Introduction
As total and individual fatty acids in the diet have an impact on human health, interest in meat fat and fatty acids has been increasing, especially in the 21st century. Because the consumption of fat and cholesterol has been reported to be linked to cardiovascular disease, obesity and cancer (Micha et al., 2010; Pan et al., 2012), the reduction of total fatty acid intake and replacement of saturated fatty acids (SFA) with polyunsaturated fatty acids (PUFA) have been recommended. However, not all SFA are linked to hyper-cholesterol or obesity. Stearic acid (C18:0) has been shown to have no effect on plasma cholesterol level (Ulbricht and Southgate, 1991), although myristic (C14:0) and palmitic acids (C16:0) have a cholesterol raising effect. Oleic acid (C18:1) has a similar effect as PUFA in lowering serum cholesterol (Ulbricht and Southgate, 1991). Additionally, the consumption of high-oleic acid ground beef increases HDL-cholesterol concentration (Gilmore et al., 2011). Therefore, a higher proportion of oleic acid in beef is desirable (Pavan and Duckett, 2013). In this regard, it is essential to obtain the fatty acid profile of all major beef muscles to make conclusive and comprehensive statements regarding the role of beef fat in health.
Intramuscular fat (IMF) plays an important role in meat quality due to its positive effects on the sensory palatability of beef (Joo et al., 2013). In Korea, as well as in Japan, highly marbled beef is preferred by beef the market, and Hanwoo producers continue to develop effective production and feeding systems to increase marbling level in loin muscles. In general, Hanwoo cattle are fattened indoors with high energy diets. It has been reported that animals fed a high-grain diet had muscles containing higher concentrations of n-6 PUFA while those fed grass diets had muscles with increased n-3 PUFA concentrations (Daley et al., 2010; Wood et al., 2008). The fatty acid profile of IMF affects the overall palatability of beef because it determines meat quality and sensory traits (Joo et al., 2013; Wood et al., 2008). According to Smith (2016), the fatty acid that contributes to the softness of the fat in Korean cattle is oleic acid.
In our previous study, we reported the chemical composition and meat quality traits of ten primal cuts from Hanwoo steer (Jung et al., 2005; Jung et al., 2006). The results indicated that chemical components including fat contents are significantly different among individual Hanwoo cuts. Needless to say, the fatty acid profile varies for individual muscle cuts, and the fatty acid profiles for individual muscle cuts are needed for researchers to accurately evaluate health and palatability. However, most studies have evaluated the beef fatty acid profile by focusing on the longissimus muscle. Therefore, the objective of this study was to evaluate the fatty acid profile of ten major muscles from high and low marbled Hanwoo carcasses. Also, the relationship between the fatty acid profile and sensory traits was investigated.
Materials and Methods
Ten Hanwoo steer carcasses were selected from commercial plants and fabricated into 10 primal cuts. The ten carcasses consisted of five carcasses with two quality grades, quality grade 1++ (high marbled: HM) and 2 (low marbled: LM), which were primarily determined according to the degree of marbling using the Korean Beef Marbling Standard (BMS). The 10 primal cuts consisted of Ansim (tenderloin), Dungsim (loin), Chaekeut (sirloin), Moksim (neck), Abdari (chuck), Udun (outside round), Suldo (inside round), Yangjee (brisket, plate, flank), Satae (shank), and Kalbi (rib). The 10 muscles were sampled from 10 sub-primal cuts dissected from each of the 10 primal cuts. The 10 muscles are PM (Psoas major), LT (Longissimus thoracis), LL (Longissimus lumborum), SS (Semisponals), TB (Triceps brachii), SM (Semimembranosus), GM (Gluteus medius), RA (Rectus abdominis), SF (Superficialis flexor), and IC (Internal and external intercostal). The 10 muscles, sub-primal cuts and primal cuts are shown in Table 1.
Fat content was determined using the modified method described by Folch et al. (1957). Lipid was extracted from 3 g homogenized meat sample with 30 mL of Folch solution I (chloroform:methanol = 2:1, v/v). The homogenate was filtered with Whatman no. 1 filter paper. The filtered solution was stirred with 0.88% of NaCl and allowed to separate into two layers. After washing the wall of the measuring cylinder with 10 mL of Folch solution II (chloroform:methanol:H2O = 3:47:50), the final volume of the lower layer was recorded. The upper layer (methanol and water layer) was removed using an aspirator, while 10 mL of the lower layer (chloroform containing lipid extracts) was transferred to a dish to dry at 50℃. The weight of the dish was measured before and after drying. Fat content was computed from the weight difference for the dish.
After the extraction of intramuscular lipids, lipid methyl esters were prepared via saponification with 1.0 N methanolic NaOH and subsequently methylated with boron trifluoride in methanol. Fatty acid methyl esters (FAME) were analyzed using a HP6890N (Hewlett-Packard, USA) gas chromatograph equipped with a HP7683 (Hewlett-Packard) automatic sampler. FAME separations were accomplished using a 100 m SP2560 (Supelco, USA) capillary column (0.25 mm i.d. and 0.20 μm film thickness). For the separation of FAME from the samples, the following temperature program was applied with nitrogen as a carrier gas at a flow rate of 1 mL per min. Column oven temperature increased from 50 to 180℃ at 10℃ per min, from 180 to 220℃ at 5℃ per min, 220 to 240℃ at 2℃ per min, and then held at 240℃ for 20 min. The injector and detector were maintained at 250℃. Sample injection volume was 1 μL and the analysis was performed in duplicate. Individual fatty acids were identified by comparison of the retention times with standards (Supelco 37 components FAME Mix, USA). Results were expressed as the percentage of the total fatty acid detected based on the total peak area.
Meat color (CIE L*, a* and b*) was measured on the muscle surface using a Minolta Chromameter (CR-300, Minolta Co., Japan) that was standardized with a white plate (Y=93.5, x=0.3132, y=0.3198). The average value from five random locations on the sample surface was used for statistical analysis.
Drip loss was measured as the weight loss during the suspension of a standardized (2 cm diameter × 2 cm thickness) sample in a plastic box (18 × 15 × 10 cm) at 4℃ for 24 h (Joo et al., 2002). Drip loss was computed from the weight of the drip and that of the sample, then expressed as percentage loss based on the initial sample weight. The cooking loss was determined by the weight loss during cooking. The sample (2 cm diameter × 2 cm thickness) was placed in a plastic bag and broiled in a water bath at a temperature of 90℃ for 30 min. Samples were then surface dried and weighed. The cooking loss was calculated using the following equation:
Sarcomere length was determined using the method described by Cross et al. (1981). Briefly, samples were placed in solution A (0.1 M KCl, 0.39 M boric acid, and 5 mM ethylenediaminetetra acetic acid in 2.5% glutaraldehyde) for 2 h, and then transferred to solution B (0.25 M KCl, 0.29 M boric acid, and 5 mM ethylenediaminetetra acetic acid in 2.5% glutaraldehyde) for 17-19 h. Individual fibers were torn into pieces and placed onto microscope slides with a drop of solution B. The slide was then placed horizontally in the path of a vertically oriented laser beam, yielding an array of diffraction bands on a screen. These bands were perpendicular to the long axis of the fibers, and an average of 10 sarcomere lengths was obtained for each meat sample. Sarcomere length (μm) was calculated using the standard formula (Cross et al., 1981).
Tenderness was measured as the Warner-Bratzler shear force (WBSF, kg/cm2) using Instron Universal Testing Machine Model 3343 with a V-shaped shear blade. From each of six samples, cross sectional areas as close as possible to 0.5 cm × 4.0 cm (approximately 2.0 cm2) were cut across the fibers to measure cutting force. Samples were placed at right angles to the blade. The crosshead speed was set to 100 mm/min. The full scale load was 50 kg.
Samples from each treatment were evaluated by an 8-member trained expert descriptive attribute sensory panel in the Meat Science Laboratory at Gyeongsang National University. Panelists evaluated the samples for tenderness, juiciness, flavor, and overall palatability using a 9-point hedonic scale. Evaluation was performed on a single sheet, and four lines for sensory traits were marked with the following parameters: tenderness = very tough (0) to very tender (9); juiciness = very dry (0) to very juicy (9); flavor = dislike extremely (0) to like extremely (9); overall palatability = very bad (0) to very good (9).
Data from three replicates were analyzed by analysis of variance (ANOVA) using statistical analysis systems (SAS, 2002). ANOVA was for the mathematical model using SAS 9.2 (SAS Institute, Inc., USA). Duncan’s multiple range tests were used to determine significance among means. Correlation analysis was performed using the CORR procedure of SAS to evaluate the correlation between individual fatty acids and the sensory panel traits or the meat quality traits.
Results
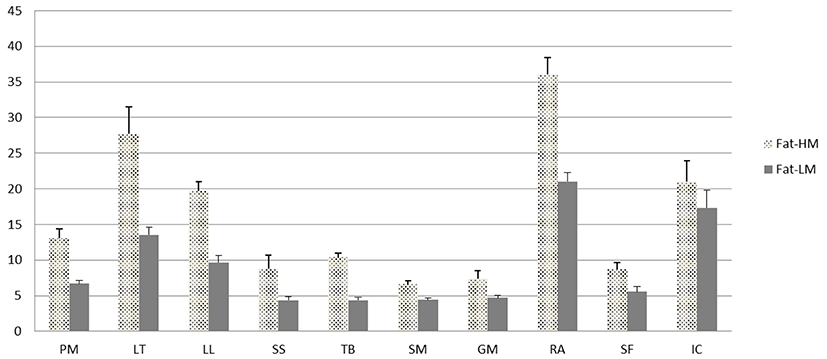
There were significant (p<0.001) differences in fat content among the 10 muscles from HM (quality grade 1++) and LM (quality grade 2) Hanwoo carcasses (Fig. 1). The fat content of muscles from the HM carcass was significantly (p<0.001) higher compared to muscles from the LM carcass. Among the muscles from HM carcass, the highest fat content was found in RA, followed by LT, IC, LL, PM, TB, SS, SF, GM, and SM. However, among the muscles from LM carcass, IC had higher fat content than LT. From these results, RA, IC, LT, LL, and PM were classified as high-fat muscles, while TB, SS, SF, GM, and SM were categorized as low-fat muscles.
The fatty acid profiles of the 10 muscles from Hanwoo steers are summarized in Table 2. The proportions of SFA, MUFA and PUFA were significantly (p<0.001) different among the 10 muscles due to differences in all fatty acids except EPA. The highest SFA proportion was found in PM (48.1%) and the lowest was in SS (38.5%) and RA (38.1%) (p<0.001). PM had the highest proportion of lauric (C12:0), myristic (C14:0), palmitic (C16:0), stearic (C18:0), and arachidic (C20:0) acids, but the lowest proportion of myristoleic (C14:1), palmitoleic (16:1), oleic (C18:1n-9), and eicosenoic (C20:1n-9) acids (p<0.001). Oleic acid comprised the majority of MUFA, and the proportion was significantly (p<0.001) higher in IC, TB, SF, RA, and SS than in other muscles. Total PUFA proportion in SS did not differ from proportions in TB, SM, and GM, but was greater than in PM, LT, LL, RA, SF, and IC (p<0.001). SS also had the greatest proportions of linoleic (C18:2n-6), eicosatrienoic (C20:3n-6), and arachidonic (C20:4n-6) acids. The proportion of linoleic acid, the majority of n−6 fatty acid, was significantly (P<0.001) lower in the high-fat muscles (IC, LT, and LL). However, the proportions of linolenic (C18:3n-3), eicosapentaenoic (C20:5n-3, EPA), and docosahexaenoic (C22:6n-3, DHA) acids were negligible and not significantly different among high-fat and low-fat muscles. Consequently, the high-fat muscles had lower n-6/n-3 ratios compared to the low-fat muscles (p<0.001).
a-eMean values in the same row with different superscripts differ significantly (*p<0.05, **p<0.01, ***p<0.001, ns: not significant).
1RMSE, Root mean square error; 2SFA, Saturated fatty acids; 3MUFA, Monounsaturated fatty acids; 4PUFA, Polyunsaturated fatty acids
The comparison of fatty acid composition between high-fat muscles from HM and LM Hanwoo carcasses is shown in Table 3. Among all fatty acids, only oleic acid was significantly (p<0.05) different between HM and LM for all 5 high-fat muscles, and HM muscle had a higher proportion of oleic acid than LM muscle. There were no significant differences in the proportions of palmitic acid, EPA, and DHA between HM and LM for all 5 high-fat muscles (p>0.05). LM muscles had a significantly (p<0.05) higher proportion of SFA than HM muscles due to the higher proportion of stearic acid. On the contrary, HM muscles had a significantly (p<0.01) higher proportion of MUFA than LM muscles due to the higher proportion of oleic acid. However, there were no significant differences in n-6/n-3 ratio between HM and LM for all 5 high-fat muscles (p>0.05).
a,bMean values within a row with different superscripts differ significantly (*p<0.05, **p<0.01, ***p<0.001, ns: not significant).
1SFA, Saturated fatty acids; MUFA, Monounsaturated fatty acids; PUFA, Polyunsaturated fatty acids
The comparison of fatty acid composition between low-fat muscles is shown in Table 4. No significant differences in SFA proportion between HM and LM muscles were observed in SS and SF muscles (p>0.05). There were also no significant differences in palmitic acid between HM and LM for all low-fat muscles except the SS muscle (p>0.05). However, all 5 low-fat muscles showed significant (p<0.01) differences in MUFA proportion between HM and LM due to significant (p<0.01) differences in the proportion of oleic acid. Among the 5 low-fat muscles, only the SM muscle had significant differences in n-6/n-3 ratio between HM and LM due to significantly (p<0.05) lower proportions of linoleic, eicosadienoic (C20:2n-6), eicosatrienoic, and arachidonic acids in HM muscle.
a,bMean values within a row with different superscripts differ significantly (*p<0.05, **p<0.01, ***p<0.001, ns: not significant).
1SFA, Saturated fatty acids; MUFA, Monounsaturated fatty acids; PUFA, Polyunsaturated fatty acids
The correlations between fatty acid proportion and meat quality traits are shown in Table 5. The saturated fatty acids such as lauric, myristic, and palmitic acids were positively correlated with CIE a* (redness), but negatively correlated with drip loss (%). In particular, lauric and myristic acids had strong correlations with drip loss (%) (r=−0.224; p<0.05 and r=−0.277; p<0.01, respectively) while palmitic acid had a strong correlation with CIE a* (r=0.356; p<0.001). Consequently, SFA had significant correlation with CIE a* (r=0.281; p<0.01) and drip loss (%) (r=−0.233; p<0.001). The CIE a* was negatively correlated with DHA (r=−0.214; p<0.05) and n-6/n-3 ratio (r=−0.241; p<0.05). Cooking loss (%) had a significantly (p<0.05) negative correlation with linoleic, eicosadienoic, and eicosatrienoic acids, resulting in significant correlation with PUFA (r=−0.233; p<0.05) and n-6/n-3 ratio (r=−0.209; p<0.05).
*p<0.05, **p<0.01, ***p<0.001.
1SFA, Saturated fatty acids; MUFA, Monounsaturated fatty acids; PUFA, Polyunsaturated fatty acids
The correlations between fatty acid proportion and sensory panel scores are presented in Table 6. Tenderness was positively correlated with saturated fatty acids but negatively correlated with polyunsaturated fatty acids. A strong correlation was observed between tenderness and SFA (r=0.389; p<0.001) and PUFA (r=−0.572; p<0.001). There were no significant correlations between all sensory panel traits and oleic acid, resulting in no significant correlation with MUFA (p>0.05). Contrarily, all sensory panel scores had significant (p<0.001) correlations with polyunsaturated fatty acids including linoleic, eicosadienoic, eicosatrienoic, arachidonic, and DHA, resulting in strong correlations between sensory panel scores and n-6/n-3 ratio (p<0.001). Finally, overall palatability was positively correlated with SFA (r=0.262; p<0.01), but negatively correlated with PUFA (r=−0.567; p<0.001) and n-6/n-3 ratio (r=−0.487; p<0.001). However, overall palatability had no significant correlation with oleic acid and MUFA (p>0.05).
*p<0.05, **p<0.01, ***p<0.001.
1SFA, Saturated fatty acids; MUFA, Monounsaturated fatty acids; PUFA, Polyunsaturated fatty acids
Discussion
The fat contents of 10 muscles from HM and LM Hanwoo carcasses obtained in the present study are similar to those obtained in a previous study (Jung et al., 2015), with the exception of the highest fat content (36.07%) for RA. In the previous study, the fat content of the Yangjee primal cut, which consisted of 7 sub-primal cuts including RA, was 15.85%. The fatty acid profile of RA was expected to be different from that of the Yangjee primal cut due to the higher fat content. According to Wood et al. (2008), a major factor affecting the fatty acid composition of animal muscles is the total amount of fat. In this regard, our results suggest that the differences in fatty acid composition among the 10 muscles from HM and LM Hanwoo steers (Table 2) were attributable to the differences in the intramuscular fat content of the 10 muscles (Fig. 1).
When the 10 muscles were classified as high-fat and low-fat muscles, larger differences in IMF content between HM and LM were observed in high-fat muscles compared to low-fat muscles (Fig. 1). This implies that the IMF content of longissimus muscle (i.e., marbling of loin) has a stronger influence on the IMF contents of high-fat muscles such as RA (Upjinsal), LT (Kotdungsimsal), IC (Kalbisal), LL (Chaekeutsal), and PM (Ansimsal) compared to those of low-fat muscles such as SS (Moksimsal), TB (Abdarisal), SM (Udunsal), GM (Boseopsal), and SF (Arongsatae). The results also suggest that the fatty acid profiles for HM and LM differ within individual muscles due to differences in fat contents (Table 3 and 4). This is consistent with the previous reports that increasing the total lipid content in muscle increased the content of individual fatty acids (Dinh et al., 2010; Wood et al., 2008). Pavan and Duckett (2013) reported that the proportion of the neutral lipid fraction (SFA and MUFA) was higher with increasing total lipid content in muscle. Our data also showed that total PUFA proportion was higher in low-fat muscles, but lower in high-fat muscles (Table 2).
The results for the fatty acid profile in high-fat and low-fat muscles are consistent with those found in an earlier study that compared the fatty acid profiles and sensory properties of LD, TB, and SM muscles from Hanwoo and Angus beef (Cho et al., 2005). The three muscles differed significantly in SFA, and oleic and linoleic acids were higher in TB than in LD. Cho et al. (2005) also reported that low-fat TB had lower proportions of saturated fatty acids such as stearic acid and higher proportions of n-6 and n-3 polyunsaturated fatty acids such as linolenic and arachidonic acids compared to high-fat LD. Contrarily, LD had a higher proportion of SFA and a lower proportion of PUFA. Our data also showed that high-fat muscles had lower n-6/n-3 ratios than low-fat muscles. The higher proportion of SFA and lower proportion of PUFA in high-fat muscles in the present study confirmed the conclusion by Wood et al. (2008) that the total amount of fat is a major factor affecting the fatty acid composition of animal muscles.
In this study, the fatty acid profile varied by IMF content within individual muscles (Table 3 and 4). The proportion of oleic acid was higher in HM muscles while LM muscles had a higher proportion of SAF. It was presumed that the high proportion of oleic acid was due to the feeding of Hanwoo cattle with high-concentrated diets. The amount of marbling and the concentration of MUFA increase dramatically with time on feed in grain-fed cattle in relation to the activity of stearoyl-CoA desaturase (SCD) (Smith et al., 2016). According to Ntambi (1999), a key enzyme involved in the cellular synthesis of MUFA from SFA is the membrane-bound SCD, which inserts a cisdouble bond in the Δ-9 position of fatty acid substrates. Because the accumulation of oleic acid and MUFA is related to higher SCD activity (Wang et al., 2005), high-concentrate diets for Hanwoo cattle certainly stimulated the activity of adipose tissue SCD. Consequently, our results suggest that the high proportion of oleic acid and MUFA and the lower proportion of SFA in HM muscles were due to the enhancement of Δ-9 desaturation with high-concentrate diets.
Previously, our study showed that Hanwoo muscles differed in the amount of IMF and meat quality traits as well as sensory properties (Jung et al., 2015). Consequently, it was suggested that Hanwoo beef palatability could be improved by increasing fat content in muscles due to the resulting increase in the tenderness, flavor, and juiciness of the meat (Jung et al., 2016). In this study, differences in fatty acid composition between muscles and correlations between fatty acids and meat quality traits were observed (Table 5). It is possible that variations in the amount of marbling fat and fatty acid composition explain the differences in meat quality traits. It is well known that muscles differ in the amount and fatty acid composition of the main lipid fraction, neutral lipid (triacylglycerol) and phospholipid, resulting in differences in meat quality such as shelf-life and flavor (Wood et al., 2004). In addition, our results clearly showed that saturated fatty acids such as lauric, myristic, and palmitic acids had positive correlations with CIE a* but negative correlation with drip loss (%). The results showed that 10 muscles from quality grade 1++ cuts had less red color and better water-holding capacity compared to same muscles from quality grade 2 cuts.
Finally, SFA was positively correlated with tenderness and overall palatability, but PUFA was negatively correlated with all sensory traits in this study (Table 6). These results were similar to those from Cho et al. (2005), who reported the positive correlations between saturated fatty acids and all sensory traits, but negative correlation between unsaturated fatty acids and all sensory traits. However, there was no significant correlation between oleic acid and all sensory traits, resulting in no correlation between MUFA and all sensory traits. This result was unexpected because an increased level of IMF was reported to have a positive influence on sensory qualities (Fernandez et al., 1999). Larick and Turner (1990) also reported a positive correlation between cooked beef fat flavor and oleic acid, which is the main fatty acid in the IMF in cattle and sheep. Although the correlations between the oleic acid content and sensory properties are generally accepted, our results clearly showed that the proportion of oleic acid was not correlated with all sensory traits. Cho et al. (2005) also did not report a significant correlation between oleic acid and any sensory traits. As Hanwoo beef has a high proportion of oleic acid with a high level of marbling, further investigations are necessary to identify the relationship between oleic acid content and sensory traits. Additionally, the negative correlation observed between n-6/n-3 ratio and all sensory traits suggests that total n-6 contents have a negative influence on all sensory traits including tenderness, juiciness, flavor, and overall palatability.













