Introduction
Acute myocardial infarction (AMI) is one of the most deadly manifestations of cardiovascular disease which occurs when there is an imbalance between supply and demand for oxygen that causes myocardial hypoxia (Geng et al., 2015). The heart needs oxygen but it is also susceptible to oxidative stress, which occurs during post-ischaemic reperfusion. Oxidative stress and inflammation play a central role in the pathogenesis of myocardial infarction (Neri et al., 2015) antioxidants are extensively consumed during infarction and O2 abruptly increases metabolism in the absence of normal defenses. Inflammatory processes are involved in cardiovascular injury resulting from ischemia. Ischaemia causes alterations in the defence mechanisms against oxygen free radicals; at the same time, production of oxygen free radicals increases.
ISO, a synthetic catecholamine and a β-adrenergic agonist, causes severe oxidative stress in the myocardium, which causes infarct-like necrosis in heart muscles (Lalitha et al., 2013). Among the mechanisms suggested in cardiac lesions resulting from the induction of isoproterenol, the production of cytotoxic free radicals in autoxidation of catecholamines is one of the most important causal factors (Mnafgui et al., 2016). Higher levels of catecholamines consume the energy reserve of the heart’s muscle cells, leading to irreversible cell damage and ultimately to complex biochemical and structural changes that cause necrosis (Geng et al., 2015; Senthil et al., 2007). Myocardial infarction with ISO is performed in experimental studies to evaluate many cardiac dysfunctions and to examine the efficacy of various natural and synthetic cardioprotective agents. Thus, similar to those observed in human myocardial infarction, many metabolic and morphological changes may occur in cardiac tissues of experimental animals (Khalil et al., 2015).
Kefir is one of the fermented dairy products containing lactic acid and acetic acid bacteria and microorganisms such as yeast. Kefir has been produced in countries of Central Asia, Russia and the Caucasia for many years and is widely used as a beverage and drug in the treatment of various diseases (Kilic et al., 1999). In addition to healthy individuals, it is recommended to consume for individuals with gastrointestinal and metabolic diseases, hypertension, ischemic heart disease, and for allergic patients (Koroleva, 1988a). During kefir fermentation, bioactive compounds are formed which are beneficial to health as a result of the symbiotic relationship between bacteria and yeast (Nurliyani et al., 2015). Kefir has a significant bioactivity and has been used as an antioxidant (Guven et al., 2003; Uchida et al., 2010), immunoregulators and antigenotoxic (Prado et al., 2015), hypolipidemic (Jascolka et al., 2013), and an anti-inflammatory agent. Due to its composition, kefir is mainly regarded as a probiotic source (Nalbantoglu et al., 2014).
AMI is a common disease with severe morbidity and mortality. Although kefir has antioxidant and hypolipidemic effects (Guven et al., 2003; Huang et al., 2013), and it is known that fermented dairy products (such as yogurt and sour milk) reduce the rate of cardiovascular diseases (Sonestedt et al., 2011), studies investigating the effect of kefir on myocardial infarction are limited. With this study; we investigated whether cardiac marker enzymes, lipid profile, and levels of GSH, MDA, AOPP have a protective effect on kefir in isoproterenol-induced acute myocardial infarction in rats.
Materials and Methods
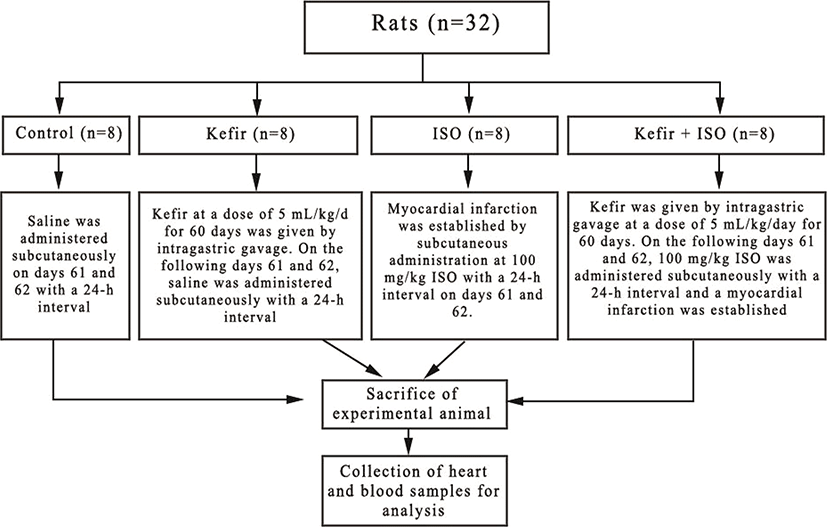
The test subjects of this study were obtained from the Van Yuzuncu Yil University Experimental Animal Unit. Thirty-two female Wistar Albino rats weighing 250-300 g, approximately 7-8 weeks old, were used for the study. Prior to the experiment, rats were adapted to the environment for 7 days. Rats were at the dark during the initiation of the experiement and were housed in cages with continuous feed and fresh water in the rooms set at ambient temperature of 22±2°C. The study was carried out with the approval of the Experimental Animals Local Ethics Committee of the Van Yuzuncu Yil university, which was given in 07/07/2017 and numbered 2017/06. Rats were randomly selected and divided into 4 groups as 1-control group, 2- kefir group, 3-isoproterenol group (ISO) and 4-kefir+isoproterenol group (kefir+ISO) shown as (Fig. 1). Control group (8 rats): Saline was administered subcutaneously on days 61 and 62 with a 24-h interval. Kefir group (8 rats): Kefir (Fahmy and Ismail, 2015) at a dose of 5 mL/kg/d for 60 days was given by intragastric gavage. On the following days 61 and 62, saline was administered subcutaneously with a 24-h interval. ISO group (8 rats): Myocardial infarction was established by subcutaneous administration at 100 mg/kg ISO (Sudha et al., 2013) with a 24-h interval on days 61 and 62. Kefir+ISO group (8 rats): Kefir was given by intragastric gavage at a dose of 5 mL/kg/day for 60 days. On the following days 61 and 62, 100 mg/kg ISO was administered subcutaneously with a 24 h interval and a myocardial infarction was established.
Pasteurized milk at room temperature was used to prepare the kefir. 20 grams of kefir granules were added and mixed in a 1/2 liter milk. The container lid was closed and allowed to ferment for 20 hours at room temperature. At the end of the fermentation, the granules were separated by a plastic filter, the filtrate granules were used for a new kefir.
Twelve hours after the last isoproterenol administration, all rats were given 90 mg/kg ketamine i.p., were decapitated, and blood samples were taken from them with anticoagulant and anticoagulant-free tubes. The hearts were immediately removed for histopathological examination and stored in 10% formaldehyde.
Serum levels of creatine kinase (CK), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), triglycerides, total cholesterol, very low density lipoprotein (VLDL), low density lipoprotein (LDL), high density lipoprotein (HDL) and glucose were measured by autoanalyzer, whole blood malondialdehyde (MDA), glutathione (GSH) and plasma advanced oxidation protein products (AOPP) levels were determined spectrophotometrically (Gutteridge et al., 1995; Jain et al., 1989; Beutler et al., 1963; Rizzi et al., 1988; Witko-Sarsat et al., 1999).
After necropsy, cardiac tissues were collected for histopathological evaluation, fixed in 10% formalin solution for 48 hours and then washed with tap water for 10 hours. After passing through the alcohol and xylene series for routine tissue fixing, samples was embedded in paraffin blocks. 4 µm thin slices were taken from each block and were prepared on the slides. They were then stained with hematoxylin-eosin (HE histopathological examination) followed by the Masson trichrome to evaluate the fibrous tissue in the adhesions with greater precision and examined under a light microscope.
The descriptive statistics for the features studied are mean and standard deviation. Kruskal Wallis test was used to compare the groups in terms of these characteristics. Duncan test was performed to identify different groups. The statistical significance level in the calculations was 5% and the statistical package program SPSS (ver. 21) was used for the calculations.
Results
The triglyceride, total cholesterol, HDL, VLDL, LDL and glucose levels of control, kefir, ISO, kefir+ISO groups were given in Table 1. No significant changes in lipid profile and glucose levels were found between ISO and kefir+ISO groups. The triglyceride and VLDL levels of the kefir group were significantly lower (p<0.001) than controls. There was no significant difference in HDL level between all groups.
The averages of ALT, AST, CK and LDH activities of control, kefir, ISO, kefir+ISO groups were presented in Table 2. In this study, serum AST, CK, and LDH as cardiac marker enzymes were significantly increased in ISO administrated rats. With the addition of kefir, the activity of all these enzymes decreased and the toxic effects of ISO decreased significantly (p<0.001).
The averages of GSH, MDA, AOPP levels for control, kefir, ISO, and kefir+ISO groups were shown in Table 3. In the kefir+ISO group, MDA, and AOPP levels decreased significantly (p<0.001) and GSH level increased (p<0.05) compared to the ISO group.
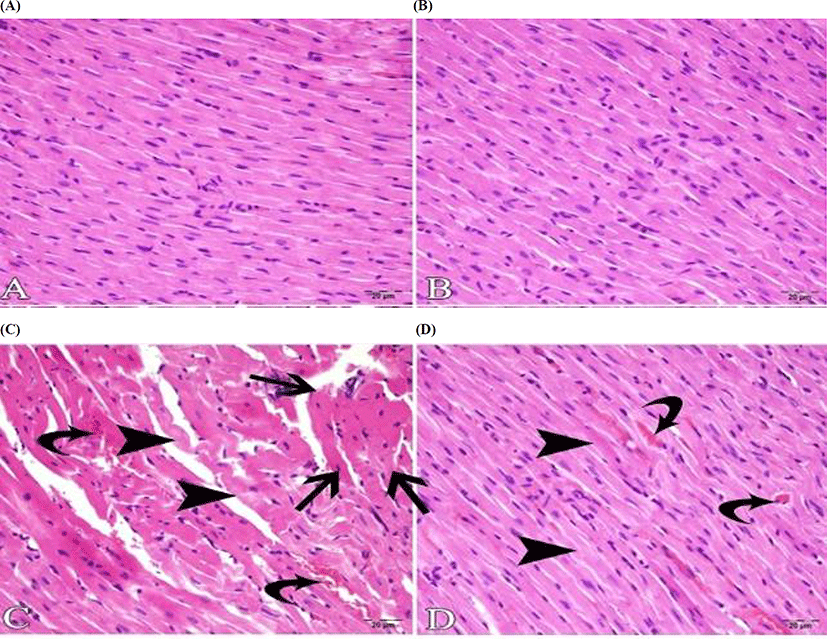
Histopathological examination of the cardiac tissue showed that the endocardium, myocardium and pericardium of the control (Fig. 2A) and kefir groups (Fig. 2B) were in normal histological structure. In the ISO group, due to hemorrhagic, necrotic myocarditis, it was observed that the structure of muscle fibers was deteriorated locally. As for the myocardium, severe bleeding, hyaline degeneration in muscle fibers, and interstitial edema and mononuclear cell infiltration in the myocardium with Zenker necrosis were observed (Fig. 2C). In the kefir+ISO group, it was observed that these inflammatory events were very obvious, with very few inflammatory cells in the myocardium. No necrosis was seen, although there was hyperemia in the veins and mild hyaline degeneration in some muscle fibers (Fig. 2D).
Discussion
High serum total cholesterol levels are generally considered to be a risk factor for cardiovascular diseases (Liu et al., 2006). It is therefore important to prevent cardiovascular diseases, especially to reduce elevated serum cholesterol levels. It is possible to effectively reduce high levels of cholesterol with pharmacological agents, but these drugs are not only expensive, but they also have side effects (Jones et al., 2004).
In recent years, new approaches to reducing high levels of cholesterol have been discussed. Among these applications, the use of probiotic bacteria also has an important place. Probiotics (Hotel and Córdoba, 2001), which are defined as microorganisms that provide beneficial effects to the host’s health when taken in sufficient amounts, are quite remarkable because of decreased lipid and cholesterol levels (Ooi et al., 2010). Lactic acid bacteria exhibit hypocholesterolemic effect with various mechanisms: By binding bile acids and cholesterol to bacterial cells, exogenous cholesterol absorption is inhibited in the small intestines (Liu et al., 2006). As a function of the hydrolase activity of bacterial bile salt, bile salt resorption is suppressed by deconjugation (Xiao et al., 2003), cholesterol is assimilated to reduce the level of luminal cholesterol suitable for absorption (Pereira and Gibson, 2002). Lactobacillus bacteria can also produce ferulic acid that stimulates the inhibition of hepatic HMG CoA reductase (Tomaro-Duchesneau et al., 2012a; Tomaro-Duchesneau et al., 2012b) and the elimination of acid sterols (Kim et al., 2003).
Some investigators have observed that levels of total cholesterol, triglycerides, LDL, VLDL were significantly reduced and HDL cholesterol was elevated (Angelis-Pereira et al., 2013) but some investigators reported that kefir had no effect on the lipid profile (St-Onge et al., 2002). James et al. (1999) indicated that every 1% reduction in cholesterol reduces the risk of heart disease by 2-3%, thus the regular consumption of fermented milk containing a suitable Lactobacillus acidophilus strain has a potential reduction of coronary heart disease risk by 6-10%.
High calcium intake from dairy products or dietary supplements has been shown to lower serum triglyceride and LDL-cholesterol levels in humans. This significant increase in calcium intake might be responsible for the hypocholesterolemic effects of kefir drink, as several studies have shown that high calcium intake improves the serum lipid profile, mainly via reducing serum triglyceride and LDL-cholesterol levels (Denke et al., 1993; Soerensen et al., 2014). In this study, only the triglyceride and VLDL levels of the kefir group were found to be significantly lower (p<0.001) than the controls. Kefir appears to have a reducing effect on triglycerides and VLDL. On the other hand, while in most studies with ISO-induced myocardial infarction, the level of triglycerides (according to controls) was increased (Abbas, 2016; Shahat et al., 2016; Dhivya et al., 2017), this phenomena was not observed in this study; on the contrary, the triglyceride levels of the ISO and ISO+kefir groups were significantly lower than the control and kefir groups (p<0.001). In this study, it has been observed that after the application of ISO, the feet of animals were stranded, where they had a quiet posture and did not feed. Triglyceride levels may also have decreased because the animals have not been fully fed for 48 hours. Ji and Friedman (2003) analyzed fasting triglyceride levels at 12, 18 and 24th hour in rats, and they found that fasting triglyceride levels increased at 18 h to 12 h but decreased significantly at 24 h to 12 h (p<0.05). Nwokocha et al. (2017) also found that the triglyceride level of the ISO group was lower than the controls (p<0.05). The highest cholesterol level was found in the ISO group, although the decrease was observed with the addition of kefir, no significance was determined. It has been reported that different strains of lactic acid bacteria may have different effects on serum cholesterol concentration (Anderson and Gilliland, 1999).
The microbial composition of the kefir granules varies according to the origin of the kefir granules in culture and production methods (Pintado et al., 1996). For this reason, the conflicting results obtained with kefir may depend on the variability of kefir granules used in experimental studies and production methods. It is also stated that the duration of kefir addition is also important (Liu et al., 2006). In this study, kefir was given for 60 days. There were no statistical changes in total cholesterol levels between the control group and the kefir group, nor between the ISO group and the kefir+ISO group. The reason is that the hyperlipidemic situation is not the case because all animals in the study are fed a normal diet. Again, the LDL level of the ISO group was found to be higher than the other three groups (p<0.001), although there was no signifycant difference between the ISO and kefir+ISO groups even though the kefir administration started to decrease LDL level.
Hyperglycemia is common during acute myocardial infarction (Lavi et al., 2008; Marfella et al., 2005; Nordin et al., 2005; Lobo and Shenoy, 2015). In the early hours of acute myocardial infarction there is a greater release of catecholamines and glucose intolerance. These anomalies negatively affect the results (Diaz et al., 1998). Experimental and clinical investigations have demonstrated that rapid increases in serum glucose increase the size of the infarction (Esposito et al., 2002; Williams et al., 1998), which impairs left ventricular function and causes microvascular obstruction (Jensen et al., 2011). Hyperglycemia has been reported to increase interstitial fibrosis and apoptosis of myocytes (Shiomi et al., 2003). Ischemic preconditioning is a powerful endogenous mechanism that protects myocardial infarction. Hyperglycemia has been shown to block the cardioprotective effect of ischemic preconditioning by closing the KATP channels (Kersten et al., 2001).
It has been reported that kefir has a potency to reduce hyperglycemia. Although its mechanics are not fully understood, it has been shown that kefir is effective because of its immunomodulatory properties with bioactive components such as exopolysaccharides, peptides and antioxidants. The exopolysaccharide is a biopolymer that lowers blood glucose level. By covering the intestinal microvilli, it inhibits the absorption of glucose and therefore does not increase glucose in the body. Another mechanism is that the exopolysaccharide modulates insulin signaling through c-AMP. Increased c-AMP in pancreatic cells contributes to increased insulin secretion from pancreatic β-cells (Hadisaputro et al., 2012).
Probiotic use in diabetic patients did not show a significant difference in fasting blood glucose, serum triglyceride, total cholesterol and LDL-cholesterol levels (Mazloom et al., 2013). Ejtahed et al. (2012) reported that consumption of probiotic yogurt in type 2 diabetic patients improves fasting blood glucose, HbA1c and antioxidant status. Maeda et al. (2004)in vitro studies have shown that kefir reduces blood glucose level.
In this study, it was observed that the blood glucose level was significantly higher in the ISO given groups than in the control group (p<0.05). As such, elevated blood glucose compared to controls is an expected condition for ISO-induced myocardial infarction (Shenoy and Lobo, 2015). It was seen that blood sugar level that rose did not fall with kefir. It was only determined that the glucose level of the kefir group was similar to that of the control group and that there was no significance between the control group and the kefir group. Since there is no difference between the diabetic animals’ blood characteristics used in this study, a significant change in kefir blood glucose can not be expected under normal conditions.
The myocardium contains some markers that allow a wide range of diagnoses of myocardial infarction. When insufficient oxygen or nutrients can not be supplied, cytosolic enzymes leak into the bloodstream and increased serum concentrations (Abbas, 2016). Cardiac enzymes are enzymes present in myocardial cells. When the myocard is damaged for whatever reason, these enzymes are released in the blood and the level of enzyme increases.
Creatine kinase is one of the first markers identified as biochemical markers for myocardial damage (Duma and Seigel, 1965). It has a clinical sensitivity of 90% for the diagnosis of acute myocardial infarction. It is released within 12 hours after the onset of symptoms of acute myocardial infarction, reaching its maximum in 24-36 hours in the serum and normalizing in 48-72 hours (Al-Hadi and Fox, 2009). LDH is a delayed marker of myocardial damage, which begins to increase within 12-24 hours after injury and peaks within 2 to 3 days (Ojha et al., 2010). Increased ALT and AST activity in the bloodstream may sometimes reveal damage to cardiac tissue (Alam et al., 2017).
The application of ISO causes damage to heart, and increased permeability in the cell membrane or destruction of the cell results in the release of cardiac enzymes into the bloodstream. The increase of these cardiac enzymes in myocardial infarction induced by ISO has been demonstrated in all studies (Abbas, 2016; Alam et al., 2017; Ahmed et al., 2017). In this study the activity of cardiac marker enzymes decreased with the addition of kefir (p<0.001).
Oxidative stress in cardiac and vascular myocytes is defined as damage to cells as a result of increased ROS formation and/or decreased antioxidant reserve (Dhalla et al., 1992). The increase in the formation of ROS is caused by degraded mitochondrial reduction of molecular oxygen, secretion of ROS by white blood cells, endothelial dysfunction, autoxidation of catecholamines, radiation or air pollution. The deleterious effects of ROS are mainly due to the fact that ROS causes changes in the subcellular organelles and causes intracellular Ca2+ overload.
There is a growing interest in finding natural antioxidants from food. It is a fact that it protects the body against attacks by free radicals and is believed to delay the progression of many chronic diseases (Pryor, 1991). Antioxidants from natural sources are more preferred than those produced chemically, since some synthetic antioxidants have been reported as carcinogenic (Imaida et al., 1983). There are studies that report that kefir has antioxidant activity (Liu et al., 2005a; Cenesiz et al., 2008; Delen et al., 2015; Fahmy and Ismail, 2015; Yener et al., 2015). Protein content and probiotic microflora are among the main factors affecting the antioxidant properties of fermented milk (Najgebauer-Lejko and Sady, 2015). It has been reported that some proteins in dairy products have antioxidant potential (Wong and Kitts, 2003). Peptides derived from milk protein hydrolyzates inhibit lipid oxidation. The specific peptidic structure of specific groups of side chains of amino acid residues or antioxidant peptides can lead to the chelation of prooxidative metal ions and the termination of radical chain reactions (Peña-Ramos and Xiong, 2001; Liu et al., 2005a; Liu et al., 2005b). The reduction capacity of a compound is an important indicator of its potential antioxidant activity (Meir et al., 1995). The reduction power of milk is increased significantly by the fermentation of kefir, which is attributed to lactic acid bacteria that can exhibit an excellent reducing power (Liu et al., 2005b).
MDA levels are also used as an indicator of lipid peroxidation in biological material. Because; MDA is the only consequence of lipid peroxidation and changes in its level indicate changes in lipid peroxidation (Goncu, 2010). It has been shown that kefir inhibits lipid peroxidation and, therefore, reduces MDA levels in different pathological conditions (Guven et al., 2003; Ozcan et al., 2009; Yener et al., 2015; Fahmy and Ismail, 2015). In this study, it was also found that high MDA level decreased in the group given only isoproterenol by giving kefir (p<0.05). Kefir appears to have reduced lipid peroxidation and antioxidant activity.
Oxidation of proteins results in the covalent modification of proteins with reactive oxygen derivatives or oxidative stress products. AOPP has been used in recent years as a novel marker for protein oxidation and is described as products of cross-linked proteins containing dithyrosine. Oxidative stress is a sensitive marker for determining the degree of protein oxidation and the effect on proteins (Karacan, 2013). A limited number of studies (Li et al., 2014; Ulla et al., 2017) found that isoproterenol elevates AOPP levels in isoproterenolone. In this study, the AOPP level of the isoproterenol group was higher than other groups statistically at the p<0.001 level. The amounts of AOPP in the Kefir+ISO group approached the control group when kefir was given. Based on this result, kefir appears to reduce oxidative stress by inhibiting the oxidation of the protein.
Glutathione synthesized as a tripeptide (glutamic acid, cysteine, glycine) in the cell provides a reducing power to cells using NADPH. More than 99% of glutathione is present in reduced form. It is an important antioxidant in the cell because of its thiol group and its high concentration of cysteine (0.1-10 mM). Several pathological conditions were investigated in different studies (Guven et al., 2003; Ozcan et al., 2009; Yener et al., 2015; Fahmy and Ismail, 2015). In this study, it was observed that in the group that received ISO, the level of GSH decreased with respect to the control group and increased with the treatment with kefir. It can be said that kefir is an effective antioxidant and due to the presence of cysteine in its structure (Menestrina et al., 2016), which increases the synthesis of glutathione.
Conclusion
This is one of the premise studies that examined the effect of kefir in the ISO-induced myocardial infarction. Kefir was given for 60 days and the protective effect of kefir was investigated in detail by applying ISO. Compared to the ISO group, the levels of AST, CK, LDH, MDA, AOPP of kefir+ISO group were decreased significantly and the level of GSH increased. There were no significant changes in lipid profile and glucose levels between these two groups. In conclusion, looking at the findings of cardiac enzymes activities and histopathological changes of the myocardium, it can be said that the administration of kefir preserves the heart tissue from the toxic damage of the ISO by its antioxidant function.