Introduction
Muscle fiber characteristics strongly influence meat quality because skeletal muscles mainly consist of muscle fibers that can be characterized by their morphological traits, and contractile and metabolic properties (Lee et al., 2010). Morphology traits such as total number of fibers and cross-sectional area of fibers are major determinants of muscle mass and meat quality. Contractile and metabolic properties of muscles can be differentiated by muscle fiber types. Thus, meat quality is strongly related to fiber type compositions in muscles (Joo et al., 2013). Fundamentally, muscle fibers can be divided into three major categories (type I, slow-twitch oxidative or SO; type IIA, fast-twitch oxidative glycolytic or FOG; and type IIB, fast-twitch glycolytic or FG) depending on their histochemical, physiological, and biochemical properties (Peter et al., 1972; Schiaffino and Reggiani, 1996). All these three fiber types are observed in most muscles of cattle. Their relative fiber type compositions can influence appearance quality traits (AQT, such as meat color, drip and purge loss, and amount of intramuscular fat) and eating quality traits (EQT, such as tenderness, flavor, juiciness, and palatability) (Joo et al., 2013). Although many studies have reported fiber type compositions in various cattle breeds (Calkins et al., 1981; Costa et al., 2017; Hwang et al., 2010; Ozawa et al., 2000; Totland et al., 1988), very few studies have investigated fiber type compositions in Hanwoo cattle.
Recently, muscle profiling of Hanwoo beef is becoming important because beef industry in Korea has shown a trend toward marketing individual muscles (Jung et al., 2016). It is obvious that muscle fiber characteristics have significant influence on fat content and connective tissue, resulting in various AQT and EQT. Jeremiah et al. (2003) have found that 30 anatomically defined bovine muscles are significantly different in EQT. In addition, fiber type compositions in bovine muscles can be potentially affected by several factors, including breed, genotype, sex, hormone, growth performance, diet, muscle location, exercise, and ambient temperature (Joo et al., 2013). Gotoh (2003) has reported that histochemical properties of muscles in Japanese Black cattle can change depending on functional demands during growth period. They can also change depending on the feeding system. Therefore, understanding the relationship between composition of muscle fiber types and meat quality traits is needed to improve the production of quality beef meat. Manipulation of muscle fiber characteristics can have profound impacts on profitability of the beef industry. The objective of this study was to determine the relationships between histochemical characteristics and meat quality traits of eight primal cuts of Hanwoo steers.
Materials and Methods
Eight major muscles including longissimus lumborum (LL), psoas major (PM), semimembranosus (SM), semitendinosus (ST), gluteus medius (GM), triceps brachii (TB), rectus abdominis (RA) and superficialis flexor (SF) from nine Hanwoo (Korean native cattle) steers with 3 quality grades (QG) (QG 1++, 1+, and 1; 3 cattle for each QG) were obtained at a commercial meat processing plant in Korea. Approximately 10 g of each muscle was taken for histochemical analysis within 1 h post-mortem. It was frozen in isopentane chilled with liquid nitrogen. After 24 h of chilling, eight muscles were removed to investigate meat quality traits. Meat color (CIE L*a*b*), drip loss (%), Warner-Bratzler shear force (WBSF), fat content, moisture content, myoglobin (Mb) content, collagen content, and sarcomere length of these muscles were evaluated for meat quality traits.
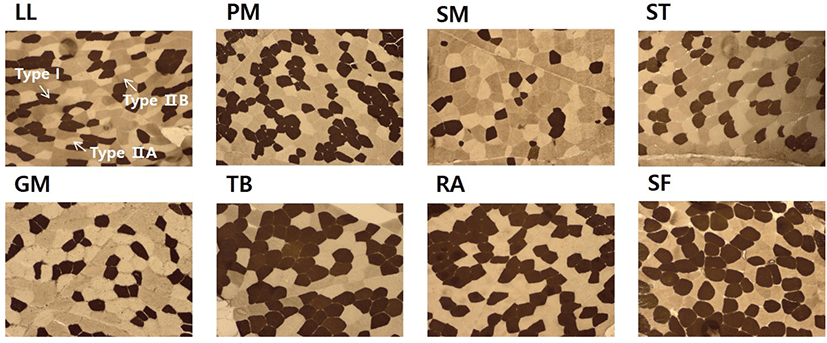
Transverse serial sections of 10 μm in thickness were cut from entire blocks (1.0×1.0×1.5 cm) with a cryostat microtom (HM525, Microm GmbH, Germany) at −20°C. The sections were subsequently used for histochemical analysis of myosin adenosine triphosphatase (mATPase) following alkaline (pH 10.70) and acid (pH 4.63) preincubation using the method of Brooke and Kaiser (1970) with slight modifications. An image analysis system (Image-Pro®plus 5.1, Media Cybernetics Inc., USA) was used to examine the stained sections. The muscle fibers were classified into fiber type I, IIA, and IIB according to the nomenclature of Brooke and Kaiser (1970). Approximately 500 fibers per sample were counted to analyze the muscle fiber characteristics. Fiber number percentage (FNP), fiber area percentages (FAP), and fiber diameter (FD) were determined. FNP refers to the ratio of counted fiber number of each fiber type to the total counted fiber number. FAP was the ratio of total cross-sectional area of each fiber type to total fiber area measured.
Moisture content of the sample was determined using the oven drying method (AOAC, 2000). Fat content was determined after extracting fat from 3 g of homogenized meat sample using the procedure of Folch et al. (1957). Myoglobin (Mb) concentration was measured using the method of Warriss (1979) with modifications as described previously (Hwang et al., 2010). Collagen content was determined by AOAC method (2000) with modification as described previously (Hwang and Joo, 2017).
Meat color (CIE L*, a*, and b*) was measured on the surface of muscles, using a Minolta Chromameter CR-300 (Minolta Co., Japan) that was standardized with a white plate (Y = 93.5, X = 0.3132, y = 0.3198). Drip loss was determined by suspending muscle sample with standardized surface area in an inflated plastic bag for 24 h at 2°C using the method of Honikel (1987) with modifications. Cooking loss was determined as weight loss after broiling in a water bath at 70°C for 30 min. Warner-Bratzler shear force (WBSF) values were determined using an Instron Universal Testing Machine (Model 4400, Instron Corp., USA) after samples (1.3-cm diameter cores) obtained from steaks were cooked to internal temperature of 70°C for 30 min. Sarcomere length was determined according to the method of Cross et al. (1981) with modifications as described previously (Hwang et al., 2010).
Experimental data were analyzed by analysis of variance (ANOVA) procedure of statistical analysis systems (SAS, 2002). Duncan's multiple range test was used to determine significant differences among means at 5% level of significance (SAS, 2002). Pearson correlation coefficients were used to describe the relationship between meat quality traits and muscle fiber characteristics using partial correlation coefficients (SAS, 2002).
Results and Discussion
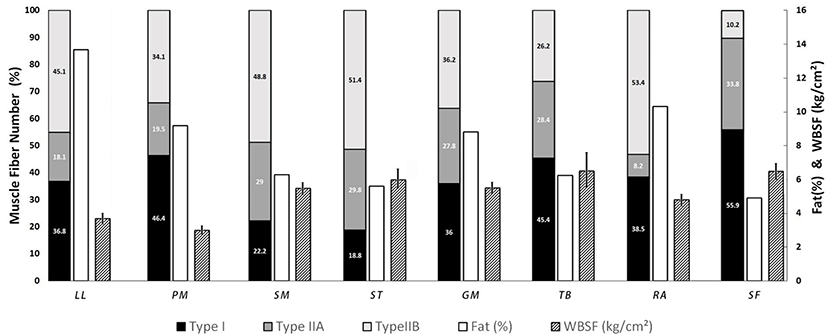
Muscle fiber characteristics including FNP, FAP, and FD are summarized in Table 1. As expected, a clear difference in composition of fiber types was observed among these eight muscles. SF had the highest FNP of type I, followed by PM, TB, RA, LL, GM, SM, and ST. FNP of type I was 55.9% in SF. It was almost three times higher than that in ST (18.8%). There was no significant (p>0.05) difference in FNP of type I between PM and TB. However, FNP of type IIA was significantly (p<0.05) different between PM and TB. SF had the highest FNP of type IIA while RA had the lowest FNP of type IIA. LL and PM had significantly (p<0.05) lower FAP of type IIA compared to other muscles. There was no significant difference in FNP of type IIA among SM, ST, GM, and TB. Meanwhile, higher FNP of type IIB was observed in ST (51.4%), SM (48.8%), and LL (45.1%), while SF had the lowest FNP of type IIB (10.2%) among these eight muscles (p<0.05).
A-GMeans±SE with different superscripts in the same row are significantly different (p<0.05).
1LL, Longissimus lumborum; PM, Psoas major; SM, Semimembranosus; ST, Semitendinosus; GM, Gluteus medius; TB, Triceps brachii; RA, Rectus Abdominis; SF, Superficialis flexor
Dissimilar results were observed for FAP of fiber types among these eight muscles. SF had the highest FAP of type I while ST had the lowest FAP of type I. There was no significant (p>0.05) difference in FNP of type I between LL and RA, although their FAPs of type I were significantly (p<0.05) different. In contrast, FAPs of type I in PM and TB were significantly (p<0.05) different, although there was no significant (p>0.05) difference in FNP of type I between the two. FAPs of type IIA were significantly (p<0.05) different among muscles. However, FAP of type IIA in TB was not significantly (p>0.05) different from that in SM. GM muscle showed the highest FAP of type IIA fiber (37.4%), while LL had the lowest FAP of type IIA fiber (14.5%). Consequently, FAPs of type IIB varied significantly (p<0.05) among these eight muscles. The FAP of type IIB ranged from 9.9% in SF to 58.7% in ST. FAPs of type IIB in LL, SM and ST exceeded 50%.
The composition of muscle fiber type varied considerably among these eight major muscles from Hanwoo steer. These variations in fiber type composition were expected because many previous reports showed that muscle fiber characteristics were different depending on various factors, including breed (Ryu et al., 2008), selection (Larzul et al., 1999), gender (Ozawa et al., 2000), growth performance (Gondret et al., 2006), diet (Gotoh, 2003), and muscle location (Totland and Kryvi, 1991). Muscle fiber compositions in LD (longissimus dorsi), PM, and SM muscles from highly marbled Hanwoo steer have been determined in our previous study (Hwang et al., 2010). It was found that FNP and FAP of type IIA and IIB were higher in SM muscle while those of type I fibers were lower in PM muscle than those in other muscles (Hwang et al., 2010). In the present study, FNP and FAP of type IIB and IIA were also higher in SM than those in PM. However, PM had higher FAP of type IIA compared to LL. In addition, SF and TB muscles had higher FAP of type I compared to PM muscle. These differences might be due to variations in intramuscular fat content in muscles. Our previous study used only QG 1++ Hanwoo steer, the highest marbled beef (Hwang et al., 2010). However, Hanwoo steers with three QGs (QG 1++, QG 1+, and QG 1) were used in this study. This suggests that compositions of muscle fiber type in bovine muscles could be influenced by QG of beef carcass which is determined mainly by intramuscular fat content.
Kim et al. (2000) have reported that PM muscle has lower percentage of type IIB fibers than LD muscle (15.9 vs 29.5%). However, no significant difference in the type I or IIA composition was observed between these two muscles from intact Hanwoo bulls (Kim et al., 2000). Their higher proportions of type I fiber (43.4%) but lower proportions of type IIB (29.5%) in LD muscle compared to our results (34.4% and 51.1%, respectively) might be due to different sexes of Hanwoo used in the two studies (i.e., bulls in their study but steers in this study). Proportions of type I in LT (longissimus thoracis) muscle of Japanese Black and Brown steers are 31.0% and 18.1%, respectively (Gotoh, 2003). Those of type IIB are reported to be 50.0% and 63.5%, respectively (Gotoh, 2003). In the present study, FAPs of fiber types in LL muscle of Hanwoo steers appeared to be similar to those of Japanese Black steer. Ozawa et al. (2000) have reported that relative percentages of βR, αR and αW fiber types in LT muscles of Japanese Black steers are 27.2, 17.3, and 55.5%, respectively. Compared to the results of Japanese Black steers, type I fibers in LL muscles of Hanwoo steers were present at approximately similar percentages. However, a higher percentage for type I fiber but a lower percentage for type IIA fiber were observed in Hanwoo steers. It is currently unclear whether these differences are due to breed or sampling position in the muscle or analytical procedure used.
An obvious difference in FAP of fiber types among the eight muscles was observed in this study. Especially, very low FAPs of type I, IIA and IIB were observed in ST, RA, and SF muscles, respectively. FNP of type I and IIB in ST were 18.8 and 51.4%, respectively, similar to results of Totland et al. (1988) showing that proportions of type I and IIB in ST ranged from 10 to 30% and from 34 to 58%, respectively. Iwamoto et al. (1991) have also reported a low proportion of βR fiber type (26.0%) but a high proportion of αW fiber type (46.7%) in ST muscle of Japanese black steer. In general, ST muscle (a typical white) shows a transformation of type IIA fibers into IIB fibers during the first few months after birth, whereas type I fibers are nearly unaffected by the age of cattle (Wegner et al., 2000). Therefore, the lower FAP of type I but higher FAP of type IIB in ST compared to other muscles of Hanwoo steer maybe due to transformation of type IIA fibers into type IIB fibers without changing FNP of type I during early stage of growth.
Oury et al. (2010) have reported that oxidative enzyme activities in RA are higher than those in LT. However, glycolytic enzyme activity in RA is lower than that in LT, resulting in unusual large cross-section surface of SO fibers and very low oxidative activity of intermediate IIA fibers (Oury et al., 2010). RA muscle has significantly higher proportions of SO fibers (37.8 vs 28.0%), offset by significantly lower proportions of FOG fibers (8.3 vs 19.6%) compared to TB muscle (Oury et al., 2010). In this study, very low FAP of type IIA (8.1%) with the longest FD of type I in RA among eight muscles was also observed. Generally, FNP of type IIB is increased with transformation of small type IIA to large type IIB fibers (Ashmore et al., 1972). Thus, it is possible that the very low proportion of type IIA in RA muscle is due to transformation of type IIA to type IIB fibers and large size of type I fibers.
On the other hand, the lowest FAP of type IIB in SF muscle was in stark contrast to that in RA. In general, the skeletal muscle contracts in a manner of fast or slow-twitch under energy supply from oxidative and glycolytic metabolisms with heterogeneity of muscle fiber (Pette and Staron, 1990). The muscle is mainly composed of type I fibers situated in a deep position adjacent to bone. It could play a major role in maintaining posture by stabilizing extended joints in cattle (Totland and Kryvi, 1991). According to Gotoh (2003), in flexor digitorum superficialis muscles (a passive type of antigravity muscle), type I fibers occupied 58% in forelimbs and 39% in hind limbs because muscles in hind limbs retained propelling power in addition to antigravity activity. In the present study, SF muscle dissected from forelimbs had the highest FNP of type I (55.9%) and type IIA (33.8%) among the eight muscles obtained from Hanwoo steer.
In addition, differences in diameter of three fiber types in eight muscles of Hanwoo steers were confirmed in this study. As expected, the diameter of fiber type IIB was longer (p<0.05) than that fiber type I or IIA in all muscles except RA and SF muscles. RA and SF muscles had the longest diameter of type I (58.5 and 57.5 μm, respectively). However, the longest diameter of type IIB was observed in TB (62.9 μm) and LD (61.9 μm). These results were consistent with those of Oury et al. (2010) reporting that RA muscles appeared to have significantly higher proportions of SO fibers and mean surface area than those of TB muscles. However, the diameter of type I in TB was significantly (p<0.05) longer than that in LL, similar to results of Totland and Kryvi (1991) showing that muscle involved in maintenance of upright and standing position such as TB in the forepart are particularly rich in type I fibers. There was no significant difference in diameter of type I or IIB among SM, ST, and GM muscles (p>0.05) which were obtained from the hindpart. However, these diameters were significantly (p<0.05) shorter than those of TB. This result confirmed previous finding showing that average cross-sectional area of type I fiber was about 15% larger in muscles of the forepart than that of the hindpart (Totland and Kryvi, 1991).
Meat quality traits of the eight muscles from Hanwoo steer are summarized in Table 2. As expected, there were significant (p<0.05) differences in fat content among the eight muscles. LL had the highest fat content, followed by RA, PM, GM, SM, TB, ST, and SF. Contrarily, SF had the highest moisture and collagen contents while LL had the lowest contents. Mb content was significantly (p<0.05) higher in SF and PM muscles compared to that in other muscles. There were also significant (p<0.05) differences in color measurements among muscles. Especially, the highest a* value was observed in PM and SF muscles which also had the highest Mb contents while SM had the lowest a* value and Mb content. LL had the highest L* value while SF had the lowest L* value. SM had the highest drip loss (%) and cooking loss (%) whereas LL and PM had the lowest values of drip loss (%) and cooking loss (%), respectively. PM had the longest sarcomere length but the lowest WBSF value while SF had the highest WBSF value but the shortest sarcomere length.
A-EMeans±SE with different superscripts in the same row are significantly different (p<0.01).
1LL, Longissimus lumborum; PM, Psoas major; SM, Semimembranosus; ST, Semitendinosus; GM, Gluteus medius; TB, Triceps brachii; RA, Rectus Abdominis; SF, Superficialis flexor
2Warner-Bratzler shear force
A few studies have compared meat quality traits between different muscles in Hanwoo cattle. Our previous studies have shown that PM muscle had higher Mb and mitochondria concentrations compared to LD and SM muscles (Hwang et al., 2010; Jeong et al., 2009). Results of the present study confirmed that Mb content in PM muscle was significantly (p<0.05) higher than that in LL and SM muscles. However, there was no significant (p>0.05) difference in Mb content between PM and SF muscles. These results suggest that the higher Mb content in PM and SF muscles might be due to a higher percentage of type I fiber among eight muscles. Gotoh (2003) has reported that PM muscle contains more red myofibers (type I + IIA) than white myofibers (type IIB). However, a reverse tendency is shown in LD muscle (Gotoh, 2003). Depending on muscle type, discoloration of beef muscle after exposure to oxygen accounts for much of the variance in color stability (Hood, 1980). The stability of meat color is higher in LD muscle than that in PM muscle (Gotoh, 2003). In this study, rapid decreasing in a* and b* values in SF, RA, TB, and PM muscles during cold storage was expected because muscles of low color stability had more oxidative muscle fibers with red color (Jeong et al., 2009). The highest L* value of LL muscle compared to SM and ST muscles which had higher percentage of type IIB but lower percentage of type I was probably due to higher fat content (white marbling).
Lower drip loss and cooking loss were observed in LL, RA, and PM muscles which had higher fat content compared to other muscles. However, SM muscle with the lowest fat content showed the highest drip loss and cooking loss. This result supports results of a previous report showing that fat content is related to water-holding capacity of meat (Joo et al., 1995). Highly marbled meat has been observed to have less drip loss in pork (Joo et al., 2000) and beef (Jung et al., 2015). Our results support findings of Joo et al. (2013) showing that increasing intramuscular fat content will decrease drip loss and purge loss of meat. In addition, muscle fiber composition can influence intramuscular fat content and water-holding capacity of meat (Joo et al., 2003). Our data also confirmed findings of Gotoh (2003) showing that percentage distribution of type I fibers had a significantly positive correlation with percentage of intramuscular fat. Conversely, type IIB fibers had a significantly negative correlation with intramuscular fat (Gotoh, 2003). The relationship between fiber type composition and intramuscular fat content has been well documented (Gotoh, 2003; Joo et al., 2013).
Our previous study has revealed that PM muscle has lower WBSF but longer sarcomere length while SM muscle has higher WBSF but shorter sarcomere length (Hwang et al., 2010). Similar results were obtained in this study. In addition, results of this study revealed that SF had the highest WBSF but the lowest sarcomere length among the eight muscles. Although FAP of type I and Mb content were higher in both PM and SF, SF showed significantly higher WBSF than PM. This is certainly due to higher content of collagen in SF compared to that in PM. These results suggest that the correlation between fiber type composition and tenderness (WBSF) could be reversed by increasing the number of muscle samples. Many studies have investigated fiber type compositions in less than three muscles and reported positive correlation between fiber type I and shear force with negative correlation between type IIB and shear force (Hwang et al., 2010; Ozawa et al., 2000; Ryu and Kim, 2005; Sirin et al., 2017). These findings are different from observations of the present study showing lower WBSF of PM muscle (with higher FAP of type I) but higher WBSF of ST, TB, SM, and GM muscles (with higher FAP of type IIB). Further studies are needed to clarify this issue because current data on this topic are limited.
Results of correlations between muscle fiber characteristics and meat quality traits are summarized in Table 3. Fat content was negatively correlated with FNP of type IIA (r = −0.65) but positively correlated with type IIB (r = 0.37). Similar tendencies were found between fat content and FAP of fiber types. In contrast, moisture content had positive correlation with type IIA but negative correlation with type IIB. Drip loss and cooking loss were negatively correlated with FAP of type I (r = −0.51 and r = −0.53, respectively) but positively correlated with type IIA (r = 0.49 and r = 0.57, respectively). Mb content was positively correlated with FAP of type I (r = 0.79) but negatively correlated with type IIB (r = −0.60). The a* value also showed the same tendency as Mb content. It was positively correlated with type I but negatively correlated with type IIA and IIB. Interestingly, b* value was negatively correlated with all three fiber types. However, L* value had negative correlation with type I but positive correlation with type IIB. Collagen content was positively correlated with FAP of type I (r = 0.27) and FNP of type IIA (r = 0.24) but negatively correlated with FAP of type IIB (r = −0.36). Sarcomere length was negatively correlated with FAP of type IIA (r = −0.63) but positively correlated with type IIB percentage (r = 0.40). However, FAP of type I was not significantly related to sarcomere length. In contrast to sarcomere length, WBSF was positively correlated with FAP of type IIA (r = −0.40) but negatively correlated with type IIB fiber (r = −0.39). However, FAP of muscle fiber type I had no significant correlation with sarcomere length.
*p<0.05, **p<0.01, ***p<0.001
1Warner-Bratzler shear force
The correlation between muscle fiber types and fat content found in the present study was not in agreement with our previous study that investigated three Hanwoo muscles (LD, PM, and SM) (Hwang et al., 2010). In our previous study, fat content had negative correlation with FAP of type IIA (r = −0.33) and IIB (r = −0.31) but positive correlation with FAP of type I (r = 0.39) (Hwang et al., 2010). However, in this study, somewhat different correlation tendencies between fat content and muscle fiber types were found. There was no significant correlation between fat content and FAP of type I. However, FAP of type IIB showed positive correlation with fat content. The positive correlation between type IIB fiber and IMF content in this experiment might be due to higher percentage of type IIB in LL muscle, and LL muscle also showed a higher IMF content compared to the other muscles. The contrast correlation results between fat content and fiber type compositions in this study compared to previous studies was unexpected. Muscle fiber type IIB might be negatively correlated with fat content if the number of muscles investigated was limited to three or less muscles, including LL muscle. This hypothesis was verified when the correlation coefficients were calculated based on data from only LL muscle.
It is generally accepted that both type I and IIA fibers contain more Mb than type IIB fiber. These fibers contribute to the redness of meats (Gotoh, 2003). Our previous study has shown that Mb content is positively correlated with type I fiber but negatively correlated with type IIB (Hwang et al., 2010). Results of this study confirmed findings of previous studies, although type IIA fiber had negative correlation or lacked correlation with Mb content (r = −0.16). The lack of correlation between Mb content and type IIA fiber might be due to very low percentage of type IIA fibers in RA muscles with higher Mb contents. The high percentage of type IIA fibers in SM and ST muscles with lower Mb contents might have also influenced the lack of correlation. In relation to meat color, a* value showed a tendency similar with Mb content (i.e., negative correlation between a* value and type IIA fiber, r = −0.23). Further studies are needed to determine the correlation between muscle fiber types and Mb content as well as meat color of Hanwoo steer.
Early reports (Garcia-Bunuel and Garcia-Bunuel, 1967; Kovanen et al., 1984; Laurent et al., 1978) have well demonstrated that the concentration of collagen is higher in slow muscle than that in fast muscle. Results of the present study also showed that collagen content was positively correlated with type I and type IIA fibers but negatively correlated with type IIB fiber. It is generally accepted that collagen type composition and concentration are strongly correlated with meat tenderness (Cho et al., 2017). Thus, the lack of correlation between WBSF and FAP of type I fiber (r = −0.03) was unexpected. It might be due to differences in diversity of eight muscles in relation to proportion of type I and WBSF (Fig. 2). For example, PM had high proportion of type I fiber with low WBSF while ST showed low proportion of type I with high WBSF. These results suggested that the diversity of FAP of type I and WBSF among these eight muscles might have contributed to the lack of correlation between WBSF and FAP of type I fiber.
In conclusion, correlations between fiber type composition and meat quality traits were significantly affected by the number of muscles used to calculate these correlations. Correlation coefficients calculated with data from eight muscles were clearly different from those calculated with data only from LL muscles. When correlations were computed with data from all eight muscles, the lack of correlation were observed between fiber types and meat quality traits, different from correlations obtained from data of only LL muscles. Consequently, our results suggest that correlation for each individual muscle should be used to improve meat quality and profitability of retail beef cuts, especially for loin cut.













