Introduction
Reactive oxygen species (ROS) are products of cellular metabolism in living organisms (Yigit et al., 2014), and high levels of ROS can cause lipid peroxidation (Yamaguchi et al., 1994), protein carbonylation (Repine et al., 1981) and DNA damage (Cerutti, 1985). Meanwhile, ROS may lead to a series of diseases, such as diabetes, hypertension, cancer and atherosclerotic cardiovascular disease (Finkel et al., 2000; Memarpoor-Yazdi et al., 2012). Recently, more and more researchers had focused on extraction of novel, safe and natural antioxidants, for instance, carotenoids and vitamin A (Choudhry et al., 2016; Korekar et al., 2014; Maadane et al., 2015; Páramo et al., 2012). Such antioxidants are mainly obtained from vegetables, fruits, foods (Geetha et al., 2002; Podsedk, 2007), and are regarded as the second antioxidant defense line in living organisms (Yigit et al., 2014). In addition to these antioxidants, there also contain antioxidant glutathione peroxidase (GSH-Px) enzyme, superoxide dismutase (SOD) and catalase (CAT) (Lin et al., 2005; Surai et al., 1996; Wilson et al., 1992). As the first antioxidant defense line, these components act as ROS scavenging to protect the cell structure with the second antioxidant defense line together. Therefore, bioactive proteins from foods have gained extensive attentions due to their safety and health benefits.
Considering its rich and highly digestible proteins (Lechevalier et al., 2017), egg is an important nutrient due to many desirable functional attributes in daily diet (Baldwin et al., 1986). Of these, hen eggs produce many bioactive ingredients during incubation stage (Surai et al., 2002). Proteins derived from hen eggs and embryos mainly include ovalbumin, (Duan et al., 2013), GSH-Px, SOD, and so on (Suria et al., 2002). Researchers showed that these proteins had various activities, such as, antioxidant, antibacterial and anti inflammatory activities (Duan et al., 2014a; Duan et al., 2014b; Erdmann et al., 2008; Miguel et al., 2007; Mine et al., 2007). While embryos are developing, more oxygen was consumed, increasing the risk of being affected by ROS. Previous study has obtained the antioxidant peptide from embryo, and the peptide showed a high inhibition on linoleic acid autoxidation and ABTS radicals (Duan et al., 2014a). Furthermore, the differences of antioxidant capacity in different tissues of chick embryo are also detected during each embryonic development of the entire stage (Chay et al., 2011; Surai et al., 2000). However, the information of antioxidant protein during embryonic development of the entire stage is still unclear. Thus, the objective of this study was to isolate and characterize a natural antioxidant protein from fertilized eggs.
Materials and Methods
Fertilized eggs were obtained from a hatchery (Changlv Native Products Co., China). Eggs were incubated at 37.8°C and 60% relative humidity in incubator (Photoshop Solar Energy Co., China). All eggs were turned through 90° every 90 min. Fertilized eggs were collected on days 0, 5, 7, 10, 12, 14, 16, 18, 20 of incubation. Six samples on same day were homogenized in a high-speed tissue triturator (Langyue instrument, China) and were freeze-dried at −80°C for 24 h. The totally dried samples were stored at −70°C for further analysis. All the testing chemicals were of analytical grade and purchased in Hefei Chemical Reagents Company (China).
The method to extract protein was conducted according to the method of Zhong et al. (2014) with some modifications. Dried samples were defatted by 20 volume (W/V) of acetone for 48 h and dried at 4°C for 12 h. Dried powder was collected and stored at −80°C. Based on previous work, proteins were extracted from the defatted dried powder by mixing with 31 volumes of PBS (0.2 M, pH 6.8) and maintaining at 55°C for 93 min. After centrifuged at 6000 rpm/min for 15 min at 4°C, the supernatant was collected and dialyzed against deionized water using a dialysis bag of 8-14 kDa for 48 h, the retentate were lyophilized and stored at −80°C for further analysis.
DPPH radical scavenging activity (RSA) was measured according to the method described by Je et al. (2005). 2.0 mL of each sample (10 mg/mL in PBS buffer) was added to 2 mL of 95% ethanol containing 0.1 mM DPPH radicals. The mixture was placed in the dark for 30 min, and the absorbance of the reaction solution was determined at 517 nm. The control was operated in the same way where 95% ethanol was used replaced the sample solution. Each experiment was repeated 3 times to determine the average value. The scavenging effect was calculated as the following equation:
Where Ac is the absorbance of the control, As is the absorbance of the sample mixture.
The O2−• radical scavenging activity was assayed according to the method described by Duan et al. (2014). Briefly, reaction solution was made up of sample solution (1.5 mL, 10 mg/mL protein in PBS buffer) and Tris-HCl buffer (4.5 mL,50 mM, pH 8.0) were incubated at 25°C for 10 min, later 300 uL of 3 mM pyrogallic acid (dissolved in 10 mM HCl) was added. The absorbance of reaction mixture at 325 nm was measured immediately at 30 s intervals. The autoxidation rate constant (Kb) was calculated from the curve of A325 nm vs time. Each experiment was repeated 3 times to determine the average value. The inhibitory actions of test samples against the autoxidation rate indicated their O2−• scavenging abilities.
The Hydroxyl radical scavenging activity was assayed according to the method described by Li et al. (2008) with a few modifications. The •OH was generated by mixing 1.0 mL FeSO4 (5 mM) and 1.0 mL H2O2 (0.1%), then, added 1 mL phenanthroline solution (5 mM), Mixed well and incubated at 37°C for 1 h, the absorbance of mixed solution was measured at 536 nm. The percentage scavenging effect was calculated as the following equation:
Where A1 absorbance of the solution containing FeSO4, H2O2, phenanthroline solution; A2 absorbance of solution containing phenanthroline and FeSO4; A3 absorbance of solution which has 1 mL sample solution.
The crude protein samples on day 16 were dissolved in 10 mM phosphate buffer (pH 7.2), and centrifuged at 10,000 rpm for 15 min. The supernatant was collected and loaded on a DEAE cellulose 52 column (16×500 mm) previously equilibrated with 10 mM phosphate buffer (pH 7.2). The unbound protein was washed with the same buffer and the bound protein was eluted by increasing the concentration of NaCl (0.1, 0.3, 0.5 M) in phosphate buffer at a flow rate of 5 mL/min. After elution, the absorbance of tube was read at a wavelength of 280 nm, and four peaks including D1, D2, D3, D4 were collected. Each peak was pooled, dialyzed, freeze-dried, and the totally dried powders were used for DPPH radical scavenging assay.
The most active fraction D2 was dissolved in phosphate buffer (10 mM, pH 7.2) and applied to a Sephadex G-100 column (10×300 mm). Then, eluted with 10 mM phosphate buffer (pH 7.2) at o flow of 1 mL/min, the elution was detected at 280 nm. The peak was pooled, dialyzed, freeze-dried for further study.
The molecular weight of protein was evaluated according to method of Laemmli (1970). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 5% stacking gel and 12% separating gel. The gels were stained with coomassie brilliant blue R-250 for 2 h, then, the gels were de-stained for 24 h. The protein was separated at 80 V for 30 min and at 120 V for 100 min. A pre-stained standard protein marker (18.0-94.0 kDa) was run in the gel to determine molecular weight of the proteins.
The band was excised from the gel and identified by a LC-LTQ Orbitrap Velos Pro (Thermo Scientific, USA). The digestion of the band was detected according to Katayama (2001). After the digestion treatment, the supernatant was collected and completely dried for LC-MS analysis. Sample was separated by the Nano-HPLC liquid phase system EASY-n LC1000 and analyzed by LTQ Orbitrap Velos Pro (Thermo Scientific, USA). The mass spectrometry parameters were as follows: analysis of time 105 min, spray voltage 1.8 kV, interface heater temperature 250°C, with parent ion scanning range of 350-1800 m/z. The parameters of Information Dependent Acquisition (IDA) were as follows: fracture mode of collision-induced dissociation, normal chemical energy 35%, the q value 0.25, activation time 30 ms. The MASCOT V2.3 search engine (Matrix Science Ltd., U.K.) was adopted to analysis data, using the Gallus gallus database as search parameters.
The D2-S protein (20 mg/mL) was added to SDS-PAGE. After electrophoresis, the strip was cut and placed in a centrifuge tube, then discolored at 50% CAN/25 Mm NH4HCO3 solutions for 30 min. 15 ng/µL of trypsin and chymotrypsin were added and incubated for 40 min at 4°C and then, 5-10 µL of 25 mmol/L NH4HCO3 solution to each tube was added and water bath 37°C for 12 h. Subsequently, 1% TFA-50% ACN-50% water 200 µL were added for 1 h at 37°C water bath and transferred to another new EP tube. The lyophilized sample was dissolved in 0.1% formic acid, 2% acetonitrile, mixed and centrifugated at 13,200 rpm for 10 min at 4°C. The supernatant was subjected to Q Exactive mass spectrometer (Thermo Scientific, USA). Proteome Discoverer software was adopted to analyze the original data of the mass spectrometry to obtain the target protein sequence information.
The experiment was conducted in triplicate. All results were expressed as mean±standard deviation (n=3) and were analyzed using SPSS 16.0 software (SPSS Inc., USA). One-way analysis of variance (ANOVA) was used to analyze data, the differences between the means were separated using Duncan’s multiple range test. Results were considered statistically significant at p<0.05.
Results
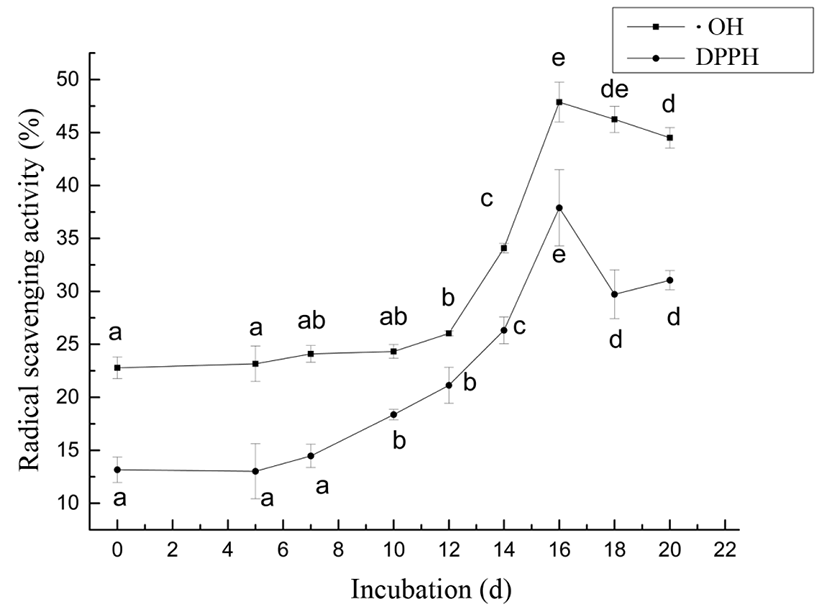
As shown in Fig. 1, the DPPH radical scavenging activity had no significant change before day 7, and then continuously increased with the change of hatching days until day 16 and reached its maximum 37.89±3.60%. Meanwhile, the protein on day 16 possessed the highest scavenging rates on •OH, 47.87±1.88% in Fig. 1. As shown in Table 1, the value of Kb on day 16 was significantly smaller than the other days (p<0.05). The results suggested that protein on day 16 had the highest capacity to scavenging radicals.
| Incubation time (d) | Kb value (*10−6) |
|---|---|
| 0 | 52.39±2.34de |
| 5 | 56.70±2.31f |
| 7 | 50.00±1.77d |
| 10 | 53.34±2.62de |
| 12 | 41.78±3.75c |
| 14 | 34.62±1.16b |
| 16 | 21.24±4.13a |
| 18 | 33.31±1.29b |
| 20 | 30.70±0.72b |
The results were expressed as means ± standard deviation (n=4), different characters (a, b) represent significant differences (p<0.05). Kb value means auto-oxidation rate of pyrogallic.
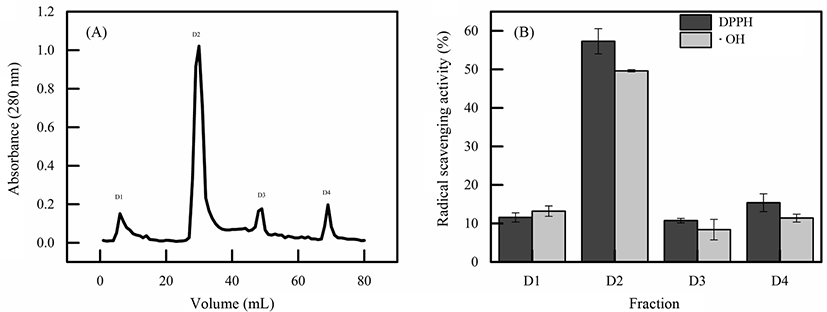
The protein on day 16 was dissolved in 10 mM phosphate buffer and applied to DEAE cellulose column, then, eluted with the same buffer. As shown in Fig. 2A, four peaks, number fraction D1 to D4, were collected, dialyzed and lyophilized separately. These fractions were detected their radical scavenging ability on DPPH and •OH (Fig. 2B). The results showed the DPPH and •OH radical scavenging ability of each peaks, among four factions, fraction D2 showed the highest ability (Fig. 2B), reached 57.32±3.28%, 49.63±0.23%. Thus, fraction D2 was used for further purification.
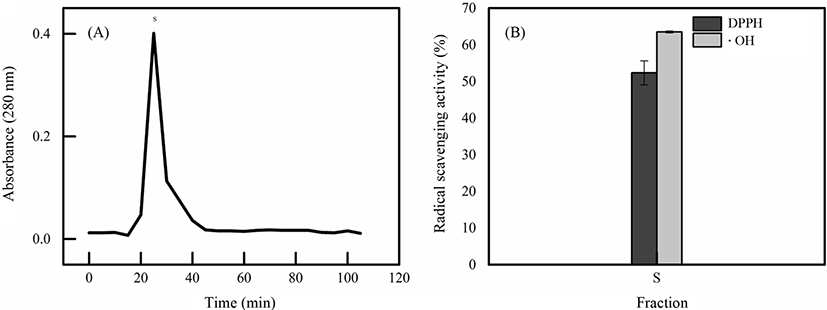
The D2 peak was dissolved in 10 mM phosphate buffer and purified further with Sephadex G-100. The active peak D2 protein was rechromatographed on Sephadex G-100 column resulted in single peak (Fig. 3A), indicating the preparation of the antioxidant protein from fertilized eggs has been accomplished. Fig. 3B showed the radical scavenging ability on DPPH and •OH of fraction D2-S were 52.34±3.27%, 63.49±0.25%, respectively.
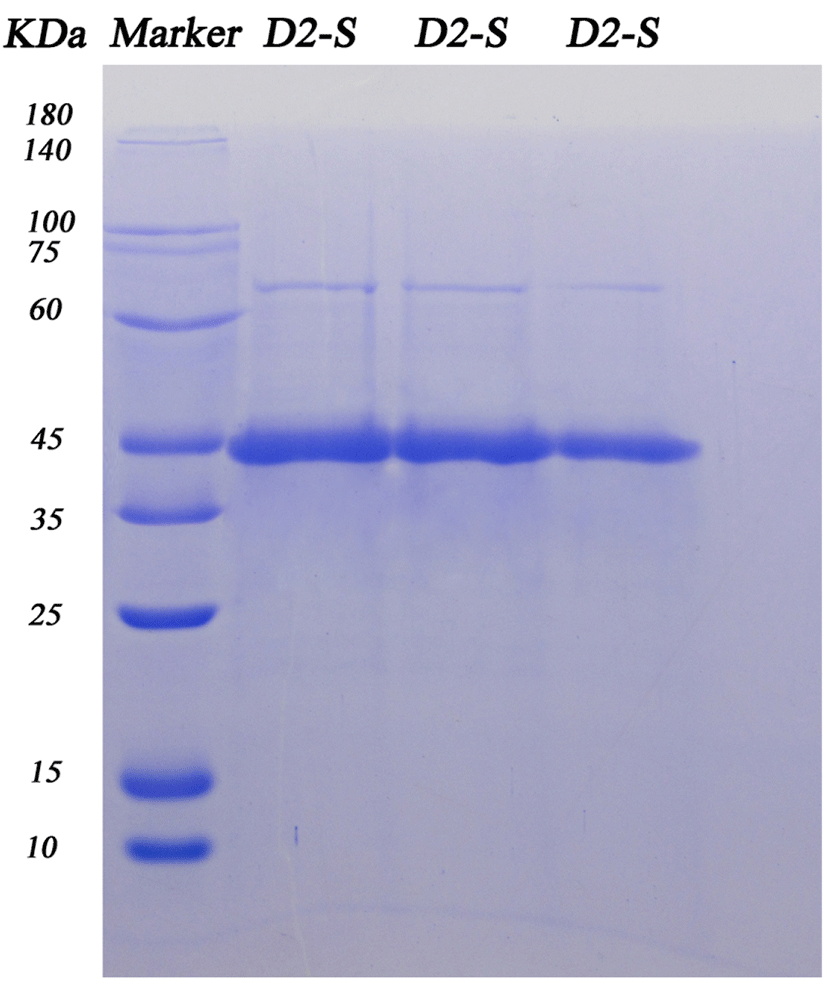
The SDS-PAGE results showed that it’s a single band and molecular weight was about 45 kDa. Fig. 4 The protein band was obtained and analyzed by EASY-Nlc 1000-LTQ Orbitrap Velos Pro (Thermo Finnigan). The purified protein was digested, isolated and analyzed by LC-MS, the obtained peptide sequences was compared with the sequences of the protein (Gallus gallus) in NCBI. The result exhibited that purified protein sequence was highly similar to ovalbumin (gi|28566340|gb| AAO43266.1|) (Table 2) and sequence coverage reached 84%, this was a more reliable identification result. LC-MS analysis of purified protein showed its molecular weight was 43.22 kDa and the isoelectric point of 5.19.
The matched peptides shown in black, the sequence coverage reached 84%. The molecular weight was 43.220 Da and the calculated pI value was 5.19 of purified protein.
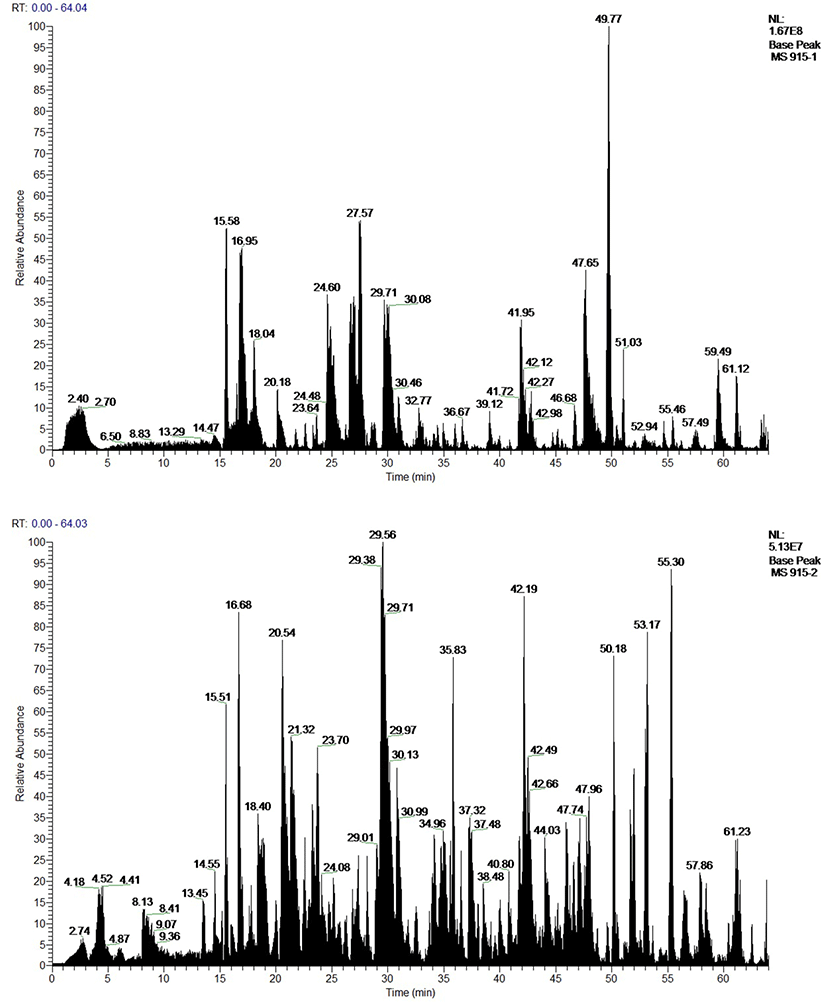
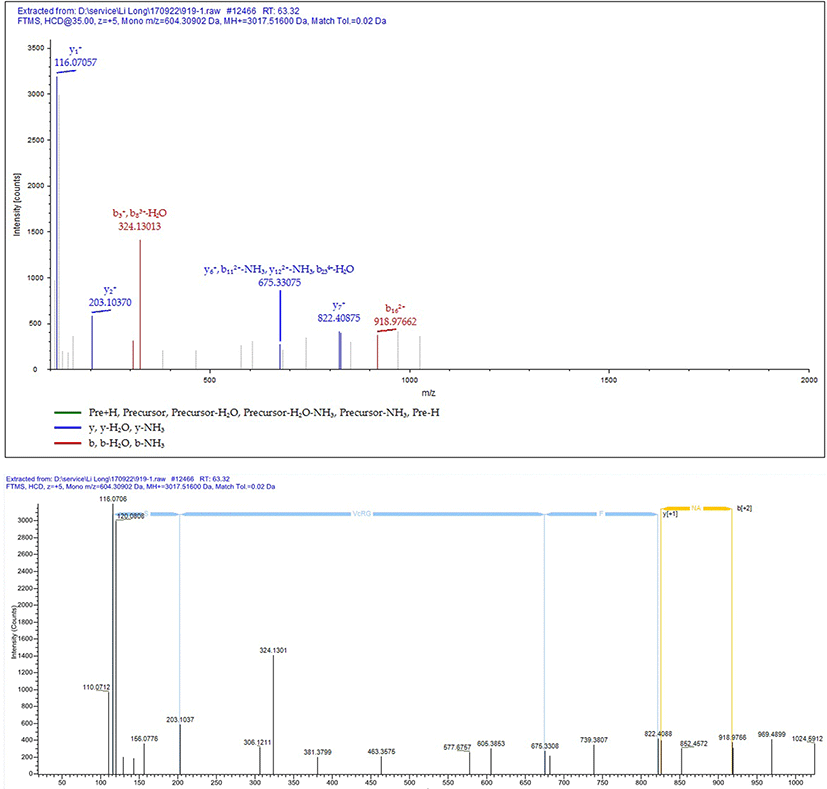
According to the theoretical sequence of the sample, we used trypsin and chemotrypsin for protease digestion and subsequent matching analysis. Fig. 5A and 5B showed TIC maps of protein D2-S digested by trypsin and chemotrysin. Fig. 5C and 5D showed C-terminal sequence secondary mass spectrometry of the protein D2-S. According to results of TIC map and secondary mass spectrometry, protein D2-S C-terminal sequence was NAVLFFGRCVSP, which was consistent with the sequence of ovabumin.
Discussion
Fertilized hen eggs are traditionally considered as dietary supplement in many Asian countries for their nutrition value. During incubation, embryo requires more oxygen to maintain energy, especially in the formation of tissues and organs, and the improvement of respiratory function (Vlecket al., 1980). In this work, the protein derived from fertilized eggs showed unequal radical scavenging capacity on DPPH, •OH, O2−• in different incubation periods, and the ability of radical scavenging was significantly increased after 10-d incubation, which was consistent with the results of Duan (2014a). The protein derived from fertilized eggs on day 16 with stronger radical scavenging capacity on DPPH, •OH, O2−•, which suggested that it had the strongest capacity to convert free radicals to stable products, prevent the radical chain reaction, and avoid cell damage caused by hydroxyl radicals-the most reactive of all the reduced forms of dioxygen (Je et al., 2007; Sivapriya et al., 2007). Therefore, the protein may be a potential antioxidant that exists in the embryo to protect embryo from ROS. Furthermore, protein derived from fertilized eggs may be an efficient antioxidant.
Based on the results of antioxidant activity, an antioxidant protein was isolated and purified from fertilized eggs on day 16. The SDS-PAGE illustrated that its molecular weight was about 45 kDa. LC-MS analysis result showed that purified protein sequence was highly similar to ovalbumin (coverage reached 84%), compared with ovalbumin sequence. In order to understand more about protein D2-S, we have studied its C-terminal sequence, result showed that protein D2-S C-terminal sequence was NAVLFFGRCVSP, which was consistent with the sequence of Ovabumin. ovalbumin is a 386-aa residue protein, molecular weight of about 43 kDa. Duan (2013) reported that the change of ovalbumin was not significantly during incubation, Xu et al. (2007) reported that the hydrolysates of ovalbumin showed a distinctly inhibitory action to •OH, O2−• and linoleic acid autoxidation. Abeyrathne et al. (2014) showed that peptide derived from ovalbumin were hydrolyzed by alcalase and trypsin showed strong iron- and copper-binding capacities and antioxidant capacity. The sequence of antioxidant protein obtained from fertilized eggs on day 15 exhibited highly similar to ovalbumin (Yang et al., 2016). Thus, we suspected that the purified protein in this study was a new antioxidant protein. There has been reported that antioxidant activity of some proteins was attributed to the presence of leucine, histidine and proline amino acid sequence (Peñaramos et al., 2004). In this study, we discovered that the content of ovabumin in 16 incubation days was 33.72±1.33 ug/mL, and the radical scavenging ability on DPPH, ·OH was 48.36±0.48%, 53.27±1.37%, respectively. Meanwhile, the purified protein possessed antioxidant capacity. The amino acids sequence was highly similar to ovalbumin and had leucine, histidine and proline amino acid. Therefore, we named this protein D2-S.
The outcome of present study revealed that antioxidant protein from fertilized exhibited antioxidative properties. Considering the antioxidant capacity of protein in the whole process of chicken embryo hatching, an antioxidant protein D2-S (MW: 43.22 kDa) isolated from fertilized eggs possessed potential antioxidant and free radical scavenging properties. However, the real mechanism of its antioxidant capacity is not clearly known. Thus, it is necessary to fully characterize this active mechanism and evaluate its capacity in food system.