Introduction
Pork cutlet is a popular processed meat consumed in Korea, and its abundant nutrients (e.g., proteins, fat, and minerals) and high water content can promote microorganism growth (Zhao et al., 2022). Accordingly, pork cutlets are potentially susceptible to cross-contamination with pathogenic bacteria, including Escherichia coli O157:H7, Staphylococcus aureus, Salmonella Typhimurium, and Listeria monocytogenes during production, processing, and transport (Hu et al., 2020). According to the Ministry of Food and Drug Safety (MFDS), frozen pork cutlets were recalled nationwide in 2020 due to contamination with enterohemorrhagic E. coli (MFDS, 2020). In general, freezing is a conventional preservation technique for improving shelf-life by inhibiting microorganism growth (Coombs et al., 2017). Additionally, previous studies have reported that microbial populations decrease with an increase in meat frozen storage period (Medić et al., 2018; Mohammed et al., 2021). However, several studies have demonstrated that all microbes are not reduced under frozen conditions. Enterohemorrhagic E. coli has been reported to survive in meat products for more than six months under frozen conditions (Ziuzina and Misra, 2016). In addition, Metzger et al. (2015) observed that S. aureus inoculated into frozen milk maintained the same microbial counts for 90 days. Similarly, Manios and Skandamis (2015) observed that the frozen beef patties inoculated with Salmonella spp. remained at their initial microbial load when thawed after 75 days. Additionally, L. monocytogenes showed minimal reduction over 12 months in frozen vegetables, indicating that it is resistant to freezing conditions (Fay et al., 2024). Therefore, freezing methods may have limitations in terms of preservation of microbiological safety, and effective preservation techniques are required to improve the hygienic quality of pork cutlets.
Preservation techniques, including thermal and non-thermal techniques, are essential for enhancing hygienic safety and quality of food; however, thermal treatment of raw meat and its products is not recommended as it can affect quality, including organoleptic and physicochemical properties and nutrient content (Lee and Yoon, 2024). Non-thermal processes, including ultrasonication, high hydrostatic pressure, cold plasma, UV, pulsed electric fields, ozone, and food irradiation, were extensively studied because of their advantages, including low-temperature processing, rapid treatment times, and minimal alteration of food quality (Jadhav et al., 2021; Zhang et al., 2019a). Among the non-thermal treatments, food irradiation is considered the most effective method for eradicating pathogens without compromising food quality (Kim et al., 2010). The approved sources of food irradiation include gamma rays using radioisotopes (60Co and 137Cs), and electron beams (EB) at ≤10 MeV and X-rays at ≤7.5 MeV from electron accelerators (Ehlermann, 2016). EB irradiation is more energy efficient than that of X-rays (energy conversion efficiency: 5%–10%) and offers a faster treatment time (103–105 Gy/s) compared to that of gamma rays (0.01–1 Gy/s; Amit et al., 2017; Levanduski and Jaczynski, 2008). EB irradiation is generally effective for relatively low-density and thin products due to its low penetrability (6–8 cm; Amit et al., 2017). Food irradiation is currently approved as a hygienic and phytosanitary technique in over 50 countries, with doses of up to 10 kGy reported to not pose any toxicological, nutritional, or microbiological risks (Ravindran and Jaiswal, 2019).
Food irradiation techniques have both positive and negative impacts on quality, affecting its physicochemical and organoleptic attributes (Indiarto et al., 2023). These effects are attributed to oxidation resulting from free radical chain reactions induced by irradiation treatment (Guo et al., 2021). Earlier studies reported that factors such as radiation source, irradiation dose, meat species, packaging, and temperature influence various quality attributes of meat, including color, 2-thiobarbituric acid reactive substances (TBARS), and pH (Cava et al., 2009; Sales et al., 2020; Yang et al., 2022; Yao et al., 2024). Therefore, the appropriate irradiation doses must be determined based on the target meat product, considering a balance between microbiological safety and quality attributes, such as organoleptic and physicochemical properties (Singh and Singh, 2020; Yim et al., 2023).
Analytical detection techniques for distinguishing between non-irradiated and irradiated foods gained increasing importance and are critical for enhancing consumer confidence, choice, and safety (Chauhan et al., 2009). These techniques can confirm the presence of radiation-derived components by identifying changes in their physical, chemical, biological, and microbiological properties (Stefanova et al., 2010). In foods containing lipids, irradiation breaks the chemical bonds of the parent fatty acids, resulting in the formation of hydrocarbons (Kwon et al., 2011). Hence, the European Committee for Standardization (CEN) established the EN1784:2003 standard based on the detection of hydrocarbons using gas chromatography/mass spectrometry (GC/MS), which was adopted as a standard by the Codex Alimentarius Commission (Delincée, 2002; Stefanova et al., 2010). However, the method is limited in terms of rapid detection due to the time-consuming extraction and purification process using organic solvents. As an alternative, headspace/solid-phase microextraction (HS/SPME)-GC/MS has been developed and applied successfully in the detection of volatile and semi-volatile organic compounds in various matrices (Lancioni et al., 2022). Therefore, hydrocarbon profiling by GC/MS was performed to detect irradiated pork cutlets and analyzed further to assess the applicability of the rapid detection method using HS/SPME-GC/MS.
The aim of the present study was to determine the influence of EB irradiation on E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes to enhance the hygienic quality of pork cutlets, as well as evaluate quality attributes such as color, TBARS, and pH. Furthermore, the hydrocarbon profiles of EB-irradiated samples were compared using GC/MS analysis, and the applicability of rapid detection for major hydrocarbons using HS/SPME-GC/MS was evaluated.
Materials and Methods
Plate count agar, potato dextrose agar, tryptic soy broth (TSB), mannitol salt agar, and xylose lysine deoxycholate agar were obtained from Difco (Detroit, MI, USA). 5-Bromo-4-chloro-3 indoly-β-D-glucuronide agar was obtained from KisanBio (Seoul, Korea). Listeria-Selective Agar Base and Listeria-Selective Supplement were obtained from Oxoid (Hampshire, UK). Butylated hydroxyanisole, thiobarbituric acid, trichloroacetic acid, n-hexane, florisil, n-dodecane, 1-dodecene, n-tridecane, 1-tridecene, n-tetradecane, 1-tetradecene, n-pentadecane, 1-pentadecene, n-hexadecane, 1-hexadecene, 1,7-hexadecadiene, n-heptadecane, 8-heptadecene, and n-eicosane were supplied by Sigma-Aldrich (St. Louis, MO, USA).
In the present study, commercially available uncooked, breaded, and frozen pork cutlets were purchased from a local grocery store in Jeongeup, Korea. The formulation of pork cutlets consisted of pork loin (50%) coated in a breading mixture that includes breadcrumbs, vegetable oil, dextrose, yeast, cornstarch, wheat flour, sodium polyphosphate, gluten, white sugar, refined salt, and onion powder. To determine the D10 value, 25-g samples were individually placed in stomacher bags (BagFilter 400S, Interscience, Saint-Nom, France), sterilized with 25 kGy of irradiation, and inoculated with pathogenic microorganisms. For physicochemical property and hydrocarbon analyses, approximately 100 g of each sample was sealed in retortable pouches (10×15 cm) made of polyester/aluminum/polypropylene. According to the method of Yeom et al. (2024), the investigation of D?? values involved EB irradiation doses of 0, 0.5, 1, 1.5, and 2 kGy, whereas studies on physicochemical properties and irradiation detection methods applied EB irradiation doses of 0, 2, 4.5, 7, and 10 kGy. EB irradiation (0.5–10 kGy) of samples was conducted using an ELV-8 electron accelerator set at a voltage of 10 MeV at the Advanced Radiation Technology Institute (Jeongeup, Korea), with samples placed on dry ice in a single layer and EB-irradiated at a dose rate of 0.5 kGy/scan by adjusting the conveyor belt speed.
To determine the D10 values of foodborne pathogens, we used a method based on An et al. (2018) with minor modifications. Briefly, E. coli O157:H7 ATCC43889, S. aureus KCCM 11335, S. Typhimurium UK1 ATCC 68169, and L. monocytogenes KCCM 40307 were individually cultured in TSB at 37°C for 24 h. The bacteria counts of each foodborne pathogen were adjusted to 106–107 cells/mL by centrifugation (3,264×g, 10 min, 4°C) followed by two washes with phosphate-buffered saline. Pre-sterilized samples (25 g, irradiated at 25 kGy) were artificially inoculated with suspensions of individual pathogens in stomacher bags (BagFilter 400S, Interscience) under aseptic conditions. The inoculated samples were treated with EB irradiation dose at 0–2 kGy and homogenized using a stomacher (BagMixer 400w, Interscience) for 60 s. The homogenate samples were serially diluted and inoculated into 5-bromo-4-chloro-3 indoly-β-D-glucuronide agar, mannitol salt agar, xylose lysine deoxycholate agar, and Listeria-Selective Agar Base with Listeria-Selective Supplement for E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes, respectively. After incubation (37°C, 24–48 h), the pathogen colonies were manually counted, and the Log CFU/g sample was calculated. The D10 values were estimated from the slope of a linear regression curve of the surviving microorganisms versus the irradiation dose.
To assess the total aerobic bacteria (TAB) and yeasts and molds (Y&M), a 25-g sample was homogenized with 225 mL of sterile 0.85 (w/v)% NaCl solution in a stomacher for 1 min. Microbial counts were determined by incubating TAB and Y&M on plate count (37°C, 24 h) and potato dextrose (25°C, 72 h) agars, respectively. Plates of each microbe were counted manually, with a detection limit of less than 10 CFU/g (1 Log CFU/g). Results are expressed as Log CFU/g.
The Hunter’s color values of the EB irradiation in pork cutlets were assessed using a colorimeter (CM-5, Konica Minolta, Osaka, Japan), which was calibrated with a standard white plate (A-210, Konica Minolta), and recorded using SpectraMagic NX software (CM-S100w, Konica Minolta).
The lipid oxidation levels of the EB-irradiated pork cutlets were evaluated by measuring the TBARS levels as described by Yeom et al. (2024). Briefly, 5 g of sample, 50 μL of 7.2% butylated hydroxyanisole, and 15 mL of distilled water were placed in a 50-mL conical tube and homogenized at 11,000 rpm for 20 s using a homogenizer (T 25 digital ULTRA-TURRAX; IKA, Staufen, Germany). Subsequently, 2 mL of the homogenate was reacted in a water bath (90°C, 15 min) by adding 4 mL of a solution containing 20 mM thiobarbituric acid in 15% trichloroacetic acid, and the reaction was stopped by cooling for 15 min on ice followed by centrifugation at 1,175×g for 15 min. TBARS values were obtained by measuring the absorbance at 532 nm using a spectrophotometer (Libra S70, Biochrom, Cambridge, UK) and expressed as malondialdehyde (MDA) μg/g sample.
To determine pH, a 25-g sample was homogenized in 225 mL of distilled water (10-fold dilution). Thereafter, the supernatant of the homogenate was collected by centrifugation (3,264×g, 10 min, 4°C). The pH of the EB-irradiated samples was measured using an Orion 3 Star pH meter (Thermo Fisher Scientific, Waltham, MA, USA).
Hydrocarbon analysis of the EB-irradiated sample was conducted following the European standard EN 1784:2003 (CEN, 2003) with modifications. Briefly, fat extraction was performed three times by combining 100 g of sample and 200 mL of n-hexane in a 500-mL flask and mixing in a shaking incubator (150 rpm, 25°C, and 30 min). The fat extract was concentrated using a vacuum rotary evaporator (EYELA N-1200B, Rikakikai, Tokyo, Japan) and stored at 4°C until analysis. Extracts containing 1 g of fat were loaded onto an open column packed with 30 g of florisil and eluted with 90 mL of n-hexane. The eluate was concentrated to 1 mL and analyzed using a GC/MS analyzer under the conditions described in Supplementary Table S1. The hydrocarbon concentrations were calculated using an internal standard (n-eicosane, 4 μg/mL).
HS/SPME-GC/MS analysis of EB-irradiated samples was conducted according to the method of Lee et al. (2020). Briefly, the EB-irradiated samples were placed in 5-g aliquots in 20 mL-vials and capped. Next, an SPME autosampler (MPS 2, Gerstel, Mühlheim, Germany) with DVB/CAR/PDMS fiber was used to extract the hydrocarbons. Extraction conditions were a temperature of 120°C and a string speed of 250 rpm for 10 min to equilibrate the level of volatile compounds in the headspace. Subsequently, the hydrocarbons in the headspace were absorbed by fiber for 60 min at the same stirring speed and temperature, followed by desorption for 15 min in the GC injector. Hydrocarbons were analyzed according to the conditions described in Supplementary Table S2, and the quantification and identification were performed based on standards.
Results were expressed as mean±SD, and statistical analysis was performed by analysis of variance (ANOVA) using MINITAB software version 20.1.3 (Minitab, State College, PA, USA). Tukey’s post-hoc test was conducted to assess significant differences among groups (p<0.05). Multivariate statistical analyses were performed using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca). GC/MS data were analyzed using partial least squares discriminant analysis (PLS-DA) to visualize variations between groups, and the major hydrocarbons contributing to discrimination were identified based on the variable importance in the projection (VIP) score.
Results and Discussion
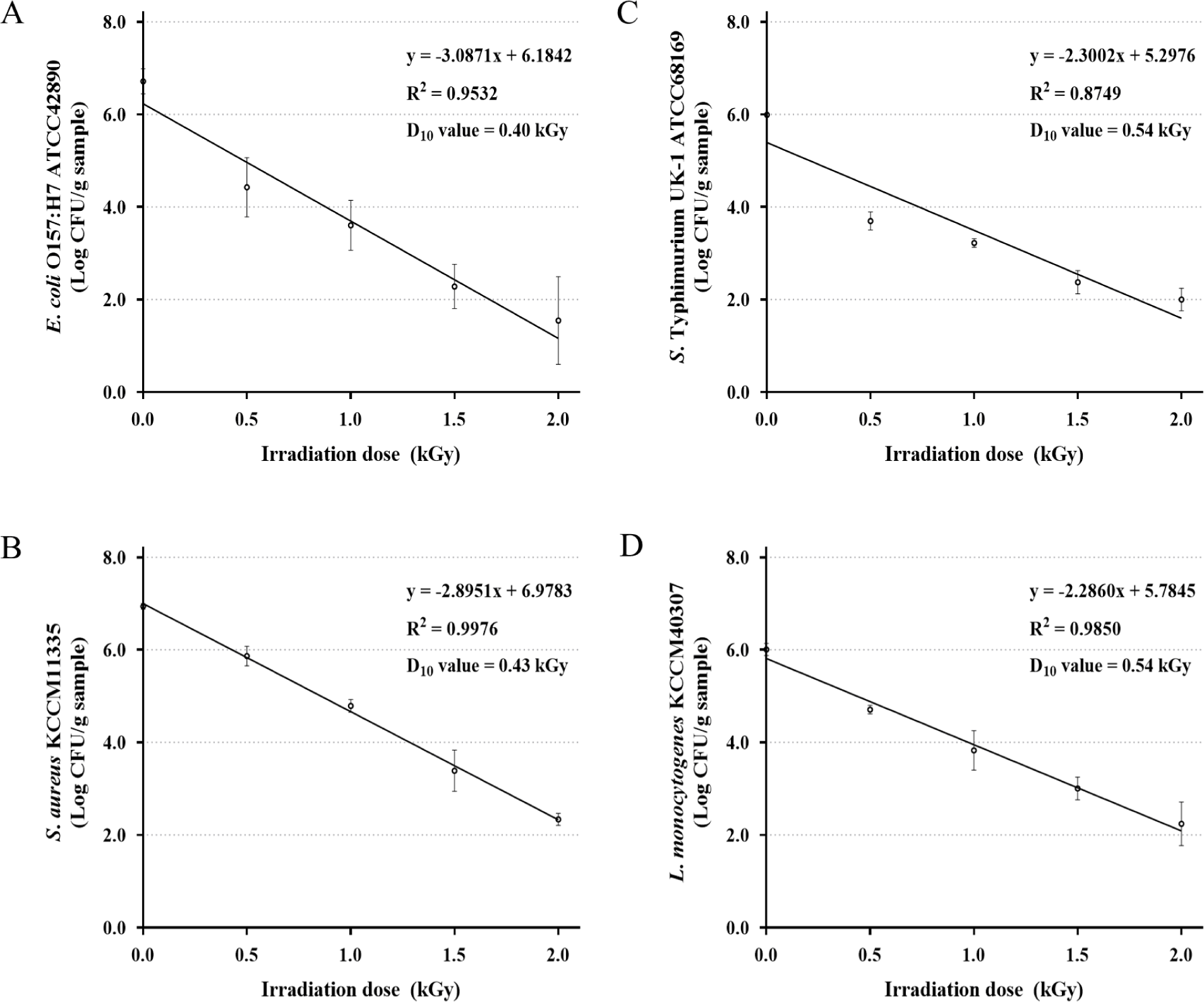
The D10 value is defined as the irradiation dose needed to reduce the population of pathogenic microorganisms by 90% or 1 Log CFU (Yeom et al., 2024). In this study, the D10 values of E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes were determined using a linear regression model assessing viable bacterial counts (Log CFU/g) as a function of the different doses (0, 0.5, 1, 1.5, and 2 kGy). The R2 coefficients of the linear regression curves were obtained as 0.9532, 0.9976, 0.8749, and 0.9850 for E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes, respectively. Based on these linear regression models, the average D10 values were calculated as 0.40, 0.43, 0.54, and 0.54 kGy for E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes, respectively (Figs. 1A, B, C, and D). Mahapatra et al. (2005) reported that D10 values for the food-borne pathogens were 0.24–0.47 kGy for E. coli O157:H7, 0.26–0.45 kGy for S. aureus, 0.37–0.70 kGy for S. Typhimurium, and 0.25–0.77 kGy for L. monocytogenes. Robichaud et al. (2021) found D10 values in the frozen infant formula to be 0.44, 0.42, 0.97, and 0.92 kGy for the same pathogenic bacteria. In addition, Gautam and Venugopal (2021) reported that D10 values in frozen seafood and food showed irradiation doses of 0.30–0.98, 0.48–0.71, 0.47–0.70, and 0.43–0.66 kGy for E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes, respectively. The differences in D10 values indicate that radiation sensitivity depends on microbial species and the food matrix, which is consistent with the findings of the present study. The D10 value of pathogenic microbes can provide an accurate estimate of the lethal/death dose, which is vital for ensuring safety from microbes when employing food irradiation as a preservation technique (Thayer, 2000). The National Advisory Committee on Microbiological Criteria for Food (NACMCF) requires that non-thermal processing methods used as an alternative to conventional pasteurization must achieve a 5-Log CFU reduction in targeted pathogens such as E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes (NACMCF, 2006). Here, the 5-D10 values were estimated as 2.00, 2.15, 2.70, and 2.70 kGy for E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes, respectively. Therefore, this finding suggests that a minimum EB irradiation dose of 2.70 kGy is required to enhance the hygienic quality of pork cutlets by eliminating these four pathogens.
The effect of the EB irradiation at different doses (0, 2, 4.5, 7, and 10 kGy) on the TAB and Y&M populations in pork cutlets is shown in Fig. 2. The TAB and Y&M of the pork cutlets decreased significantly with increasing EB irradiation doses. Specifically, the TAB and Y&M counts in the non-irradiated samples were 4.44 and 3.23 Log CFU/g, respectively. However, the TAB counts in the irradiated samples at 2 and 4.5 kGy decreased to 3.33 and 1.93 Log CFU/g, respectively. Similarly, the Y&M counts also decreased to 2.40 and 1.20 Log CFU/g with EB irradiation at 2 and 4.5 kGy, respectively. In samples irradiated above 7 kGy, TAB and Y&M counts were less than 1 Log CFU/g. An et al. (2018) reported that the TAB and Y&M populations were undetectable in frozen ducks irradiated with EB irradiation at 7 kGy, which is consistent with that of the current study. Similarly, Yim et al. (2023) found that the TAB count was undetectable in marinated ground beef after X-ray irradiation at 7 kGy. Furthermore, Shin et al. (2014) reported that no microbial growth was observed in raw pork and pork products treated with X-ray and EB irradiation at 6 and 8 kGy, respectively. The sterilization mechanisms of EB irradiation in meat were attributed to damage to the genetic material (RNA or DNA) induced both directly and indirectly (Ravindran and Jaiswal, 2019). The direct effects involve the breaking of bonds between base pairs in the genetic material, which can cause mutations or lethal reactions. Contrastingly, indirect effects are caused by free radicals and reactive oxygen species formed from the radiolysis of dihydrogen oxide, damaging genetic material and disrupting bacterial function (Indiarto et al., 2023). Both the direct and indirect effects increased with the increasing EB irradiation dose, resulting in enhanced sterilization of microorganisms. Therefore, our findings suggest that EB irradiation at up to 10 kGy is an effective preservation treatment for reducing TAB and Y&M in pork cutlets.
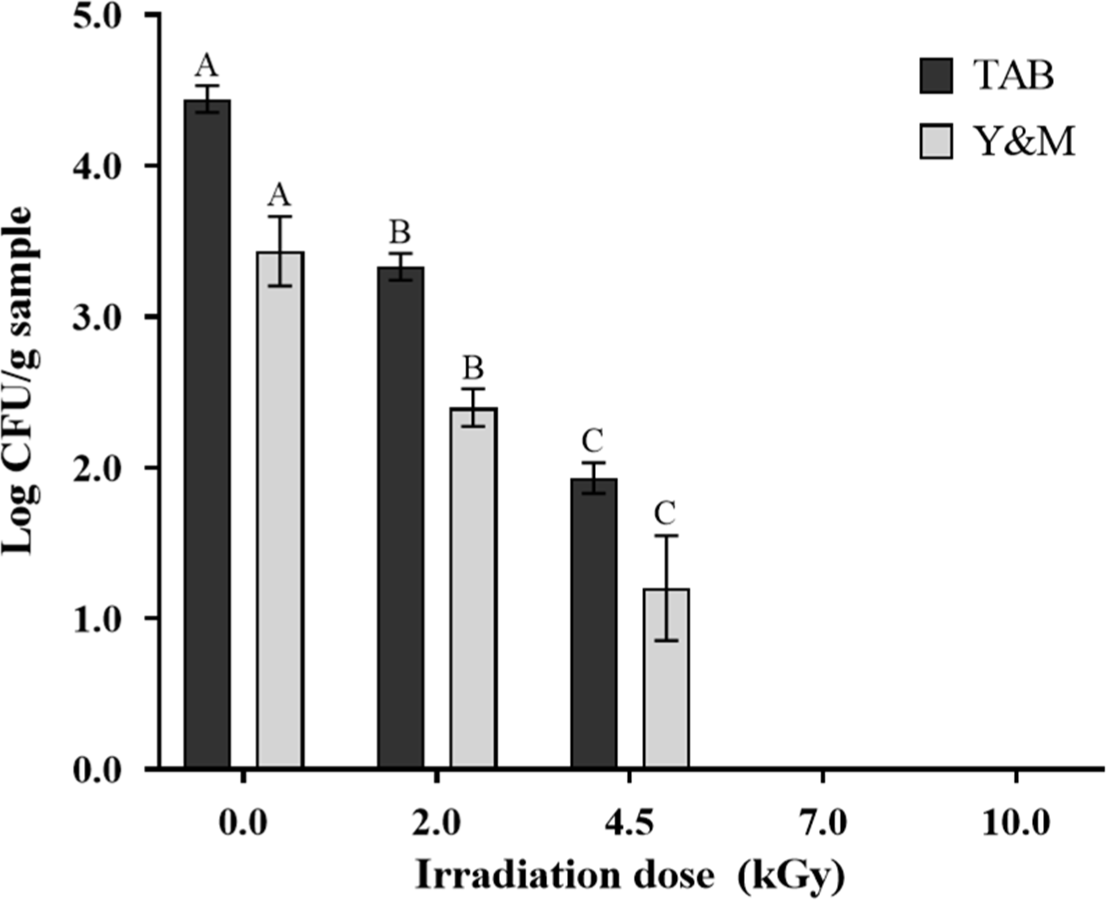
Table 1 presents the Hunter’s color values of the EB-irradiated pork cutlets. The CIE L*-value of the pork cutlet products did not exhibit a clear correlation with the EB irradiation dose. However, samples irradiated with 2–10 kGy exhibited a decrease in CIE b*-value compared to the control. Contrastingly, compared with those of control, the CIE a*-value decreased by 15.70% at 2 kGy but significantly increased by 20.06%, 30.81%, and 29.65% for EB irradiation at 4.5, 7, and 10 kGy, respectively. In meat, the redox state of myoglobin (Mb) is a key determinant of color, which varies depending on the ligand-bound form of Mb (Kim et al., 2024). Meat color is influenced primarily by the relative concentrations of three Mb forms: deoxy-Mb (reddish-purple), reddish oxy-Mb (red), and met-Mb (brown). When oxy-Mb is abundant, it can be oxidized to met-Mb by free radicals generated during irradiation, leading to a reduction in the CIE a*-value (Arshad et al., 2020). Conversely, in samples with high met-Mb levels, continued radical reactions can induce structural degradation of oxidized met-Mb, resulting in the formation of red-colored carboxy-Mb (Brewer, 2004). Indeed, Yim et al. (2023) found that the CIE a*-value of marinated ground beef increased following treatment with more than 5 kGy of X-ray irradiation (p<0.05), which was attributed to the formation of CO-heme pigment complexes in the meat after irradiation treatment. Similarly, Li et al. (2017) observed that the CIE a*-value of vacuum-packed fresh pork was increased significantly by gamma irradiation at 5 and 7 kGy. In addition, Feng et al. (2016) reported that the CIE a*-value of uncured cooked turkey increased significantly after EB irradiation at 1.5, 3, and 4.5 kGy. The findings indicate that the effects of irradiation on the CIE a*-value of meat depend on the initial redox state of Mb, as well as other factors, such as meat type, muscle composition, and oxygen availability (Faustman et al., 2023). Therefore, the findings of the present study suggest that the decrease in the CIE a*-value of pork cutlets at 2 kGy resulted from the oxidation of oxy-Mb to met-Mb, whereas the subsequent increase at 4.5–10 kGy was likely due to CO-Mb formation.
To evaluate lipid oxidation induced by EB irradiation of pork cutlets, we used the TBARS method, which measures MDA levels generated from the oxidation of lipids in muscle. As shown in Fig. 3A, the TBARS value increased significantly with increasing EB irradiation dose. Compared with those of the non-irradiated sample (0.26±0.03 MDA μg/g), the samples subjected to 2, 4.5, 7, and 10 kGy of EB irradiation had 0.30±0.02, 0.33±0.02, 0.60±0.12, and 0.79±0.18 MDA μg/g, respectively. MDA is a three-carbon dialdehyde and a relatively stable secondary lipid oxidation product derived from polyunsaturated fatty acids serving as an important indicator of meat quality, including organoleptic properties (Amaral et al., 2018). Ham et al. (2017) demonstrated that the TBARS values of cooked beef patties and pork sausages significantly increased following irradiation with EB and gamma rays at doses of up to 10 kGy. Similarly, Song et al. (2009) also reported that the TBARS values of minced pork and pork patties increased following electron and gamma irradiation (0, 2, 5, and 10 kGy; p<0.05). However, Yim et al. (2023) found that X-ray irradiation of up to 10 kGy did not significantly change the TBARS values of marinated ground beef. Lipid oxidation can be influenced by factors, including type of animal, period of storage, irradiation dose, and packaging (Indiarto et al., 2023). Earlier studies have suggested thresholds for TBARS values to ensure consumer acceptability of meat. For instance, McKenna et al. (2005) suggested a TBARS value of 1.0 MDA μg/g as an arbitrary threshold for off-odors caused by oxidation and rancidity. However, Campo et al. (2006) reported that TBARS values of up to 2.0 MDA μg/g were acceptable to consumers. However, Zhang et al. (2019b) used two different TBARS methods to measure lipid oxidation in uncooked beef, yielding MDA values of up to 0.02–2.55 μg/g and 0.04–10.72 μg/g, with TBARS levels having no significant effects on the flavor of consumer acceptance. These inconsistent results may be due to differences in sample type, methodology, and sample amount, which indicate limitations in describing the relationships between sensory attributes and TBARS values. Although Kwon et al. (2008) found that TBARS levels in raw beef, pork, and chicken increased significantly by 5 kGy of EB irradiation before and after cooking, this did not negatively influence the organoleptic attributes of the samples cooked using a water bath. However, the frying process produces various volatile and non-volatile compounds, as well as soluble and insoluble compounds that influence flavor by lipid oxidation (Chang et al., 2020), which can induce changes in the organoleptic properties of pork cutlets. According to our findings, EB irradiation accelerates lipid oxidation in pork cutlets; however, TBARS values have a limited capacity to determine organoleptic quality. Therefore, further experiments on the evaluation of organoleptic attributes, such as consumer preference surveys and flavor compound analysis, are required to determine changes in consumer acceptance of pork cutlets after frying.
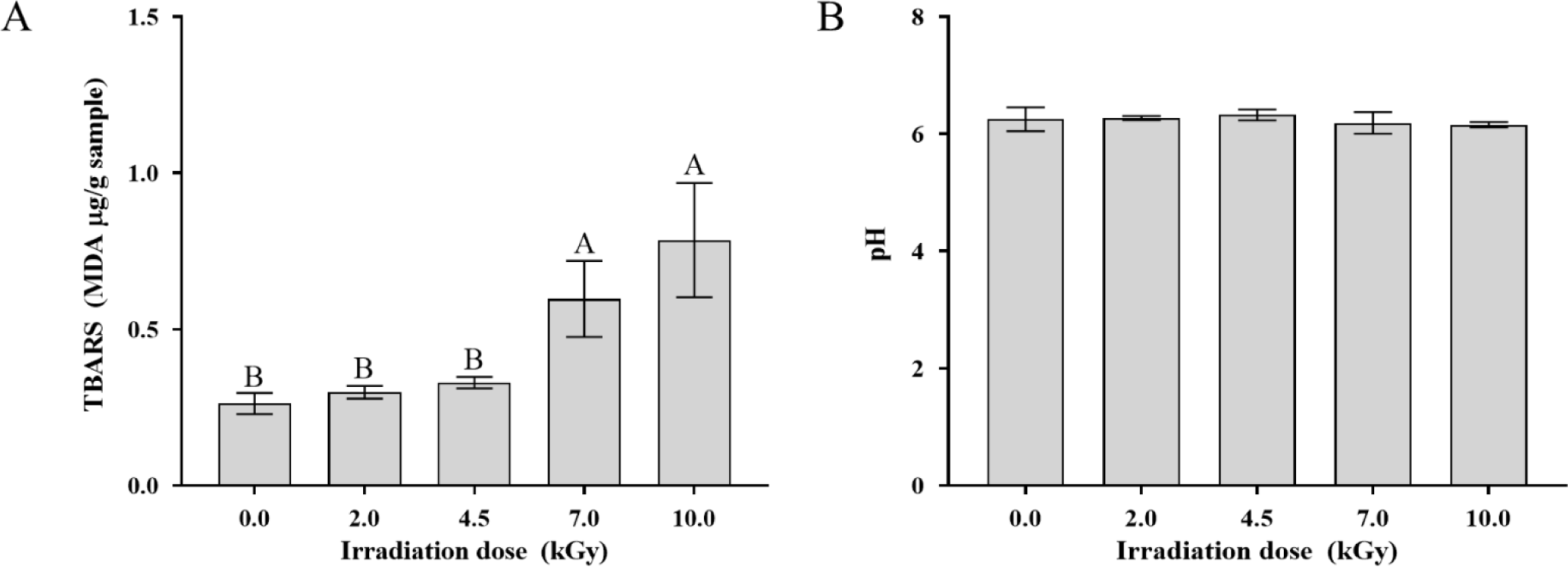
Fig. 3B shows the effect of EB irradiation on the pH of pork cutlets. The pH did not significantly change among irradiated and non-irradiated samples. pH is an important factor contributing to various meat quality traits such as tenderness, water-holding ability, microbiological safety, and color (Andrés-Bello et al., 2013). Earlier studies found that irradiation treatment does not significantly change the pH of meat or meat products, resulting in minimal or no effect on other meat-quality properties (Badr and Mahmoud, 2011; Ham et al., 2017; Wahyono et al., 2024). Similarly, the results showed that EB irradiation doses at 2–10 kGy did not alter the pH of pork cutlets, indicating no negative impact of the dose on other meat quality characteristics.
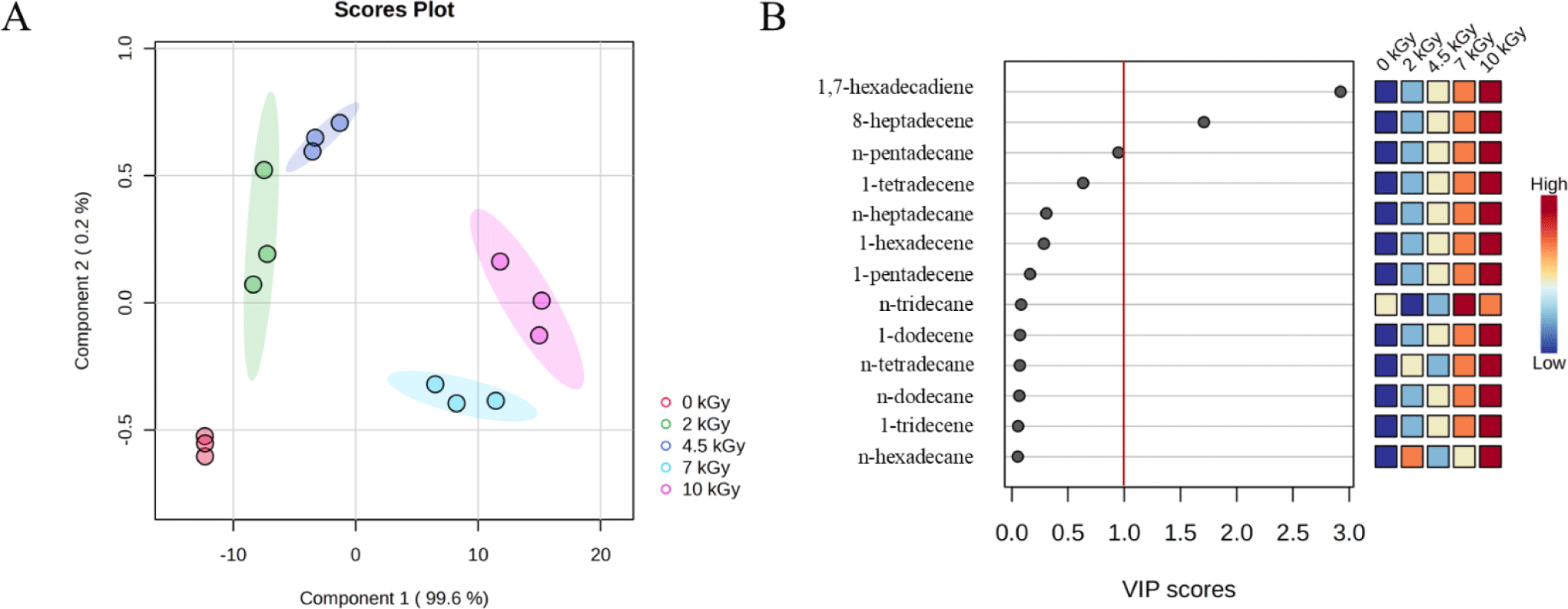
To identify markers to differentiate EB-irradiated pork cutlets, hydrocarbon profiles are presented in Table 2. The irradiated samples exhibited significant increases in the levels of seven hydrocarbons at doses up to 10 kGy: n-dodecane, 1-tridecene, 1-tetradecene, 1-pentadecene, 1,7-hexadecadiene, 1-hexadecene, and 8-heptadecene. Radiation-induced hydrocarbons in meat contain one fewer carbon atom (Cn-1) or two fewer carbon atoms with an additional double bond (Cn-2, 1-ene) than that in the parent fatty acid. The PLS-DA model and VIP scores were used to assess the importance of hydrocarbons as detection markers of the EB irradiation dose in pork cutlets (Fig. 4). As shown in Fig. 4A, the PLS-DA model explained 98.8% and 0.3% of the total variance in components 1 and 2, respectively. The PLS-DA plots confirmed that the non-irradiated and irradiated samples were clearly separated into distinct groups. Among the 13 hydrocarbons, only 1,7-hexadecadiene and 8-heptadecene showed VIP scores greater than one (Fig. 4B). A VIP score higher than one is typically used as a criterion for identifying related variables (Akarachantachote et al., 2014), indicating a stronger contribution to the discrimination of EB-irradiated samples based on the hydrocarbons identified in our findings. Thus, two hydrocarbons (1,7-hexadecadiene and 8-heptadecene) were identified as the most promising markers for distinguishing EB irradiation treatment in pork cutlets. Several studies reported 1,7-hexadecadiene and 8-heptadecene as the most prominent markers for distinguishing irradiation treatments in fat-containing foods such as duck, beef, chicken, pork, and flaxseed (An et al., 2018; Hwang et al., 2014; Kim et al., 1999; Zhang et al., 2024), which supports our findings. Additionally, the MFDS in Korea recognized 1,7-hexadecadiene and 8-heptadecene as markers to identify irradiation treatment in meat products. This decision is in line with our results which suggested that 1,7-hexadecadiene and 8-heptadecene are effective markers for distinguishing between non-irradiated and irradiated pork cutlets.
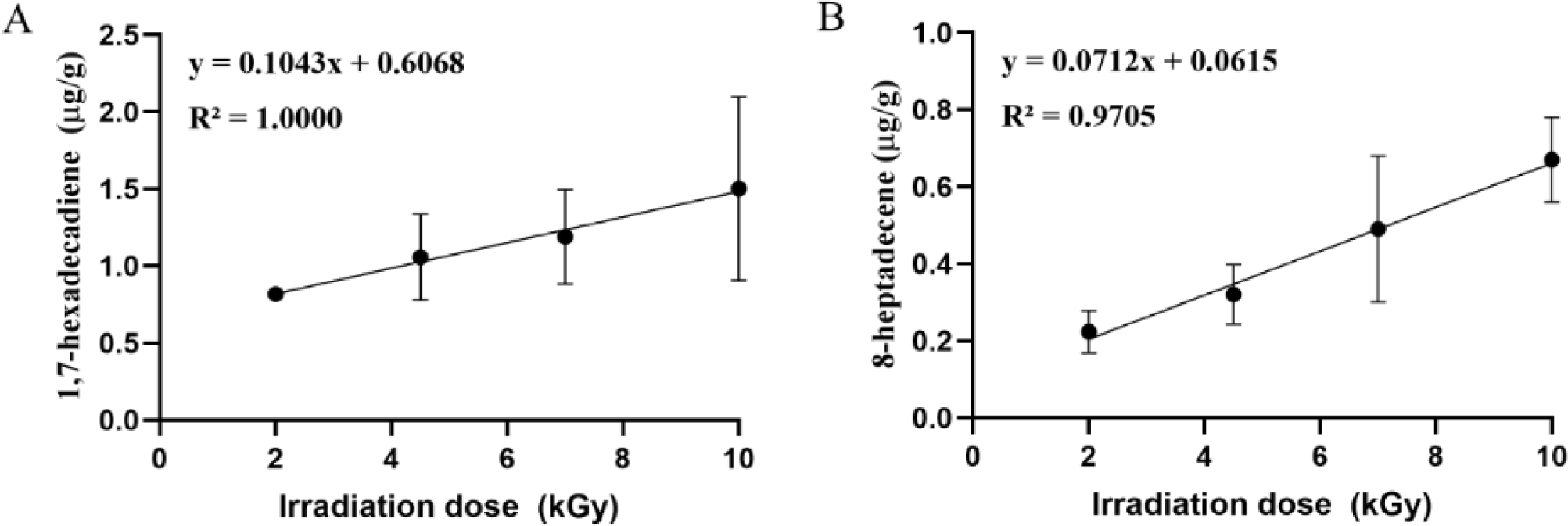
Previous studies have explored HS/SPME-GC/MS analysis of volatile compounds in irradiated meat products, mainly to investigate organoleptic properties (Li et al., 2017; Wang et al., 2022; Xu et al., 2025). The studies have focused primarily on low molecular weight compounds, such as volatile aroma compounds, while research on relatively high molecular weight compounds, such as 1,7-hexadecadiene and 8-heptadecene remains insufficient. Therefore, we performed HS/SPME-GC/MS analysis to evaluate the applicability of the rapid detection method of major hydrocarbons (1,7-hexadecadiene and 8-heptadecene) selected from GC/MS data (Fig. 5). Both 1,7-hexadecadiene and 8-heptadecene were detected only in EB-irradiated samples, and their concentration increased linearly as the EB irradiation dose increased (R2=1.000 and 0.9705, respectively), which is similar to the GC/MS results. Similarly, Li et al. (2010) reported a rapid and simple method for the detection of 1,7-Hexadecadiene and 8-heptadecene in chilled beef irradiated with 0.1–8 kGy of gamma rays. Furthermore, Lee et al. (2020) demonstrated that 1,7-hexadecadiene is the most potent marker for rapid detection in gamma-irradiated soybeans, with higher sensitivity (<0.1 kGy) than the GC/MS method (<0.25 kGy). Their findings indicated that 1,7-hexadecadiene was absent in heated, steamed, microwaved, sonicated, or UV-irradiated samples, but was only detectable in gamma-irradiated samples. Additionally, HS/SPME-GC/MS analysis serves as an effective alternative to traditional irradiation detection methods, such as PSL, TL, ESR, and GC-MS (Lee et al., 2020). The HS/SPME-GC/MS method is an environmentally friendly and cost-effective technique compared to GC/MS, as it does not require organic solvents and provides faster analysis times (Supplementary Table S3). Therefore, our results suggest that HS/SPME-GC/MS analysis of 1,7-hexadecadiene and 8-heptadecene is a useful method for rapidly detecting irradiation treatments in fat-containing foods such as pork cutlet, while addressing the limitations of traditional analytical methods.
Conclusion
This study demonstrated the potential of EB irradiation as a preservation treatment for pork cutlets. EB irradiation effectively reduced the pathogens including, E. coli O157:H7, S. aureus, S. Typhimurium, and L. monocytogenes in pork cutlets. Based on the D10 values, the EB irradiation dose range of 2.00–2.70 kGy was determined to achieve a 5-Log CFU reduction of these four pathogens. EB irradiation above 7 kGy reduced TAB and Y&M in the sample to below detection limits. Additionally, EB-irradiated samples exhibited no adverse effects in quality properties, including color, TBARS, and pH. GC/MS analysis of hydrocarbon profiles revealed that 1,7-hexadecadiene and 8-heptadecene were prominent radiation-induced markers for distinguishing EB-irradiated samples. Furthermore, HS/SPME-GC/MS analysis was used to overcome the limitations of GC/MS, which showed the applicability of the rapid detection method. Our research primarily focuses on the influence of EB irradiation on the hygienic quality and irradiation detection method of pork cutlets, and further experimental studies are needed to explore its effect on organoleptic and quality properties after cooking.













