Introduction
Sodium nitrite is conventionally used as a nitrite source in the manufacture of cured meat products because it is an inexpensive and easy handling (Jung et al., 2015). Nitrites play key roles in the meat industry for development of cured color and flavor, inhibition of fat oxidation, and securement of microbiological safety (Vossen et al., 2012). However, consumers increasingly prefer naturally cured meat products over conventionally one regarding the adverse health effects of synthetic curing agents including sodium nitrite (Sebranek and Bacus, 2007). Therefore, the market for naturally cured meat product has grown rapidly (Sebranek et al., 2012). However, the use of vegetable concentrates, especially celery concent- rate, in the curing process has several disadvantages compared to sodium nitrite such as high cost and increased time consuming required for the incubation step (Horsch et al., 2014).
Cold plasma, which is ionized gas, is an emerging nonthermal sterilization technology. Treatment using atmospheric pressure plasmas has been widely used for the sterilization of food and medical instruments and for surface modification of dielectric or metallic materials (Bogaerts et al., 2002; Yong et al., 2015). In addition, water can be purified by plasma treatment to remove harmful contaminants, including microorganisms (Foster et al., 2012). Plasma- treated water (PTW) can be produced via two different modes of plasma discharge, i.e., in water and above the water surface (Ma et al., 2015). The plasma interacts with the liquid (water), resulting in acidification and the generation of reactive oxygen and nitrogen species, including nitrate (NO3−) and nitrite (NO2−), which inactivate microorganisms (Liu et al., 2015; Oehmigen et al., 2011).
Recently, PTW has been suggested as a nitrite source, equivalent to a natural curing agent (Jung et al., 2015). However, the study of sausage cured with PTW was only focused on the quality analysis. Prior to application of PTW in practical application, however, it is important to assess their biological, chemical, and toxicological safety. Without food safety assurance, the foods applied by new technology cannot be used in the industry (Burdock and Carabin, 2004). Up to date, the safety of consumption of plasma-treated food has not been approved by governmental authorities. Thus, scientific evidences are needed to ensure its safety. The objective of the present study was to assess the mutagenicity and immune toxicity of PTW-treated emulsion-type sausage.
Materials and Methods
Water was treated with plasma and emulsion-type sausages were manufactured according to the methods described by Jung et al. (2015). To produce PTW, distilled water (500 mL) containing 1% sodium pyrophosphate (w/v) was irradiated by a surface dielectric barrier discharge (S-DBD) for 4 h. The carrier gas used was atmospheric air containing nitrogen and oxygen. The plasma device consisted of a ground electrode and an aluminum plate (0.6 mm thick) installed between two powered electrodes. A bipolar square waveform with 15 kHz was applied to the electrode. The total discharge area was ca. 20 cm2, and the average and the peak power were 3.14 W and 200 W, respectively.
Emulsion-type sausages were prepared using pork hind leg meat and back fat obtained from a commercial butcher. The meat was ground using a meat grinder with a 6-mm plate. The ground meat was mixed with back fat, ice water, and additive, which varied according to each treatment group (emulsion sausage cured with no nitrite source, control; emulsion sausage cured with sodium nitrite, SCS; emulsion sausage cured with PTW, SCP), in a bowl cutter. The concentration of nitrite ion in the two treatment groups (SCS and SCP) was maintained at 70 mg/kg. After storage, the meat batter was stuffed into collagen casing (2.5 cm diameter). Sausages were cooked in a water bath at 80℃ for 30 min until the internal temperature of the sausage reached 75℃. Cooked sausages were vacuum-packaged in low-density polyethylene/nylon bags; the packaged sausage was pasteurized in hot water (85℃) for 2 min and then cooled in water at 10℃.
A mutagenicity assay (Ames test) was estimated according to the method of Maron and Ames (1983) with modified. A 70% ethanol extract of each sample was prepared for use in the mutagenicity assay as follows. After sample preparation, 200 g of material was transferred to 1.8 L of 70% ethanol. After incubation for 16 h at room temperature (approximately 25℃), the extracts were filtered through Whatman filter paper No. 4 (Whatman International, Ltd., England). Then, 1.8 L of 70% ethanol was added, and the filtering procedure was repeated. Residual ethanol was removed from the samples using a vacuum evaporator (Rotary vacuum Evaporator N-11 Eyela; Tokyo Rikakikai Co., Ltd, Japan), and the samples were lyophilized (Labconco Freeze Dry System/Freezone 4.5; Labconco Co., USA). The lyophilized samples were stored in a freezer at −70℃ until use.
Salmonella Typhimurium strains TA98 and TA100, originally purchased from Molecular Toxicology, Inc. (Boone, USA), were cultured and provided by the Korea Research Institute of Chemical Technology (KRICT, Korea). Each strain was tested for histidine requirement, deep rough (rfa) characteristic, UV sensitivity (uvrB mutation), and ampicillin- or tetracycline-resistance, by R-factor before use. The strains were inoculated on Oxoid nutrient broth No. 2 (Oxoid Ltd., England) and cultured for 10 h at 37℃ with continuous agitation at 200 rpm (Vision Scientific Co., Korea). The density of the test cultures was 2 × 109 CFU/mL. The mutagenicity of the samples was assessed as follows. The sample amounts tested were 188, 375, 750, 1,500, and 3,000 μg per plate. The positive controls were 4-nitroquinoline-1-oxide (4-NQO), sodium azide (SA), and 2-aminoanthracene (2-AA). The activity of the S9 mixture was confirmed by the mutagenesis induced by 2-AA. The positive controls were dissolved in deionized distilled water (DDW) or DMSO (Sigma-Aldrich Co., USA). The direct plate incorporation method was used, with two plates per concentration. For each test sample, 100 μL of the culture (2 × 109 CFU/mL), 100 μL of the sterilized sample suspension (or control substance), and 500 μL of S9 mixture with DDW were mixed. Each test sample mixture was added to 2 mL of top agar containing histidine-biotin (kept warm at 45±2℃) and poured onto minimal glucose agar plates. After the agar solidified, the plates were incubated for 48 h at 37℃, and then the number of revertant colonies was counted. The 70% ethanol was used as a negative control. The positive controls were 4-NQO and SA when without metabolic activation and 2-AA with metabolic activation. The degree of mutagenicity was defined according to the method of Maron and Ames (1983); samples were considered positive when the number of revertant colonies was more than twice that of the negative control, and the effect was dose dependent.
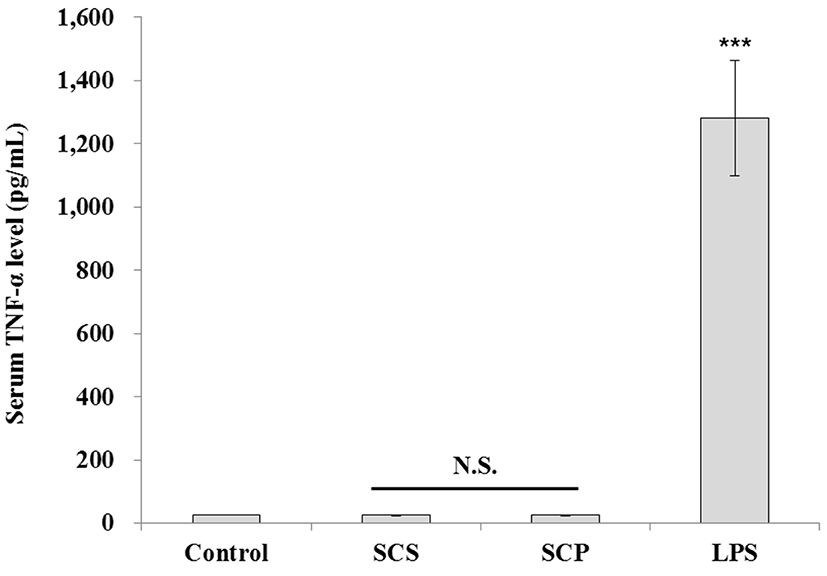
To test the immune toxicity of PTW-treated emulsified-type sausage, 8-wk-old female Balb/c mice (Oriental, Japan) were used (IACUC No. SNU-140627-1). Group I mice were administered with a normal diet, group II were administered with a normal diet containing SCS (emulsion sausage cured with sodium nitrite), group III were administered a normal diet containing SCP (emulsion sausage cured with PTW (plasma-treated water)), and group IV mice were administered with lipopolysaccharide (LPS). All animals were given free access to food. Mice were housed individually in plastic case with cedar chip bedding and kept under controlled temperature (25±1℃) and humidity (55±5%) conditions, with a 12-h light/dark cycle. Five mice were randomly chosen in each group and raised for 32 d. Then, the mice were sacrificed to detect serum tumor necrosis factor (TNF)-α levels and to count the number of Peyer’s patches in the small intestine. The concentration of serum TNF-α were measured by an ELISA kit (Mouse TNF-α DuoSet, R&D Systems, USA). Peyer’s patches in the small intestine were directly counted with the unaided eye.
Data were analyzed by one-way analysis of variance (ANOVA) using SAS software (Release 8.01; SAS Institute, Inc., USA). Differences among mean values were examined by Student’s two-tailed t-test. The results are expressed as the mean±SEM. P values less than 0.001 were considered statistically significant.
Results and Discussion
The Ames test is based on a set of S. Typhimurium strains that revert to histidine independence upon exposure to mutagens. Maron and Ames (1983) showed that a test substance can be considered a positive for mutagenicity when the number of revertant colonies is more than twice that of the negative control, and the effect is dose dependent. No mutagenicity of the test sausage samples was detected at up to 3 mg per plate (Table 1). The number of revertant colonies in the positive control was about 30, which was three times higher than that of the all test and control samples for both the TA98 and TA100 strains. There were no differences in the number of revertant colonies between the sodium nitrite-added and PTW-treated emulsion-type sausages. Based on these results, we concluded that the addition of PTW had no effect on the mutagenicity of emulsion-type sausage.
Abbreviations: 4-NQO, 4-nitroquinoline-1-oxide; SA, sodium azide; 2-AA, 2-aminoanthracene.
*Control, Emulsion sausage cured without a nitrite source; SCP, emulsion sausage cured with PTW; SCS, emulsion sausage cured with sodium nitrite.
Values are the mean±SD (p<0.05).
Tumor necrosis factor (TNF)-α together with interleukin (IL)-1, IL-6 and IL-8 is a potent paracrine and endocrine mediator of inflammation. TNF-α has been implicated in numerous acute and chronic inflammatory diseases, such as septic shock, bowel disease, Crohn’s disease, rheumatoid arthritis, atopic dermatitis, psoriasis, and Bechet’s disease (Edwards, 2004). It is primarily produced in T cells, polymorphonuclear cells, dendritic cells, and macrophages (Wallach and Kovalenko, 2009). In macrophages, TNF-α gene expression is induced by numerous physical, chemical, and biological stimuli, including ischemia, trauma, irradiation, viruses, bacteria, tumor cells, complement, cytokines such as intreleukin-1β (IL-1β), intreleukin-2 (IL-2), interferon-γ, colony stimulating factors, and by TNF-α itself (Brouckaert et al., 1993). TNF-α is also known to induce the production of other inflammatory cytokines, including IL-6 (Brouckaert et al., 1993) and IL-8 (Williams et al., 2008). Therefore, TNF-α is important effector that can be used to detect the immune toxicity of materials; thus, we examined serum TNF-α levels in animals administered with emulsion sausage cured with PTW (in their diet). The value of serum TNF-α was less than 10 pg/mL in mice in both the control and sausage-fed groups, indicating that no inflammatory response occurred, when compared to those of mice administered with LPS, the positive control. In addition, there were no differences in the length of intestine among the control, SCS, and SCP groups (Table 2). The length of intestine is one of the indicators for inflammation where the shrinkage of length is observed when inflammation occurred (Shirpoor et al., 2016).
| Sample* | Length of intestine (cm) | Peyer’s patches (average number) |
|---|---|---|
| Control | 55.50 ± 0.50 | 9.00 ± 0.00 |
| SCS | 50.33 ± 3.25 | 8.67 ± 0.58 |
| SCP | 52.33 ± 2.75 | 8.00 ± 1.00 |
*Control, Mice fed emulsion sausage cured without a nitrite source; SCP, Mice fed emulsion sausage cured with PTW; SCS, Mice fed emulsion sausage cured with sodium nitrite.
Peyer’s patches, one of the most important components of the gut-associated lymphatic system, play an essential role in induction of intestinal immune system such as IgA production. They are distributed throughout the intestinal wall and separated from the intestinal lumen by a single layer of epithelium containing microfold (M) cells, which are specialized to engulf antigens from the lumen and deliver them to antigen presenting cells (APCs) (Mowat, 2003; Neutra et al., 1996). They contain large numbers of immune cells including T cells, B cells, macrophages, and dendritic cells. When pathogenic antigens enter through the M-cells, they are captured by APCs for the processing and presentation to T cells (Kelsall, 2008; Lee et al., 2013). It is known that Peyer’s patches play an important role in the regulation of both mucosal and systemic immune systems (Macdonald and Monteleone, 2005). Therefore, in the present study, we counted the number of Peyer’s patches to assess the safety of the cured meat products manufactured with PTW. We found that the number of Peyer’s patches was similar in all study (Table 2).













