Introduction
Whey has 20% of total milk protein. It is considered as a by-product of cheese-making process. It has high biological value with plentiful amino acids (Marshall, 2004). Whey proteins are mainly composed of α-lactalbumin and β-lactoglobulin that have positive effects on health (Pescuma et al., 2008). Whey protein supplement improves protein synthesis, mineral absorption, and blood circulation (Pal et al., 2010; Pivi et al., 2007). In addition, there are various functional characteristics of whey proteins such as antioxidant capability and heat stability (de Wit, 1998).
Many attempts have been made to improve the pork industry in order to improve its production and quality. Feed additives in diets have direct effect on meat quality since pigs is a monogastric species (Kim et al., 2015c; Wenk, 2003; Wood et al., 2004). Dried whey concentrates could be obtained from soybean and milk products. Whey powder has been supplied in swine diets (Mahan, 1993; Yang et al., 2007). Several studies have been performed regarding the basic principle of dietary effect of whey protein on animals, including growth performance, nutrient digestibility, and its metabolic process (Burnell et al., 1987; Grinstead et al., 2000; Kim et al., 2015a; Theodorou et al., 2015). Ahmed et al. (2014) noted that a positive effect of dietary whey protein on beef quality such as extended shelf-life of beef. In addition, the antioxidant ability of whey protein has been demonstrated when whey powder is used as an additive in processed food (Browdy et al., 1997; Coronado et al., 2002). However, Simitzis et al. (2014) reported that whey protein supplement failed to affect meat quality of piglets.
Feeding system can influence the oxidative stability, ripening process, and sensory characteristics of meat products (Kim et al., 2014; Kim et al., 2015b; Ventanas et al., 2007). Whey has plentiful mineral compounds and some dependent protease activity is particularly affected by mineral compositions such as calpains which are calcium-dependent proteases (Goll et al., 2003; Wong et al., 1978). In particular, the plentiful taste compounds such as free amino acids and nucleotides were generated by proteolysis during ageing process and these compounds can enhance flavor of meat products (Ruiz-Ramirez et al., 2006; Toldra et al., 2000). Therefore, the aim of this study was to determine the effect of whey powder supplement on the physicochemical and texture characteristics of dry-aged meat of pigs.
Materials and Methods
The procedure for animal care and handling followed the Konkuk University Committee guideline (Korea). A total of 56 pigs (60 d old) at the beginning of the experiment were allotted to two dietary treatments (7 replicate pens per treatment and 4 pigs per pen). Pigs were divided in a completely randomized block design. Pigs were fed with experimental diets in the growing phase (42 d) and fattening phase (58 d), respectively (Table 1). The 1g/kg feed whey powder (WP) was mixed with a basal diet (Dongaone, Korea). The level of whey supplementation was following as previous study of Boudry et al. (2008). WP was purchased from Samik Dairy Co., Ltd. (Korea). Experimental diets were prepared to completely balance the nutrient requirements during all breeding phases (NRC, 1998). They were given to pigs ad libitum. Pigs were slaughtered at 180 d and the average live weight was reported as 120.3±7.4 kg without significantly difference for each treatment. After chilling for 24 h, all loins collected from the pigs for each treatment were transported to the Laboratory at University of KonKuk (Korea).
Vitamin/Mineral/etc, Supplied per kilogram of diet: 45 mg Fe, 0.25 mg Co, 50 mg Cu, 15 mg Mn, 25 mg Zn, 0.35 mg I, 0.13 mg Se, 16,000 IU vitamin A, 3,000 IU vitamin D3, 40 IU vitamin E, 5.0 mg vitamin K3, 5.0 mg vitamin B1, 20 mg vitamin B2, 4 mg vitamin B6, 0.08 mg vitamin B12, 40 mg pantothenic acid, 75 mg niacin, 0.15 mg biotin, 0.65 mg folic acid, 12 mg antioxidant.
After transportation from the slaughtering house, the loins were trimmed to remove the excess fat and skin. Dry-aging conditions were designed as following slightly modified Obuz et al. (2014) method. All samples were cut into about 500±20 g and hung up in a refrigerated room from 0 to 1℃ with a relative humidity (RH) of 70-80% for 20 d.
Drip loss of dry-aged loin was calculated using the following formula:
Drip loss = [(initial weight of dry-aged loin − weight of refrigerated dry-aged loin at 20 d) / initial weight of dry-aged loin] × 100
Moisture, crude protein, crude fat, and ash of dry-aged loin were measured by methods of AOAC (1995). Moisture content was measured using a drying oven at a temperature of 110℃ for 24 according to the gravimetric method. Crude protein content was calculated using the Kjeldahl method. Crude fat content was measured using the Soxhlet method. Crude ash was determined by using a muffle furnace at 550℃ for 3 h.
The pH of meat was measured using the following order. First, 2 g sample was homogenized in 18 mL of distilled water with a Bag mixer 400 (Interscience Co., France). The pH of homogenate was measured using a pH meter (pH 900, Precisa Co., UK).
The inner and outer color of dry-aged loin was measured using a Handy colorimeter (NR-300, Nippon Denshoku, Japan). The calibration of machine was conducted using a white plate (CIE L*=+94.48, a*=−0.67, b*=+3.31). Values of CIE L*(lightness), CIE a*(redness), and CIE b*(yellowness) were recorded.
Proteolysis index (PI) was calculated using the following formula: proteolysis index (PI) = non-protein nitrogen (NPN) × total nitrogen (TN)−1 × 100 (Careri et al., 1993). Total nitrogen (TN) content was measured using the Kjeldahl method. Non-protein nitrogen (NPN) was measured using the method of Careri et al. (1993). Briefly, 10 g minced sample was homogenized in 90 ml of distilled water. After centrifugation at 8,500 g for 15 min, 10 mL of 5% trichloroacetic acid (TCA) was added into the supernatant and incubated at 4℃ overnight. After overnight incubation, the solution was centrifuged at 8,500 g for 15 min at 5℃ and filtered through a filter paper (Whatman No. 4, Whatman Inc., USA). Non-protein nitrogen (NPN) content was measured with the Kjeldahl method.
Myofibrillar fragmentation index (MFI) was determined using the method of Culler et al. (1978) with minor modifications. Briefly, 4 g sample was homogenized with 40 mL of MFI buffer solution at pH 7.0 (100 mM KCl, 20 mM potassium phosphate, 1 mM EDTA, 1 mM MgCl2 and NaN3). The homogenate was centrifuged at 1,000 g at 2℃ for 15 min. The supernatant was removed and the pellet was centrifuged again with 40 mL of the MFI solution. Repeatedly, the supernatant was discarded and the pellet was mixed with 10 mL of MFI solution. Supernatant was filtered through a polyethylene strainer. MFI buffer solution was added to the filtrate to make protein concentration at 0.5 mg/mL. Absorbance value was measured at 540 nm using a spectrophotometer (Optizen 2120UV, Mecasys, Korea). MFI was calculated using the following formula: MFI = 200 × Absorbance.
DPPH radical scavenging activity was estimated according to Overland et al. (2011). Briefly, 5 g samples were homogenized in 20 mL methanol with a Bag mixer 400 (Interscience Co., France) and sonicated for 10 min. Sonicated mixture was centrifuged (10,000 g) at 4℃ for 10 min. The supernatant repeatedly extracted with 20 mL of methanol. Finally, the supernatant was diluted and kept in 50 mL volumetric flask. The methanol extract (0.1 mL) was reacted with 2.4 mL of methanolic DPPH solution (25 mg/ L) and kept in in a dark room (25℃) for 2 h. Absorbance value of the reaction was measured at 515 nm using a spectrophotometer (Optizen 2120UV, Mecasys, Korea). Percentage of DPPH radical scavenging activity was calculated using the following equation:
DPPH radical scavenging activity = [1 − (absorbance of sample / absorbance of control)] × 100.
TBARS of dry-aged loin was determined according to a method of Witte et al. (1970) with slightly modifications. Briefly, 2 g sample in 10 mL of 10% tricholoacetic acid (TCA) solution was mixed with 10 mL of distilled water and 0.04 mL of 0.3% butylated hydroxytoluence (BHT) solution. The mixture was filtrated through Whatman No.1 filter paper. The filtrate (5 mL) was reacted with 5 mL of TBA solution (2-thiobarbituric acid 2.88 g/L). The reaction was heated for 10 min in water bath followed by cooling. Absorbance value of the cooled reaction was measured at 532 nm using a spectrophotometer (Optizen 2120UV, Mecasys, Korea). Data was shown as malondialdehyde (MDA) meat mg/kg. TBARS was calculated using a standard curve of MDA by acidification of 1,1,3,3-tetraethxypropane (TEP).
Mineral composition of dry-aged loin was measured using a modified method of Gonzalez-Martin et al. (2002). A 1 g of freeze-dried sample was treated with 10 mL of 65% nitric acid and heated at 80℃ for 400 min. After evaporating to dry nitric acid, 10 mL of nitric acid was treated and heated, repeatedly. Finally, the dissolved sample was filled up with 100 mL of 2% nitric acid and the sample was used for mineral analysis. Mineral concentrations were determined using a spectrophotometer ICP-AES Ultima 2 (Horiba Jobin Yvon, Italy).
Meat pigment extraction was conducted according to Warriss (1979). Briefly, 4 g sample was homogenized in 20 mL of cooled 0.04 M phosphate buffer (pH 6.8) by using a homogenizer (AM-7, Nihonseiki Kaisha, Japan) at 13,000 rpm for 10 sec. The homogenate was placed at dark room (4℃) for 1 h and centrifuged (5,000 g) at 5℃ for 30 min. The supernatant was filtered through Whatman No.1 paper. The absorbance value of the filtrate was measured with spectrophotometry (Optizen 2120 UV, Mecasys, Korea). Values of Mb, OxyMb, and MetMb (%) were obtained using the following equations:
MetMb (%) = {1.395 − (A572 − A700) / (A525 − A700)} × 100;
Mb (%) = 0.369(A575/A525) + 1.140(A565/A525) − 0.941(A545/A525) + 0.015;
OxyMb (%) = 0.882(A575/A525) − 1.267(A565/A525) + 0.809(A545/A525) − 0.361,
where A525 was absorbance value at 525 nm, A572 was absorbance value at 572 nm, and A700 was absorbance value at 700 nm.
Samples at 10 mm in diameter and of 15 mm in length were fixed in muscle fiber direction. Warner-Braztler test was conducted by using a TA-XT2i texture analyzer (Stable Micro Systems, UK) equipped with a triangular slot cutting edge (1 mm thickness) at a crosshead speed of 3.33 mm/s. The condition of the Warner-Braztler test was set up according to the method of Bermudez et al. (2014).
All data were analyzed using SPSS 18.0 (SPSS Inc., 2009). Each pig was regarded as an experimental unit. Data on proximate compositions, drip loss, pH, water activity, color (lightness, redness, and yellowness), pigments, TBARS, DPPH, minerals compositions, MFI, proteolysis index, shear force, and texture parameters were analyzed by one-way analysis of variance (ANOVA) where diet was fixed as the main factor. An independent t-test was applied between means. Pearson correlation coefficient was analyzed between iron and myoglobin contents, MFI and proteolysis index by the SPSS 19.0. Statistical significance was considered when p value was less than 0.05. The p-value (< 0.1) was treated as a tendency to difference.
Results and Discussion
Proximate composition of dry-aged loin from WP-fed pigs was summarized in Table 2. There was no significant (p>0.05) difference in moisture or crude protein between the two groups. However, crude fat and ash content in the WP group were significantly (p<0.01) lower than those in the control group. Frestedt et al. (2008) indicated that the whey protein supplement could reduce fat loss and maintain the lean mass of body.
The water activity of the WP group were significantly (p<0.05) higher than that of the control group. The pH in WP group showed a higher tendency than the control group (p<0.1). In addition, drip loss in the dry aged loin from the WP group was significantly (p<0.05) lower than that the control group. The higher water activity and lower drip loss in WP group indicated that water had a higher binding strength to muscles at higher pH values of meat due to diet supplementation of whey protein (Huff-Lonergan and Lonergan, 2005).
The color of meat is associated with several factors such as pH and the iron state of pigment (Boles and Pegg). In this study, inner lightness of the WP group was significantly (p<0.05) lower than that of the control group, whereas inner redness of the WP group was significantly (p<0.05) higher than the control group. However, outer redness of the WP group was lower than that of the control group (p<0.05). Yellowness of both outer and inner dry-aged loin from the WP group was significantly (p<0.05) higher than that of the control group. According to previous studies, lightness had negative correlations with both redness and myoglobin content had a positive correlation with redness in this study (Kim et al., 2010; Newcom et al., 2004).
The effects of whey protein on trace mineral content and pigment states of dry-aged loin are summarized in Table 3. Ca2+ concentration of the WP group was significantly (p<0.05) higher than that of the control group. The amount of calcium in meat determines several factors, including skeletal muscle contraction, co-factors of enzymatic activities, and fat metabolism (Suttle, 2010). Bibber-Krueger et al. (2015) have reported that dietary calcium can stimulate Ca2+-dependent protease activity during postmortem, thus increasing the tenderness of meat. Caceres et al. (2006) have indicated that addition of calcium in meat products can increase hardness and overall acceptability. In this study, dietary supplementation with 0.1% WP increased the Fe2+ content of dry-aged loin compared to the control. The supplementation with whey protein has been reported to increase Ca2+ and Fe2+ absorption (Ahmed et al., 2014).
Myoglobin species are main factors that determine meat color (Liu et al. 1996). Gatellier et al. (2015) have reported that different diets could result in variations in pigment composition. High pigment content in meat has been found to depend on diet iron in feed (Wiklund et al., 2006). In the present study, WP group showed an increasing tendency of myoglobin content compared to the control group (p<0.1). And the iron content and total myoglobin content showed positive correlation (r=0.94, p<0.006). The changes in pigment state could be due to oxymyoglobin oxidized from myoglobin without catalyzed reaction and the conversion of oxymyoglobin to metmyoglobin by superoxide anion with oxymyoglobin (Moller and Skibsted, 2006). In this study, the composition of myoglobin species was found to be in the form of myoglobin (Mb, 25-27%), oxymyoglobin (MbO2, 35-42%), and metmyoglobin (MetMb, 33-38%). The oxymyoglobin percentage in the WP group was significantly higher than that of the control group.
Data on DPPH free radical scavenging ability and TBARS of dry-aged loin from WP fed pigs are summarized in Table 4. Although free radical scavenging ability of dry-aged loin was not significantly (p>0.05) different between the two groups, TBARS of dry-aged loin from the WP group pigs was significantly (p<0.05) higher than that from the control group. This result is different from that of Szczurek et al. (2013) in which lipid oxidation has been reported to be decreased in broiler meat with increasing diet level of whey protein. Akhrem et al. (1989) demonstrated that the positive correlation between oxymyoglobin and lipid oxidation in meat. Antioxidant ability (free radical scavenging and metal-chelating activity) of whey protein has been shown in vivo in several studies (Bayram et al., 2008; Gad et al., 2011, Haraguchi et al., 2011). Seo et al. (2011) reported that the supplemen- tation with whey protein could enhance the antioxidant ability in rats. However, DPPH free radical scavenging ability was not significantly different between the WP group and the CON group in this study. Total iron in meat exists in two forms: heme-iron (associated with myoglobin) and non-heme iron (Martinez-Torress and Layrisse, 1971). Moreover, iron content of muscle can produce prooxidant effect (Min and Ahn, 2005). Some scientists have reported positive correlation between the level of lipid oxidation and iron content (Pogge et al., 2014; Ventanas et al., 2006). Therefore, high iron content as pro-oxidant in dry-aged loin from the WP group pigs during the drying and ripening process might have contributed to the discrepancy between our results and those of other studies.
| CON | WP | p-value | |
|---|---|---|---|
| DPPH free radical scavenging activity (%) | 15.67±0.43 | 15.29±0.41 | 0.550 |
| TBARS1) | 0.58±0.69 | 0.92±0.87 | 0.041 |
TBARS unit, malondialdehyde mg/ meat kg
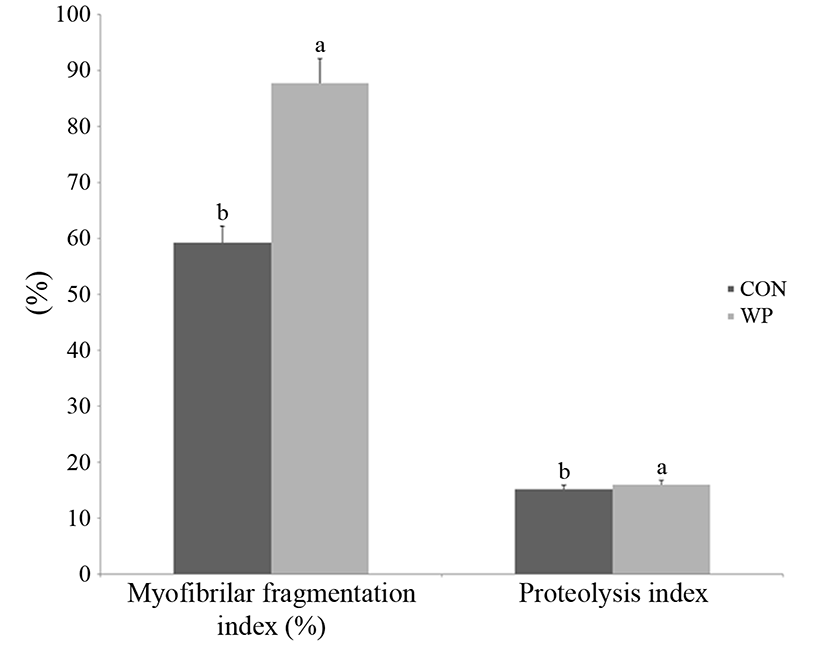
The MFI and proteolysis index of dry-aged loin from basal diet and WP fed pigs are shown in Fig. 1. Both MFI and proteolysis index of WP were significantly (p<0.05) higher than those of the control group. The MFI is an indicator of the length of myofibrils from fragmentation due to proteolysis. In the present study, MFI and proteolysis index showed positively correlation (r=0.97, p<0.001). Purchas et al. (1999) demonstrated that MFI is increased with increasing pH value and decreasing shear force in meat during the ageing process. In addition, calpains such as m-calpain and μ-calpain, which are related to convert muscle into meat, are calcium-dependent proteases (Goll et al., 2003). Positive correlation between MFI and tenderness has been reported by Vestergaard et al. (2000). Ruiz-Ramirez et al. (2006) have also used proteolysis index as a parameter to predict the texture of dry-aged ham according to the level of drying and ripening.
The effect of supplementation with whey protein on shear force of dry-aged loin after 20 d of ageing is summarized in Table 5. Shear force value of the WP group was significantly (p<0.05) lower than that of the control group. A negative correlation between shear force and MFI was found in this study, which is in agreement with the results of Vestergaard et al. (2000). Hayes et al. (2005) have reported that the addition of whey protein fraction with plentiful mineral concentrations can improve the texture properties of meat products.
| CON | WP | p-value | |
|---|---|---|---|
| Shear force (kg) | 5.35±0.17 | 4.43±0.24 | 0.038 |
Conclusion
This study noted that differences in physicochemical properties in meat were found in basal diet with 0.1% whey powder-fed pork and 30 fed pork after ageing process. Increase in calcium and iron influence on shear force and myoglogin conent of meat due to the whey powder supplementation. In addition, redness of dry-aged loin in the WP group was also increased due to increased myoglobin content. Therefore, the supplementation with whey powder could be used for the improvement of texture and sensory properties of the aged meat.













