% and the concentration of chitosan is above 1.49%, the sterilization rate decreases because the higher concentrations of sodium alginate and chitosan lead to the too high viscosity of coating solution, thus decreasing the bactericidal effect of lysozyme in the coating solution. Lysozyme contained in natural coating solution inhibits microbial growth to some extent and low-molecular-weight sodium alginate and chitosan can penetrate into bacterial cells, interfere with the transfer of genetic factors from DNA to RNA, and finally inhibit bacterial reproduction. The results were consistent with previous reports (Bensid et al., 2014; Mayachiew et al., 2010; Tang et al., 2016). The three-dimensional curve shows the arc trend, indicating that the interactive effect between B and C on the sterilization effect is significant. Based on the analysis results of the 3D response surface plot and contour map, the influencing trends of two factors of sodium alginate and chitosan on the sterilization effect can be obtained. As shown in Fig. 6 and Fig. 7, when the concentration of lysozyme is 0.08%, for the two factors (B and C), 1.37% sodium alginate and 1.62% chitosan correspond to the lowest point in 3D response surface plot, water activity of 0.879. When the concentration of sodium alginate is above 1.37% and the concentration of chitosan is above 1.62%, water activity increases because the higher concentrations of sodium alginate and chitosan lead to the unstable coating solution, thus increasing water activity in sliced Larou. The three-dimensional curve shows the arc trend, indicating that the interactive effect between B and C on water activity is significant. Based on the analysis results of the 3D response surface plot and contour map, the influencing trends of two factors of sodium alginate and chitosan on water activity can be obtained.
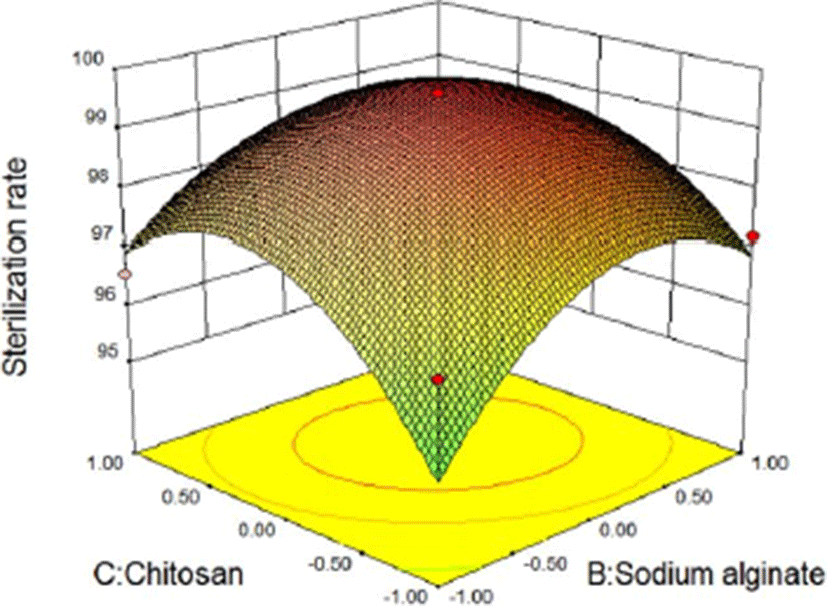
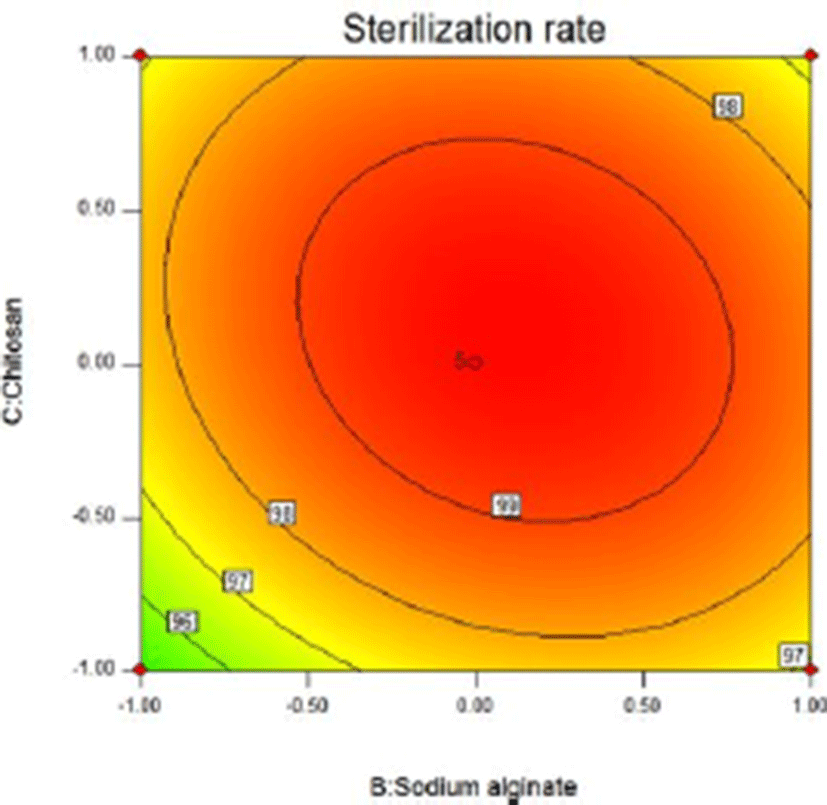
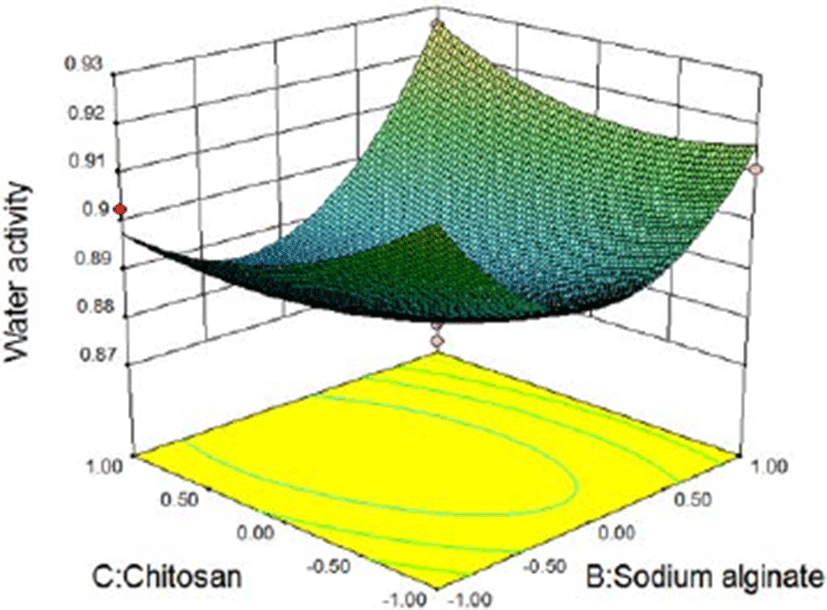
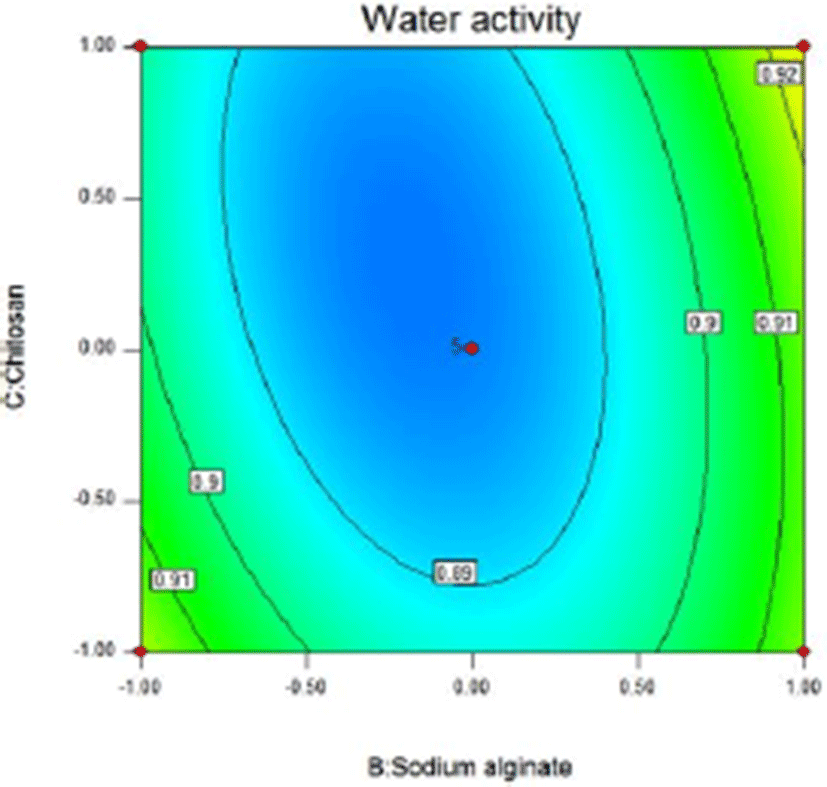
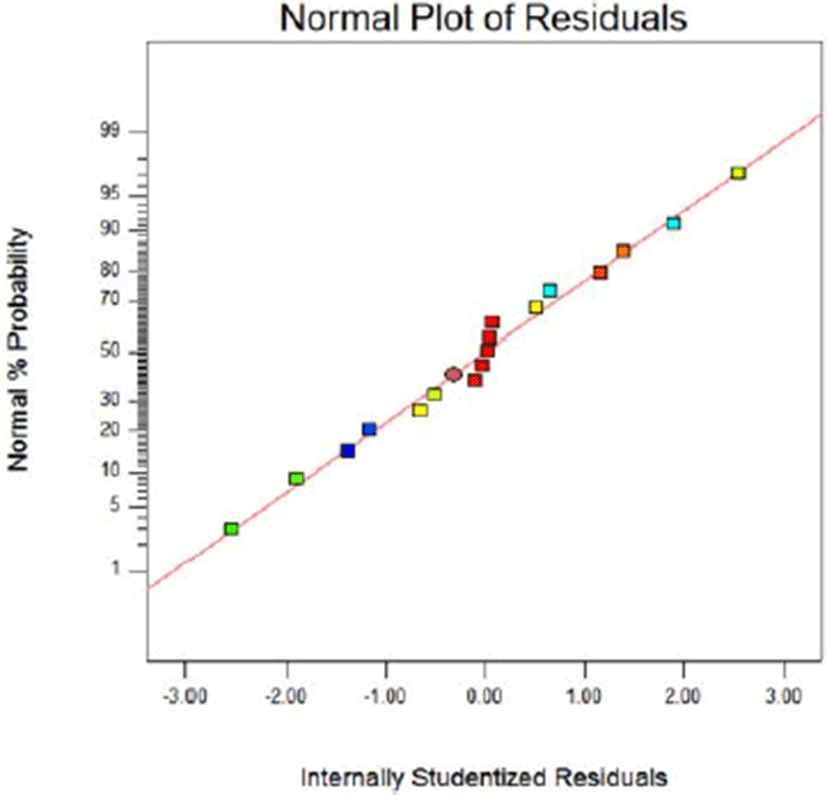
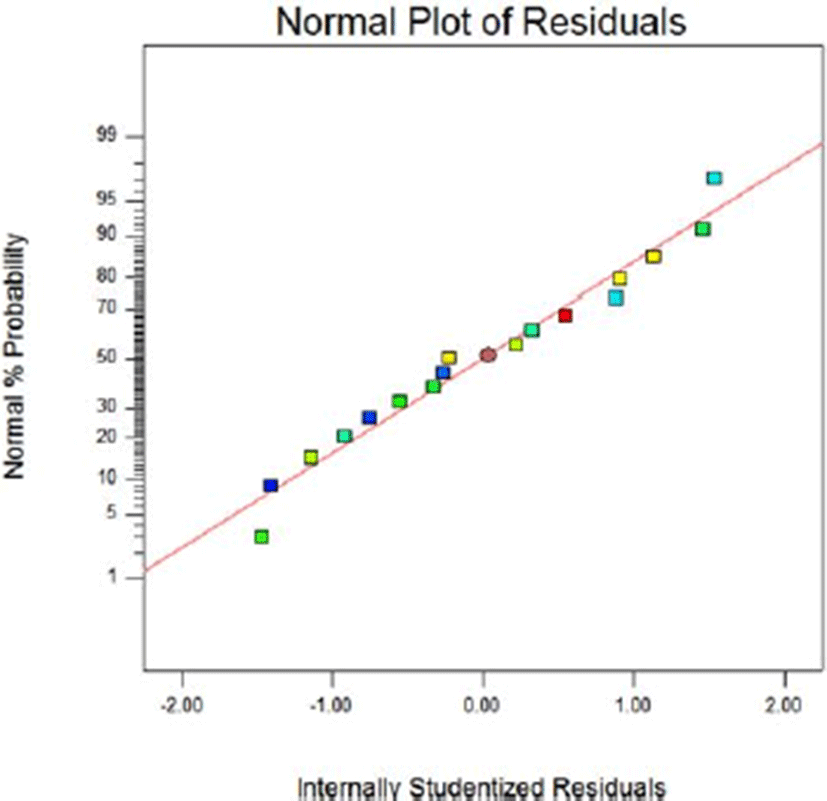
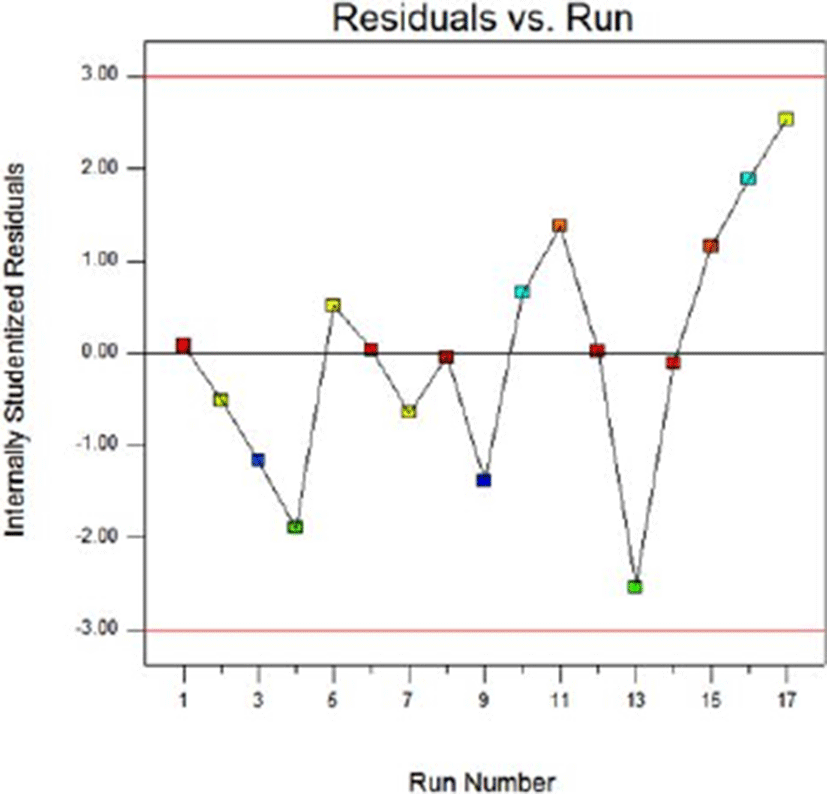
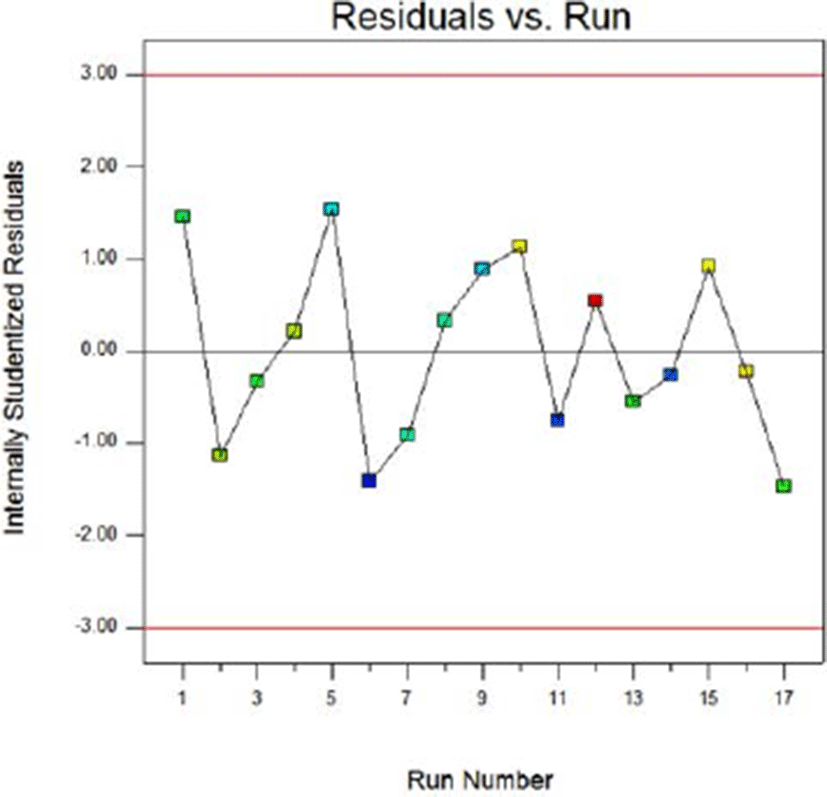
Fig. 8 and Fig. 9 show the normal probability plots of the sterilization rate and Fig. 10 and Fig. 11 show the normal probability plots of the water activity of sliced Larou. In order to better understand the fitting results, the normal probability plot can be expressed with residuals, which reflect the difference between the experimental and predicted values (Gu et al., 2015). In Fig. 8 and Fig. 10, the residuals are basically distributed around a straight line, indicating that the response transformation is not required (Kimizakis et al., 2014). The residual analysis is shown in the Fig. 9 and Fig. 11. All the data points obtained in the experiment are in the acceptable range, verifying the feasibility of the model.
According to the above model of the sterilization rate, considering the interaction of the experimental factors, the optimum parameters of the maximum sterilization rate were obtained. In the combination of 0.09% lysozyme, 1.25% sodium alginate, and 1.79% chitosan, the sterilization rate was predicted to be 99.68%. Under the above experimental conditions, the obtained sterilization rate was 99.65±0.13%, which was within the 95% confidence interval (96.3337%, 103.031%) of the predicted value. Therefore, the response surface analysis model of the sterilization rate of Larou is reliable.
According to the above model of water activity, considering the interaction of the experimental factors, the optimum parameters of the maximum sterilization rate were obtained. In the combination of 0.08% lysozyme, 1.42% sodium alginate, and 1.61% chitosan, water activity was predicted to be 0.882. Under the above experimental conditions, the obtained water activity was 0.885±0.0003, which was within the 95% confidence interval (0.864502, 0.899734) of the predicted value. Therefore, the response surface analysis model of water activity of Larou is reliable.
Based on the comprehensive consideration of the effects of two factors (sterilization rate and water activity), combined the sterilization rate with water activity, the optimal concentrations of lysozyme, sodium alginate, and chitosan were respectively determined to be 0.09, 1.40, and 1.60%. Under the above experimental conditions, the obtained sterilization rate and water activity were 99.69±0.47% and 0.887±0.0005. The results indicated the good prediction performance of the model.
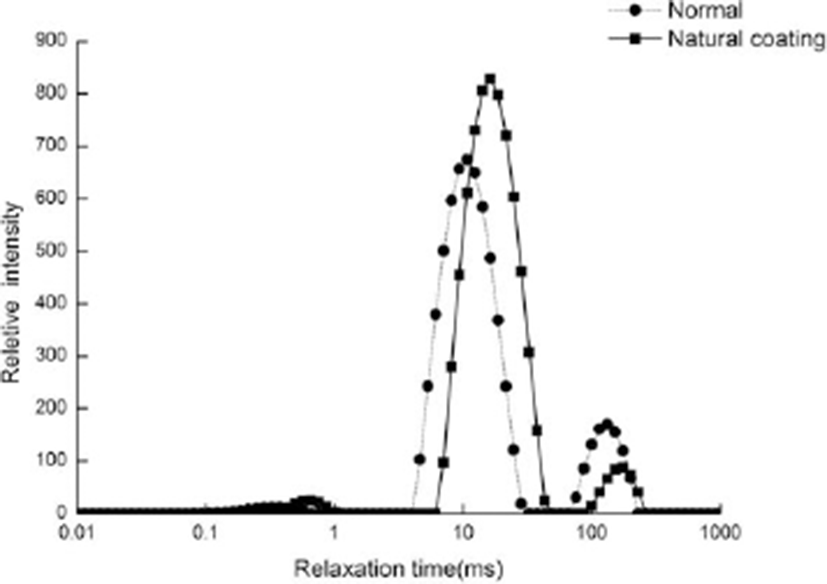
In this experiment, 4 peaks were detected in T2 relaxation pattern. According to the peak time and the proportion as well as previous reports (Borisova and Oreshkin, 1992; Tornberg et al., 2000), it can be divided into four areas: T20 (0.1–1 ms), T21 (1–10 ms), T22 (10–100 ms), and T23 (100–1,000 ms). The time difference of T20 (0.1–1 ms), T21 (1–10 ms), and T22 (10–100 ms) is not significant, but the time difference of T23 (100–1,000 ms) is significant. These changes may be caused by microbial growth and reproduction. Møller et al (2010) indicated that the migration and change of water in fermented sausages were caused by microbial fermentation. In addition, it is found that NMR relaxation property is a promising technique for controlling parameters and predicting microbial safety in meat production. As shown in Fig. 12, the peak area of sliced Larou treated with natural coating solution is less than that of untreated Larou, displaying a peak shift. The results indicated that water movement was enhanced after the treatment of natural coating solution. The proportion of each component calculated with the peak area indicated that after coating treatment, the proportions of T20 (0.1–1 ms) and T23 (100–1,000 ms) were respectively decreased by 2.0 and 8.4% (p<0.01) and the proportions of T21 (1–10 ms) and T22 (10–100 ms) were respectively increased by 3.0 and 8.3% (p<0.01). The above changes indicated that the treatment with natural coating solution changed the water distribution in sliced Larou. Bound water increased significantly and free water decreased significantly, thus increasing the sterilization rate. This result was consistent with the conclusion by Colzato M (2011). The data indicated that the coating treatment changed the moisture distribution of sliced Larou and eventually led to an extended shelf life by increasing the sterilization rate.
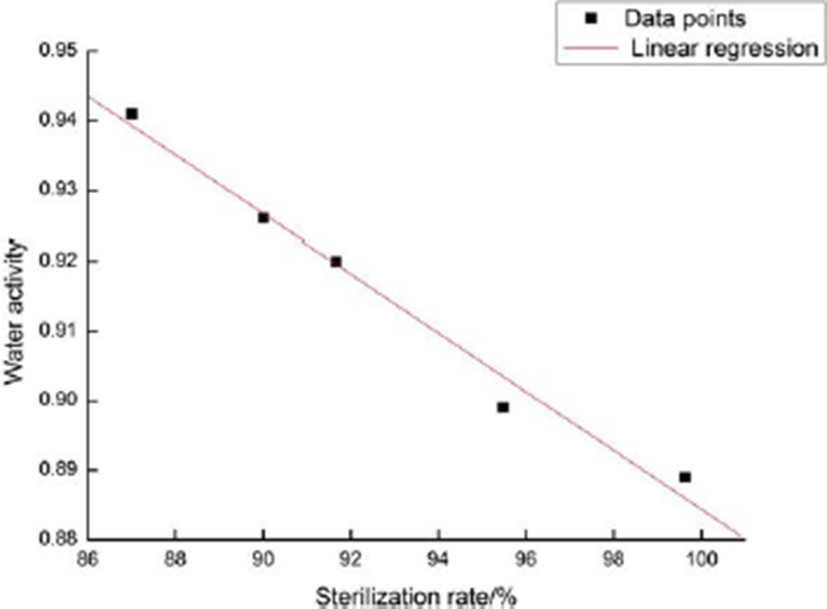
The correlation between sterilization rate and water activity is significant. Based on the data obtained in the response surface test, the relationship between sterilization rate and water activity of sliced Larou is shown in Fig. 13. The linear regression equation is y=1.27167–0.00385x and the linear relationship is the negative correlation (r=–0.99754, Sd=0.00163, p=0.000146). Under different concentrations of lysozyme, sodium alginate and chitosan in the coating solution, the higher the sterilization rate of preserved sliced Larou was, the smaller the water activity was. The correlation between the sterilization rate and water activity was significant, the decrease in water activity could inhibit the growth and reproduction of microorganisms and increase the rate of sterilization. Therefore, the sterilization rate could be used as an index to evaluate the shelf life of sliced Larou. The shelf life was correlated to water activity (Ju et al., 2016). The lower water activity indicates the higher sterilization rate and the longer shelf life.
The changes in the indexes of untreated and treated sliced Larou samples after 5–30 days are provided in Table 7. On Day 0, the total number of colonies of sliced Larou was (7.04±0.14) (Log CFU/g). During the whole storage period, the total number of colonies of the sliced Larou gradually increased(p<0.05). On Day 5, the total number of colonies of sliced Larou in the coating treatment group was reduced to (4.84±0.06) (Log CFU/g) and the POV of the treatment group was 0.0011 g/100 g higher than that of the untreated group. The POV of the treatment group on Day 30 was 0.0016 g/100 g higher than that on Day 5 and the change was not significant (p>0.05), indicating that the stability of the composite coating was higher. The coating reduced the POV of sliced Larou because the sodium alginate in the coating selectively increased the transmittance of CO2 and reduced the transmittance of O2 (Pornpimon and Sakamon, 2010). Therefore, the coating showed the better oxygen separation effect. The data of the moisture content showed that sodium alginate and chitosan membrane had the strong water absorption capacity, contributed to the good film-forming property of sodium alginate and chitosan and relieved the loss of water content in foods (Dong et al., 2006). On Day 5, the pH value in the coating treatment group was decreased. Due to the acid soluble chitosan film, Larou turned brown. Sliced Larou was in the acidic environment and pH was decreased. The pH value of treated sliced Larou showed an upward trend because meat proteins were decomposed into alkaline ammonia and amine compounds, such as organic bases, which contributed to the reproduction of bacteria. The sensory score of treated sliced Larou was higher than that of untreated sliced Larou. With the extension of storage time, the sensory score of coating treatment group meat decreased, In the 30-d storage, the sensory score showed no significant change because the natural coating liquid had a protective effect on the sliced Larou and prevented sliced Larou from decaying during storage. The rate of sterilization gradually decreases during the storage (p<0.05). It was proved that the quality of sliced Larou processed by coating was better than that of untreated sliced Larou.
Conclusion
The study aims to preserve the traditional Chinese sliced Larou by using a new natural coating solution. The effects of various factors were decreased according to the following sequence: A (the concentration of lysozyme)>B (the concentration of sodium alginate)>C (the concentration of chitosan). Under the combination of 0.09% lysozyme, 1.40% sodium alginate, and 1.60% chitosan, the final sterilization rate and the water activity of sliced Larou were respectively 99.69±0.47% and 0.887±0.0005. Response surface verification test and LF-NMR analysis proved that the model fitted well with the measured data. The test results proved that the sterilization rate was significantly correlated with water activity of sliced Larou. The data of the total numbers of colony, POV, moisture content, pH and sensory evaluation in the 30-d storage provide the theoretical basis for extending the shelf life of Larou with the natural coating solution.