Introduction
Gelatin is produced by the partial hydrolysis of the collagen contained in animal raw materials, e.g., buffalo hide (Zarai et al., 2012). Buffaloes are a representative tropical livestock which provide by-products that are well suited for gelatin manufacture. Generally, buffalo hide is thicker (6-8 mm) and stronger than that of other animals, accounting for 11.5% of the total body weight, whereas the corresponding value for cowhide is only 9.0% (Huda et al., 2011; Spanghero et al., 2004). Collagen is mayor component of hide. Thus, the collagen content of buffalo hide is higher than cowhide.
Buffalo hide collagen exhibits a high hydroxyproline content, which adds complexity to the collagen structure and has been associated with the high gel strength and thermal stability of the produced gelatin (Giménez et al., 2005; Shoulders and Raines, 2009). These properties make it difficult to optimize the extraction process by alkaline pretreatment alone; indeed, the alkaline pretreatment of hides for gelatin extraction has been found to be time consuming and produces low-purity gelatin (Ofori, 1999).
Several studies on improving the efficiency of gelatin extraction have been carried out, including those utilizing acid pretreatment. Such pretreatment can increase the yield of gelatin by degrading the triple-helical structure of collagen, which becomes unstable in the presence of crosslinking terminations, and increases the solubility of collagen (Niu et al., 2013). Acid treatment aids the release of acid-soluble proteins, fats, and other components, disrupting interactions between collagen molecules and thus increasing the extraction efficiency. The yield and functional properties of the obtained gelatin are affected by the acid type or concentration and pretreatment time (Ahmad and Benjakul, 2011). Since strong acids achieve better extraction efficiencies at lower concentrations than weak ones (Niu et al., 2013), this study was designed to determine the yields and physicochemical properties of gelatins extracted from buffalo hide pretreated with acids of different types and concentrations. The originality of this study was the right type of acid for pretreatment on gelatin extraction from buffalo hide which resulted the highest yield.
Materials and Methods
Three hides of male buffaloes (3-4 years) purchased from C.V. Panji Jaya in the Cegoroyoso village of the Pleret sub-district (Bantul, Indonesia). The physical and chemical properties of female cattle's skin may differ in pregnancy and breastfeeding. Hides were washed with tap water and soaked in a 2% for 24 h (w/v) aqueous lime solution until the hair was completely removed. The hide was scraped to remove residual fat by fleshing knife, rinsed with tap water until the surface pH of the hide reached 7-7.5, and stored at −20°C until further use (Said et al., 2011). Prior to gelatin extraction, the frozen hide was thawed at 4°C for 24 h and cut into small pieces (1×1 cm2) (Ktari et al., 2014).
Gelatin extraction was based on a modification of a previously reported method (Niu et al., 2013). Buffalo hide pieces were soaked in 0.5 M NaOH (1:4 w/v) for 2 h, followed by further soaking in hydrochloric, acetic, or citric acids (0.3, 0.6, 0.9, 1.2, and 1.5 M (1:4 w/v)) for 4 h. Subsequently, the hides were drained and washed six times until a pH of 5-6 was reached. All pretreatment processes were repeated three times for each of the two samples used.
The pretreated hides were soaked in distilled water (1:4 w/v) at 65°C (Memmert WNB7-45 type water bath) for 5 h, followed by further soaking at 70°C for 5 h in preparation for the second extraction step. The extracted gelatin was sieved (< 200 mesh) and dried in a cabinet drier at 50-55°C for 48 h until the moisture less than 12% (Mulyani et al., 2017), and the yield was calculated using the following formula (Kim et al., 2012; Ktari et al., 2014):
Each type of acid pretreatment with highest yield value was tested for gelatin properties including gel strength, viscosity, protein pattern color, functional groups by FTIR and amino acid profile.
Dried gelatin (6.67 g) was dissolved in distilled water (100 mL), and the obtained solution was magnetically stirred, heated at 60°C for 15 min, and incubated for 16-18 h at 10°C in the refrigerator. Subsequently, the strength of the prepared gel (expressed in g Bloom) was measured using a texture analyzer (Zwick/20.5, Zwick Roell AG, Germany) at a probe speed of 0.5 mm/s and 4 mm depth. The Probe with a 12.7 mm diameter flat faced cylindrical plunger (Benjakul et al., 2009; Wulandari et al., 2016).
Dried gelatin was dissolved in distilled water at 60°C for 15 min, and the viscosity of this prepared solution was measured using a digital viscometer (Brookfield, spindle No. 6) at 60 rpm at 25°C (Niu et al., 2013).
The color of dried gelatin was measured with a chromometer (Konica Minolta Sensing, Inc., Japan) utilizing the Hunter system and is expressed in terms of lightness (L*), redness (a*), and yellowness (b*). The values of L range from 100 = white to 0 = black, whereas a takes values from −50 = green to +50 = red, and b adopts values from −50 = blue to + 50 = yellow (Pranoto et al., 2007).
The moisture, protein, fat, and ash contents of the buffalo hides and gelatins were determined by proximate analysis according to AOAC guidelines (AOAC, 2005), with each measurement carried out in triplicate.
FTIR spectra (Shimadzu PC-8201) were recorded in the range of 4000-650 cm−1, using pellets containing 2 mg gelatin powder and 100 mg KBr (Kaewruang et al., 2013).
A solution of buffalo hide gelatin (2 mg/mL) was prepared by dissolving dried gelatin in deionized water. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to a modified method of Laemmli (1970) with a protein molecular weight marker (14-245 kDa; 1st Base, BIO 5150 Prestained Protein Ladder) used as a standard. Solubilized samples were mixed at 4:1 (v/v) ratio with the sample buffer (1.5 M Tris HCl pH 6.8, containing 10% SDS and 50% glycerin). The mixtures were boiling water for 2 min. The loading volumes of the sample and protein marker solutions equaled 15 and 10 µL, respectively. The separating and stacking gel concentrations were 12 and 5%, respectively. The employed electrophoresis system (ATTO PAGERUN AE-6531, ATTO Corp., Japan) utilized a constant voltage of 120 V and 15 mA/gel. After separation, the separating gel was stained with Coomassie Brilliant Blue R250 and destained using water:methanol:acetic acid (4:5:1) (Suryanti et al., 2016; Wulandari et al., 2016).
Buffalo hide gelatin (0.1 g) was heated with 6 M HCl (0.5 mL) at 112 °C for 24 h on a heating block, and the reaction mixture was filtered through a 0.45-µm membrane filter prior to analysis. A 1-µL aliquot of the hydrolyzed sample was derivatized using the Waters AccQ-Fluor reagent and subjected to HPLC analysis (Waters 2996 separation module equipped with a 260 nm wavelength, PDA detector), and the modified amino acids were separated on a Waters AccQ-Tag amino acid analysis column (Nova-Pak C18, 1.7 µm, 2.1×100 mm). The flow rate of solvent was 0.7 mL/min at 49°C temperature of analysis. The concentrations of amino acids in the standard equaled 2.5 mM, with the exception of cysteine, which was present at a level of 1.25 mM (Ktari et al., 2014).
Results and Discussion
The extraction of gelatin from buffalo hides pretreated with 0.3, 0.6, 0.9, 1.2, and 1.5 M acids resulted in variable yields (p<0.05), which ranged from 6.30±1.77 to 29.17±2.10%. The yields of gelatin obtained after HCl and citric acid pretreatments showed a similar dependence on concentration, in contrast to the results obtained using acetic acid (Fig. 1). For HCl, the yield increases with increasing acid concentration up to 0.9 M, with further concentration increases resulting in decreased yields. In the case of citric acid, the highest yield is obtained at a concentration of 0.6 M, remains relatively unchanged at 0.9 and 1.2 M, and decreases at 1.5 M. For acetic acid, the yield increases with increasing concentration up to 1.5 M. Since HCl is a strong inorganic acid and citric acid is a weak but tribasic organic acid, both produce more hydrogen ions than the weak monobasic acetic acid at the same concentration, facilitating the dissolution of the intra- and intermolecularly crosslinked collagen. As these cross-links are cleaved, the triple-helical structure of collagen is changed to random coils to afford gelatin, with optimum extraction conditions corresponding to the highest gelatin yield. Niu et al. (2013) stated that the optimum acid concentration for pretreatment depends not only on the solution pH, but also on the types and concentration of anions, i.e., on the type of acid used. For example, phosphoric acid pretreatment presumably provides a greater swelling power compared with acetic acid, destabilizing the cross-links in the telopeptide region, the amide bonds of the collagen triple helix, and the non-covalent intra- and intermolecular cross-links, which result in a compact structure (Ahmad and Benjakul, 2011).
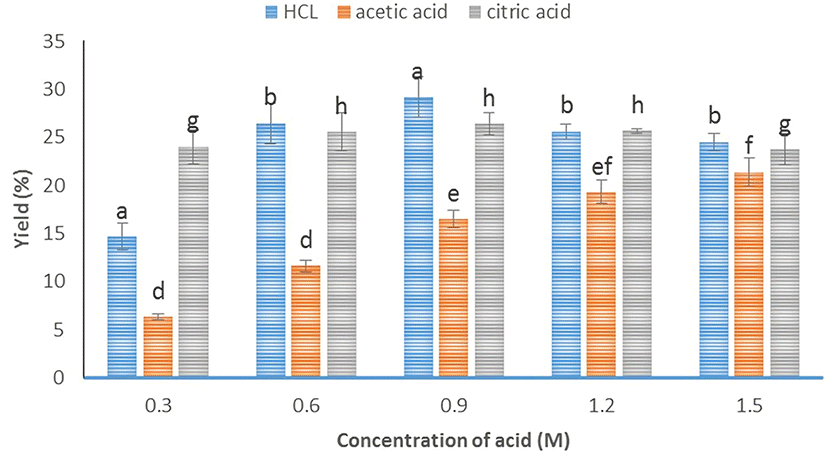
Gelatin is highly capable of forming hydrogen bonds with water molecules to form a stable three-dimensional gel, which is characterized in terms of gel strength in the gelatin industry (Nikoo et al., 2014; Zarai et al., 2012). The gel strength of buffalo hide gelatin is high, varying between 230.79±24.58 and 259.62±21.36 g Bloom (Table 1). Statistical analysis showed no significant difference between treatments (p>0.05). All acid type treatments produced gelatin with high gel strength. In comparison, the gel strengths of commercial gelatin (bovine skin gelatin, Sigma) and the Gelatin Manufacturers Institute of America (GMIA, 2012) standard equal 208.26±5.06 and 50-300 g Bloom, respectively. These characteristics of buffalo hide gelatin are attributed to its high contents of proline and hydroxyproline (Table 2), which are thought to impart stability to the triple-helical collagen structure via hydrogen bonding between free water molecules and the hydroxyl groups of the hydroxyproline residues (Ktari et al., 2014; Nagarajan et al., 2012). In general, the gel strength of buffalo hide gelatin does not significantly vary (p>0.05) between samples pretreated with 0.9 M HCl, 1.5 M acetic acid, and 0.6 M citric acid (the concentrations at which the highest yields are obtained, Fig. 1). At these concentrations, the molecular chains of collagen are broken down in an optimal way, achieving the right balance between maximum yield and high gel strength. Gelatin with shorter chains might not effectively undergo aggregation, complicating the development of junction zones, and thereby leading to poorer gel performance (Sae-Leaw and Benjakul, 2015).
aCA, AC, and HA correspond to pretreatment with citric, acetic, and hydrochloric acids, respectively. BSG, bovine skin gelatin. Results are means ± standard deviation (n=4).
bMoisture, protein, and ash values represent the means ± standard deviations of two replicates.
c-eDifferent letters in the same row indicate statistically significant differences (p<0.05). Results are means ± standard deviation (n=4).
nsnot significantly different (p>0.05).
aCA, AC, and HA correspond to pretreatment with citric, acetic, and hydrochloric acids, respectively. BSG, bovine skin gelatin. Results obtained from duplicate readings.
The viscosity of gelatin can be used as an indicator of its quality. The high viscosity indicates good gelatin quality. The viscosity increase with increasing gelling temperature, melting point and gel strength (Ratnasari et al., 2013). The buffalo skin gelatin viscosities ranged from 12.66 to 24.10 cP (Table 1), and are higher than those of commercial bovine skin gelatin (Sigma, 11.95 cP) and the GMIA standard (1.5-7.5 cP). The viscosity and hydroxyproline content of the gelatin isolated after citric acid pretreatment were higher than those of the gelatin obtained after hydrochloric or acetic acid pretreatment (Table 2). Based on the corresponding protein pattern, citric acid pretreatment produces gelatin with a molecular weight distribution similar to that observed for hydrochloric acid pretreatment, but the corresponding band seems thinner. This finding implies extensive degradation of the triple-helical structure during HCl pretreatment, which produces shorter molecular chains than those obtained after citric acid pretreatment. The viscosity of gelatin is known to be partially controlled by its molecular weight and molecular weight distribution (Shyni et al., 2014). Pretreatment using 0.3-1.5 M citric acid produced gelatin with optimal chain lengths, resulting in high viscosity and gel strength (Mulyani et al., 2016). Moreover, the gelatin obtained using the citric acid pretreatment exhibited the highest purity among the other pretreated samples, having a protein content of 93.73±0.16% and an ash content of 0.62±0.12% (Table 1).
In principle, color does not affect the functional properties of gelatin, being used to satisfy consumer preferences, and thus, having only an aesthetic value (Ratnasari et al., 2013; Shyni et al., 2014; Zarai et al., 2012). Color can be characterized in terms of lightness (L), redness (a), and yellowness (b). Buffalo hide gelatin produced using the HCl pretreatment exhibits the greatest color similarity to bovine skin gelatin. In the cases of the acetic and citric acid pretreatments, the produced gelatins are brighter than that pretreated with HCl (p<0.05). The redness and yellowness values of the gelatin obtained utilizing the acetic acid pretreatment are lower than those of the gelatin obtained using the citric or hydrochloric acid pretreatment, which is probably due to the occurrence of non-enzymatic browning reactions in the latter cases. The darkening is caused by the Maillard reactions of free amino acids released by the acids in contact with C=O components in the gelatin (Jridi et al., 2015; Mulyani et al., 2016).
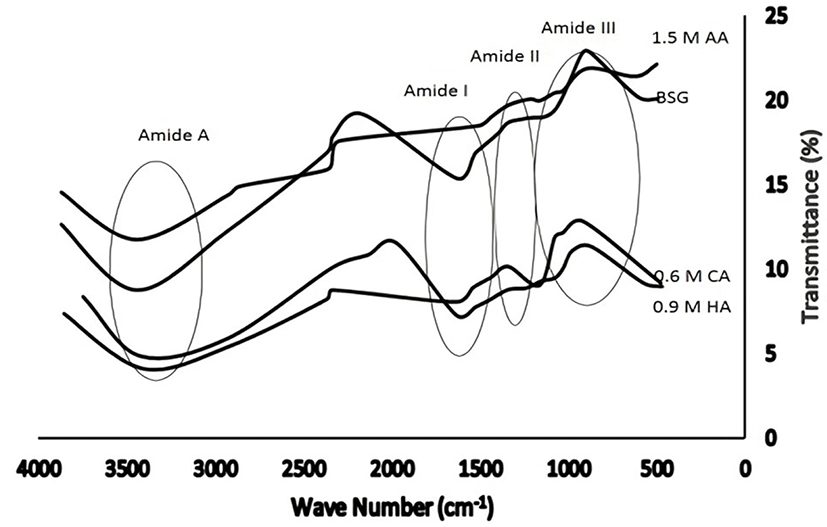
FTIR spectroscopy has previously been used to identify the functional groups and secondary structure of gelatin (Muyonga et al., 2004). The FTIR spectra of the buffalo hide gelatins obtained via the different pretreatments are shown in Fig. 2. They are similar to those of bovine skin gelatin, and thus, indicate the similarity of the corresponding functional groups. Four peaks are detected in the amide region, namely, amide A (3,410-3,448 cm−1), amide I (1,635 cm−1), amide II (1,527-1,334 cm−1), and amide III (1,242-871 cm−1) bands. The amide A band is associated with the stretching vibrations of NH groups, indicating the coiled structure of the gelatin, since stretching vibrations of free NH groups are usually observed at 3,400-3,440 cm−1 (Nagarajan et al., 2012; Sinthusamran et al., 2014). These vibrations are very useful for the IR spectroscopic analysis of protein secondary structure (Kittiphattanabawon et al., 2010). Amide II bands are ascribed to an out-of-phase combination of a CN stretch with in-plane NH deformation modes of the peptide group, whereas amide III bands are indicative of disorder in the gelatin molecules and are probably associated with the loss of the triple-helical structure (Sinthusamran et al., 2014). Among the samples, bovine skin gelatin shows the highest transmittance, and that obtained using the HCl pretreatment exhibits the lowest (i.e., the highest absorption intensity). Pretreatment with hydrochloric acid produces gelatin with a larger degree of triple-helix-to-coil structure degradation and disorder, as compared to the cases of citric and acetic acids.
The protein patterns of buffalo hide gelatins extracted using different acids (Fig. 3) show the presence of α (~100 kDa) and β (~200 kDa) chains as well as several degraded peptides (24-66 kDa). Pretreatment with 0.9 M HCl yields a gelatin with a molecular weight of 24-138 kDa, similarly to the case of 0.6 M citric acid, while the pattern observed for 1.5 M acetic acid is thinner than that of bovine skin gelatin. The formation of coil structure and short peptides, indicative of collagen degradation, is more pronounced for the HCl and citric acid pretreatments, with the highest gelatin yield obtained in the case of HCl. During extraction, collagen is converted into low-molecular-weight gelatin due to the breakdown of cross-linked collagen chains (Kaewruang et al., 2013). Zarai et al. (2012) reported that proteins with molecular weights lower than that of α chains are produced by the degradation of α, β, and g components during gelatin extraction and preparation.
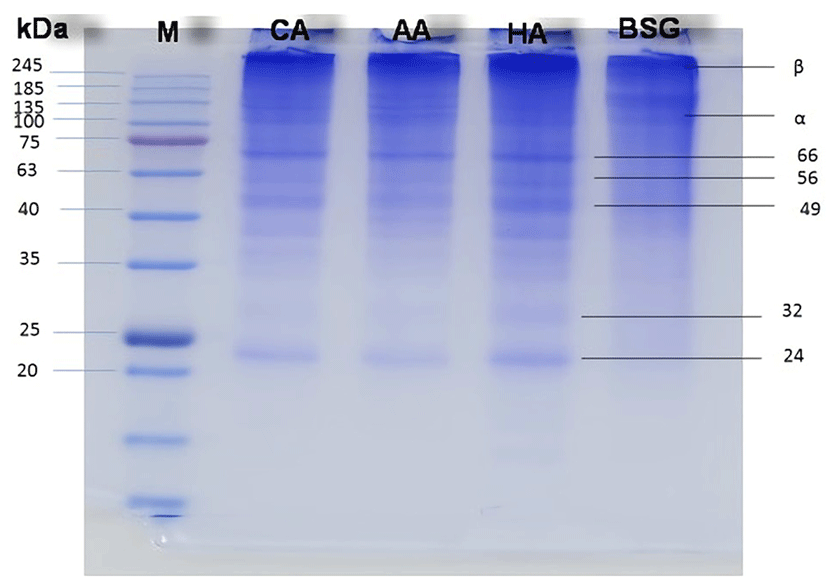
Table 2 presents the amino acid profile of the buffalo hide and bovine skin gelatins, showing that the former is dominated by glycine, proline, glutamic acid, and imino acids (proline + hydroxyproline). The proline and hydroxyproline contents of the gelatin obtained using the hydrochloric or citric acid pretreatment are higher than those of the gelatin obtained using the acetic acid pretreatment or the bovine skin gelatin. Buffalo hide gelatin also exhibits a higher gel strength than bovine skin gelatin, since the –OH groups of hydroxyproline facilitate the formation of junction zones, and thus, enhance gel strength. The higher content of imino acids and alanine observed for buffalo hide gelatin contribute to its increased viscoelasticity by promoting the formation of a triple-helical structure in the gelatin and its stabilization (Sarbon et al., 2013).
Conclusions
This study shows that the highest yield of gelatin from buffalo hide was obtained by pretreatment with 0.9 M HCl. All type of acids produce gelatin with the physicochemical properties equivalent to comercial bovine gelatin and comply with GMIA industry standards. Pretreatment with 0.6 M citric acid had the highest viscosity compared than 0.9 M HCl and 1.5 M acetic acid. Thus, the high gel strength and viscosity of the prepared gels recommend buffalo hide as an alternative source of gelatin.













