Introduction
Meat has been a vital component of the human diet since ancient times, owing to its nutritional value, palatability, and organoleptic properties. It is an excellent source of biologically available protein and essential nutrients but is highly perishable and prone to spoilage due to biochemical and microbiological reactions. Meat quality and safety are critical factors directly influencing consumers’ acceptance of meat and its products (Awad et al., 2022; Baba et al., 2021; Bekhit et al., 2021; Bougherara et al., 2023; Yehia et al., 2021). Lipid oxidation, microbial growth, and discoloration are among the main factors leading to meat deterioration and quality issues.
Fresh meat is particularly perishable due to its intrinsic properties, such as high-water activity (aw>0.95), which facilitates microbial growth and chemical reactions. Both intrinsic and extrinsic factors, including storage conditions, significantly affect meat quality and safety (Awad et al., 2022; Bekhit et al., 2021; Yehia et al., 2021). Lipid oxidation contributes to the formation of off-flavors, discoloration, and other deteriorations, reducing consumer acceptance. It is initiated by free radicals that degrade lipids, leading to the production of secondary compounds that compromise meat quality and safety. Furthermore, lipid oxidation generates genotoxic and cytotoxic compounds in meat and other foods (Awad et al., 2022).
Camels, as a sustainable meat source, can help meet the increasing global demand for food, especially in arid and semi-arid regions where other livestock struggle to thrive (Bougherara et al., 2023). Camel meat, a staple in arid regions of the Middle East and North-East Africa, has potential as an alternative red meat for global consumption (Baba et al., 2021). Recognizing this, the UN has designated 2024 as the International Year of Camelids, highlighting their potential to combat hunger and support food security and economic development in challenging environments (Kadim et al., 2008; Mohamed et al., 2024). The dromedary camel (Camelus dromedarius, one-hump) is a crucial source of meat in arid regions, thanks to its unique physiological adaptations, including tolerance to high temperatures, solar radiation, water scarcity, and limited vegetation. Camel carcasses consist of approximately 57% meat, 26% bone, and 17% fat (Kadim et al., 2008). However, camel meat, like other meats, is low in endogenous antioxidants and antimicrobials. Processing operations, such as increased surface area, loss of structural integrity, and the addition of pro-oxidants like salt, further accelerate quality deterioration (Awad et al., 2022).
Strategies to preserve meat quality and safety include controlling lipid oxidation, sequestering metal ions, and storing meat under appropriate packaging and low temperatures. Many plants possess antioxidant and antimicrobial properties. Examples of agricultural by-products with such properties include fruit peels from papaya, pomegranate, and watermelon (Al-Zoreky, 2009; Awad et al., 2022; Chamekh et al., 2020; Shiban et al., 2012; Yehia et al., 2021). Plant extracts, as alternatives to synthetic additives, can improve the functionality of meat products, support clean-label initiatives, and reduce reliance on synthetic chemicals. Plant-based solutions are rich in biologically active compounds with antioxidants, antimicrobial, and health-promoting properties (Awad et al., 2022).
Papaya (Carica papaya Linn.) is a tropical and subtropical fruit widely consumed for its delicious taste and nutritional value, particularly its high vitamin C content. Papaya processing generates approximately 8.47% peel waste, often discarded as rubbish, creating potential environmental hazards when overproduced (Ahmed and Abdel-Rahman, 2022; Nieto Calvache et al., 2016; Oliver-Simancas et al., 2024; Singla et al., 2022; Vinha et al., 2024). Food waste has become a global concern due to its environmental, ethical, social, and economic impacts. Between 2022 and 2027, the global papaya market is projected to grow significantly, leading to an increase in organic waste, including peels (Vinha et al., 2024). This highlights the need for value-added by-products from papaya processing, which could benefit both producers and the environment (Singla et al., 2022). For example, papaya peel powder has been demonstrated to reduce lipid peroxidation in beef burgers stored for three months at –18°C (Ahmed and Abdel-Rahman, 2022). Similarly, papaya leaf extracts have demonstrated antioxidant effects in goat meat emulsions (Jagtap et al., 2019). Ginger extracts have also shown peroxide-scavenging activity in camel meat burgers (Abdel-Naeem et al., 2022).
Although camel meat is an essential food source in arid and semi-arid regions, it remains one of the least studied among red meats (Khezrian and Shahbazi, 2018). Despite its nutritional and health advantages over other red meats, camel meat accounts for only 0.13% of global meat production and 0.45% of red meat from herbivores (Bougherara et al., 2023). Research on measures to enhance the safety of camel meat is scarce. While better hygiene and temperature control are logical approaches, achieving industry compliance can be challenging in many regions (Osaili et al., 2023). From a food security and safety perspective, and considering the environmental burden of food waste, it is essential to explore the potential antioxidant properties of papaya fruit peel extracts (PE) in preserving fresh camel meat under challenging storage conditions.
Materials and Methods
The following chemicals and reagents were used: methanol (A.R. 99.8%, Lab. Scan, Dublin, Ireland), glacial acetic acid (99.9%, Carlo Erba, Rodano, Italy), trichloroacetic acid (TCA, Sigma-Aldrich, St. Louis, MO, USA), anhydrous di-sodium orthophosphate (BDH, Poole, UK), EDTA disodium salt (BDH), gallic acid monohydrate (Carl Roth, Karlsruhe, Germany), Folin–Ciocalteu’s reagent (BDH), thiobarbituric acid (TBA, Loba chemie, Mumbai, India), 2,2-diphenyl-1-picrylhydrazyl (DPPH, 95%, Sisco Research Laboratories, Mumbai, India), and gum Arabic (Himedia, Mumbai, India). Distilled water (DW), sterilized or sanitized containers, glassware, knives, and forceps were used.
Local varieties of partially ripened papaya fruits (Carica papaya L., 75% yellow color) were procured from produce shops in Al-Ahsa, Saudi Arabia. The ripeness stage was grade four of maturity (Nieto Calvache et al., 2016). Fruits were washed to remove dirt, and the peels were manually separated using a clean knife. The peels were cut into small pieces, placed on aluminum foil, and dried in a laboratory oven (BINDER, Tuttlingen, Germany) at 60°C until constant weight was achieved. The dried peels were stored in heat-sealed food plastic bags at 4°C for further analysis.
Dried peels (moisture content 2.14%±0.91) were finely ground using a Waring blender. The extraction procedure followed previously established methods (Al-Zoreky, 2009; Al-Zoreky and Al-Taher, 2019). Briefly, 7.5 g of powdered peels were blended with 300 mL of 80% methanol for 2 min. The mixture was left at room temperature for 1 h with occasional blending, filtered through Whatman No. 1 paper, and stored in amber glass bottles at –28°C. Some extracts were dried at 60°C for solids content determination.
The Folin-Ciocalteu’s reagent method (Singleton and Rossi, 1965) was used to measure total phenolic concentration in PE. Diluted PE (200 μL) was mixed with Folin-Ciocalteu’s reagent (1:10, 1,000 μL) and sodium carbonate (800 μL, 7.5%). The mixture was kept in the dark for 1 h, and absorbance (A) at 765 nm was measured against a methanol blank. Standard solutions of gallic acid (0–5 mg/mL) were used to calculate total phenolic content, expressed as mg gallic acid equivalents (GAE) per g sample.
Two assays were used to evaluate the antioxidant activity of PE: DPPH and reducing power tests.
The radical scavenging activity (RSA) of PE was assessed following the DPPH method (Baliyan et al., 2022). Diluted PE (100 μL) was mixed with 3 mL DPPH (24 mg in 100 mL methanol). After 30 min in the dark, absorbance at 517 nm was measured. RSA was calculated using the formula: % RSA=(Ab−As/Ab)×100, where Ab is the absorbance of the blank and As is that of sample.
The reducing power of PE was determined following established methods (Irshad et al., 2012; Oyaizu, 1988). Various concentrations of PE were mixed with phosphate buffer (0.2 M, pH 6.6) and potassium hexacyanoferrate (1%), followed by incubation at 50°C for 20 min. After adding TCA (7.5%), the mixture was centrifuged at 800×g for 10 min. The supernatant reacted with FeCl3 (0.1%), and absorbance at 700 nm was measured.
Camel carcasses (~4.5 years old) were slaughtered at a municipal facility in Al-Ahsa, Saudi Arabia. Fore shank meat pieces were collected within 8 h post-slaughter, stored on ice, and transported to the laboratory. Fore shank meat had less fat than other meat cuts (Chang and Han, 2020). Samples were trimmed of fat and connective tissue, cut into ~12 g pieces, and stored for proximate composition analysis using AOAC (1995).
Meat pieces were treated with different marinades by dipping method. T1 (pH 4.1) consisted of 0.0625% acetic acid, 0.625% gum Arabic, and 900 ppm PE. From preliminary experiments using 225–900 ppm PE, camel meat pieces treated with 900 ppm PE had below 1 mg MDA/kg under storage at 8±1°C. Therefore, the PE level (900 ppm) was selected for subsequent meat experiments. It was twice the concentration of the in vitro DPPH test (~450 μg/mL) providing almost 76% RSA (Table 1). Untreated meat pieces were the controls. Meat samples were dipped in solutions for 10 min, manually drained, sealed in plastic bags, and stored at 8±1°C.
| PE (μg/mL) | Radical scavenging activity (% RSA) | IC50 (μg/mL) |
|---|---|---|
| 44.6 | 8.29±0.54 | |
| 222.90 | 23.80±1.47 | |
| 446.45 | 75.65±2.29 | |
| 318.15 |
Five grams of meat were homogenized with 20 mL DW, and pH was measured using a calibrated pH meter.
The assay was performed to evaluate lipid peroxidation. Meat homogenates were reacted with TCA and TBA, heated in a water bath, cooled, and absorbance was measured at 532 nm and 600 nm (Hodges et al., 1999). Results were expressed as mg MDA/kg meat (Papastergiadis et al., 2012; Pikul et al., 1983).
It was measured using the AOAC methods (AOAC, 1995), involving distillation with magnesium oxide and titration with sulfuric acid. Total volatile base nitrogen (TVBN) was calculated as the following equation:
Where V is the volume of sulfuric acid, N is the acid normality (0.01 N), and W is sample weight in g.
The Hunter CIE L*, CIE a*, and CIE b* color system was adopted. Meat surface color (Girolami et al., 2013) was assessed using a colorimeter using a MiniScan XE Plus (HunterLab, Reston, VA, USA). The color space components applied by the system are CIE L* (black: CIE L* 0 and white: CIE L* 100), CIE a* (red-green: negative CIE a* greenness and positive CIE a*), and CIE b* (yellow-blue: negative CIE b* blueness and positive CIE b*). Meanwhile, total color differences (ΔE*) were calculated using the formula (Stafford et al., 2022) below:
Results and Discussion
The use of 80% methanol as a solvent yielded 55.4% total solids from papaya peel samples, with a total phenolic content of 30.03 mg GAE/g dried peels (Table 2). Papaya fruit peels are rich in phenolic compounds, including phenolic acids and flavonoids (Siddique et al., 2018; Vinha et al., 2024). Among different solvents, aqueous methanol consistently provides higher yields and phenolic content from various plant materials, including fruit peels (Alara et al., 2021; Al-Zoreky, 2009).
| Parameter | Value |
|---|---|
| Yield (%) | 55.4 |
| Total phenolics (mg GAE/g peels) | 30.03 |
| Color | Bright yellow |
| Tannins | + |
| Flavonoids | + |
For comparison, extractions using 95% ethanol produced lower yields (8.8%) and phenolic content (~9 mg GAE/g sample) (Ahmed and Abdel-Rahman, 2022; Nieto Calvache et al., 2016; Vinha et al., 2024). Phenolics are plant-derived secondary metabolites, including phenolic acids, flavonoids, and tannins, known for their antioxidant properties (Gaye et al., 2019). Total phenolic content in papaya peels reported in previous studies ranged from <25 mg GAE/g to approximately 1 g/100 g dried peels (Gaye et al., 2019; Nieto Calvache et al., 2016; Siddique et al., 2018).
Extraction efficiency and phenolic content depend on factors such as genotype, geographic origin, extraction protocol, and the ripening stage of the fruit (Chan-León et al., 2021; Gaye et al., 2019; Vinha et al., 2024). PE also contain other bioactive compounds, such as terpenoids and tannins, which contribute to their functional properties (Siddique et al., 2018; Singla et al., 2022). As shown in Table 2, the extracted PE contained flavonoids and tannins.
Papaya peels, often considered waste, are a valuable source of polyphenols and other natural antioxidants with significant biological activity (Singla et al., 2022).
The DPPH assay was used to measure RSA, where DPPH, a stable free radical with a strong absorption band at 517 nm, reacts with antioxidants. Antioxidants, such as phenolics, reduce DPPH (purple) to DPPHH, resulting in a color change from purple to yellow and a decrease in absorbance (Baliyan et al., 2022; Blois, 1958; Siddique et al., 2018). The RSA of PE increased with extract concentration, ranging from 8.29% to 75.65% (Table 1), with an IC50 of approximately 318.15 μg/mL. Previous studies reported that PE from different varieties and regions exhibited RSA of 13% at 25 μg/mL, compared to 30% for BHT (Siddique et al., 2018). RSA values for different papaya varieties ranged from 30.26% to 35.15% (Gaye et al., 2019). It was noted that RSA decreases during post-harvest ripening, with a similar trend observed during the 10 d post-harvest (Chan-León et al., 2021).
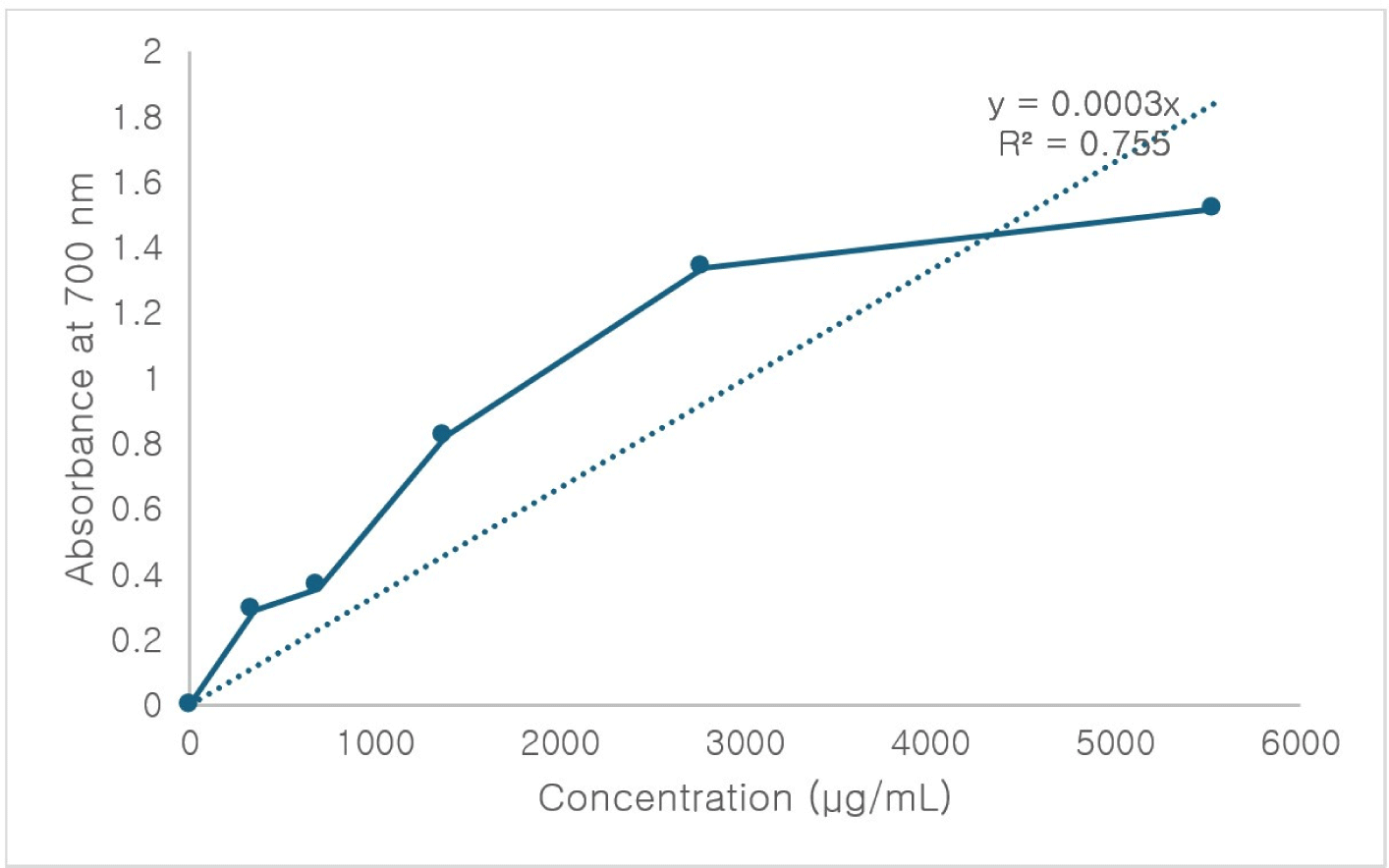
A positive relationship exists between phenolic content and reducing power activity (Siddique et al., 2018). Fig. 1 illustrates the ferric reducing ability (absorbance at 700 nm) of PE at different concentrations. The reducing power increased linearly as PE concentration doubled (r2=0.755), consistent with previous findings (Marina and Noriham, 2014). Higher concentrations of PE corresponded to greater reducing capacity, with extracts prepared using 80% ethanol showing superior reducing power compared to those extracted with absolute ethanol (Siddique et al., 2018). Interestingly, the absolute ethanol extracts contained higher total phenolics with lower reducing power compared to extracts of 80% ethanol. That could suggest that non-phenolic compounds in the 80% ethanol extracts may also contribute to ferric reducing power.
The proximate composition of fresh camel meat, as shown in Table 3, was 16.67% protein, 2.73% fat, and 1.34% ash. These values align with earlier reports (Baba et al., 2021; Kadim et al., 2022). Camel meat is nutritionally comparable to other conventional meats but has advantages such as low intramuscular fat, reduced cholesterol, and higher iron content (Baba et al., 2021). Studies from North Africa report camel meat as having 19%–22% protein and approximately 3% fat, with superior protein quality and lower fat and cholesterol compared to other animal meats (Bougherara et al., 2023). While fat content varies across camel breeds, protein content remains consistent (Al-Atiyat et al., 2016). In recognition of the importance of camelids in combating global hunger and contributing to food security, nutrition, and economic development, the United Nations designated 2024 as the International Year of Camelids (Bougherara et al., 2023; Mohamed et al., 2024). Camel meat is particularly appealing to health-conscious individuals, those managing chronic heart diseases, and individuals on weight-loss diets due to its unique nutritional profile (Osaili et al., 2023).
| Component | Content (%) |
|---|---|
| Fat | 2.73±0.46 |
| Protein | 16.67±0.97 |
| Ash | 1.34±0.358 |
Marinating is a common practice to enhance the sensory quality of meat and is also employed as a preservation technique. The dipping method involves immersing meat in a marinade at a controlled low temperature for a specific duration (Latoch et al., 2023). The current study examined the effects of PE marinade on pH, thiobarbituric acid reactive substances (TBARS), TVBN, and color parameters during camel meat storage under abusive conditions (8±1°C). Abusive temperatures are often used in studies to expedite spoilage and evaluate preservation methods in arid regions (Djenane and Aider, 2024; Osaili et al., 2023). To the best of our knowledge, this is the first study investigating the effects of PE on quality characteristics of fresh camel meat.
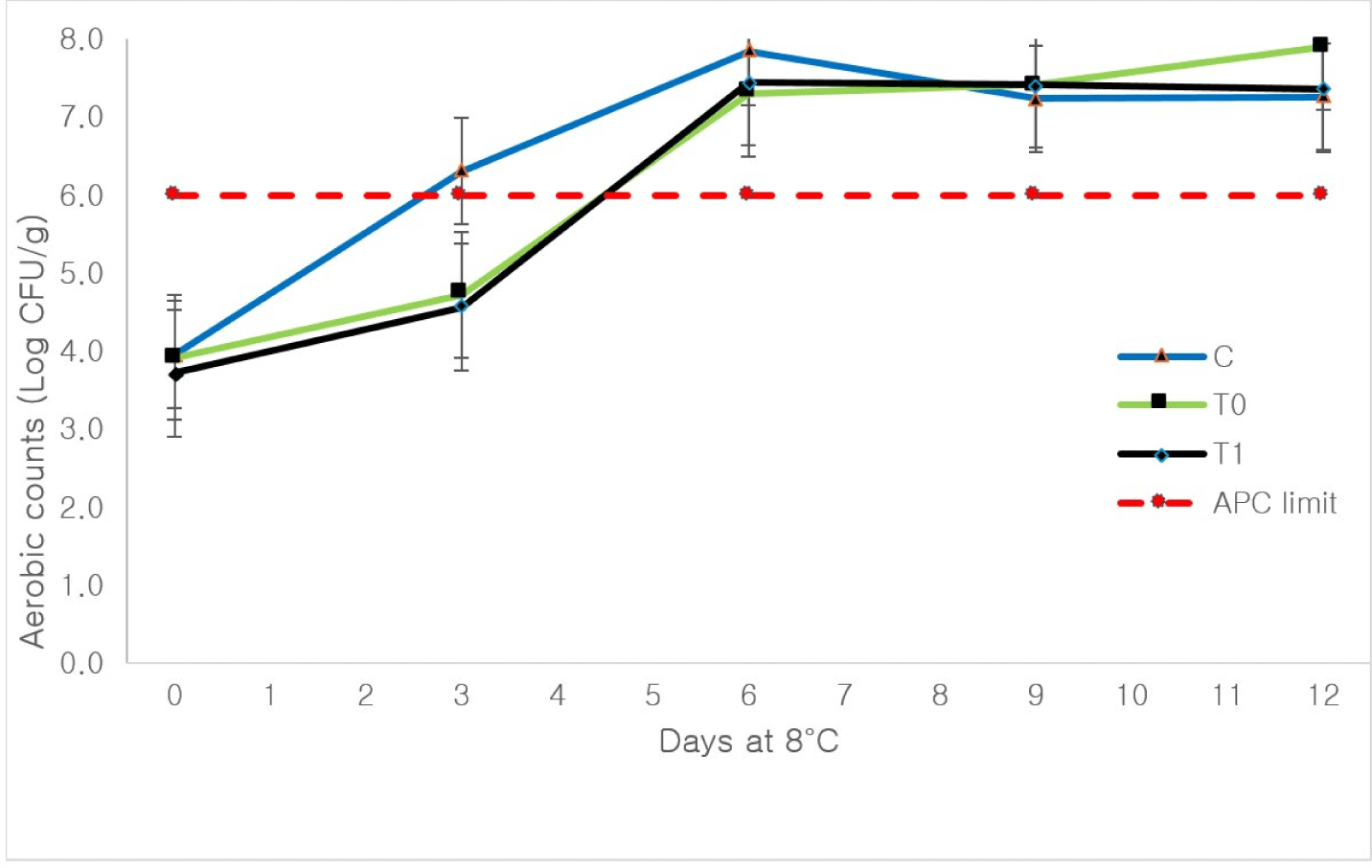
As presented in Table 4, the initial pH values were 6.07±0.06 (control), 5.83 (T0), and 5.73 (T1). These values are consistent with previous studies reporting pH values of 5.8–6.0 for camel meat across various breeds (Al-Atiyat et al., 2016). The reduction in pH in treated samples was attributed to the acetic acid in the marinade and the bioactive components of PE. Lower pH values during storage could be partially attributed to the acidic conditions of the marinade, corroborating findings from a previous study (Yehia et al., 2021). Marination is a non-thermal technique commonly applied to different meat types, where acidic marinades serve as antimicrobials and antioxidants (Latoch et al., 2023). Camel meat pH (ranging from 5.5 to 6.6) is influenced by lactic acid accumulation via glycolysis. The organoleptic properties of camel meat are significantly determined by pH, with pre-slaughter stress potentially causing a higher initial pH due to depleted meat glycogen levels (Djenane and Aider, 2024). After 3 d of storage, T1 exhibited a significantly higher pH (p<0.05) compared to the control (Table 4). A similar trend was observed after 6 d of storage, with pH values of 6.20 (control), 6.33 (T0), and 6.47 (T1). Vacuum-packed camel meat stored at 4°C or 10°C showed a pH drop from 6.0 to below 5.5 within 10 d, primarily due to microbial spoilage (Yehia et al., 2021). However, extended storage beyond 6 d at 8°C±1 resulted in pH increases for both treated and untreated camel meat samples (Table 4). In other camel meat products, such as ground meat, pH increased from 5.9 to 6.5 over 14 d of storage at 4°C (Khezrian and Shahbazi, 2018). Interestingly, under abusive storage conditions (8°C), T0 and T1 maintained stable pH levels from six to 12 d (Table 4). This pH stability in T1 coincided with the stationary phase of bacterial growth (Fig. 2), suggesting that the PE marinade may help resist pH changes under lower storage temperatures, such as 4°C. Lower storage temperatures are recommended to limit spoilage and pathogenic bacterial growth in meat (Baba et al., 2021). Regulations in the USA and EU require fresh red meat to be stored at 1°C in modified atmospheric packaging (O2/CO2, 80:20) to maintain safety (Djenane et al., 2020). However, regional compliance with such measures is often limited, with improper temperature controls in retail settings leading to potential spoilage (Osaili et al., 2023). In fresh cow meat, marination reduced pH significantly, from 5.8 to 4.8, compared to controls. Lower pH values, especially when far from the isoelectric point of meat proteins (pH ~5.0), enhance water-holding capacity and reduce cooking loss (Puolanne and Peltonen, 2013; Unal et al., 2023). In camel meat stored at 1°C in high O2/CO2 packaging, pH remained stable (5.7–5.9) during the first 3 d of storage (Djenane et al., 2020).
The MDA, a secondary product of polyunsaturated fatty acid oxidation, is commonly used as an index of lipid peroxidation (Awad et al., 2022; Reitznerová et al., 2017). The TBARS assay is widely used to measure MDA, where MDA reacts with TBA and is quantified by absorbance at 532 nm (Hodges et al., 1999).
Table 5 shows MDA concentrations in camel meat stored at 8°C for 12 d. Camel meat is reportedly more resistant to oxidative damage (lower TBARS) than beef under cold storage (Baba et al., 2021). The initial MDA concentration (0.90 mg/kg) was higher than values reported in other studies (~0.40 mg/kg) for camel meat stored at 4°C (Abdel-Naeem et al., 2022; Jouki and Khazaei, 2012). Various intrinsic and extrinsic factors influence MDA levels during storage (Awad et al., 2022; Bekhit et al., 2021). During 6 d of storage, MDA levels increased significantly in control due to oxidative activity. Myoglobin in camel meat, acting as a pro-oxidant, likely contributed to lipid oxidation (Maqsood et al., 2015). In contrast, the T1 treatment reduced MDA levels immediately (80% lower than the control and 74.3% lower than T0). Over 6 d, T1 consistently demonstrated antioxidant activity, maintaining significantly lower MDA levels (95.1% and 98% lower than T0 and control, respectively). Natural antioxidants in plant extracts like T1 can mitigate lipid oxidation, extend the shelf life of meat products, and reduce the formation of harmful genotoxic and cytotoxic compounds (Awad et al., 2022). Papaya peel powder, for example, maintained MDA values below 0.6 mg/kg in beef burgers stored at –18°C for three months (Ahmed and Abdel-Rahman, 2022). T1 antioxidant activity was due to its phenolic content (Table 2, Fig. 1), with additional contributions from acetic acid and gum Arabic. Gum Arabic stabilizes natural food colors in acidic solutions and prevents aggregation or sedimentation (Jian et al., 2017). After 6 d at 8°C, T1 maintained MDA levels at 0.09 mg/kg, below the acceptable threshold, whereas MDA in the control and T0 decreased after 6 d, possibly due to oxidation of MDA into organic acids and alcohols (Khezrian and Shahbazi, 2018; Maqsood et al., 2015).
The TVBN is a quality indicator for meat and fish freshness (Bekhit et al., 2021). Table 6 summarizes TVBN trends in camel meat stored at 8°C for 12 d. At zero time, T1 and T0 reduced TVBN to 23.29 and 23.62 mg/100 g, respectively. A previous study on vacuum-packaged camel meat treated with 2% fruit acids (pH 2.7) maintained TVBN below 10 mg/100 g at 4°C or 10°C for 30 d (Yehia et al., 2021). After 3 d at 8°C, T1 and T0 exhibited significantly lower TVBN values (~20 mg/100 g) compared to the control. Despite its lower acidity (pH 4.1) compared to fruit acid formulations (pH 2.7), T1 effectively reduced TVBN. Aerobic bacterial counts (Fig. 2) in T1 and T0-treated meat were below the EU limit (<Log 6 CFU/g) for fresh meat, while control samples exceeded this threshold after 3 d. However, bacterial counts exceeded the EU maximum after 6 d for control and treatments (Fig. 2). Microbial proliferation and endogenous proteolytic enzymes contribute to TVBN development by producing nitrogenous compounds such as amines (Bekhit et al., 2021). T1 antioxidant activity (Table 5) and the presence of gum Arabic and phenolic phytochemicals likely contributed to its ability to reduce TVBN levels. After 6 d, TVBN levels in T1-treated meat remained significantly lower (p<0.05) than T0 and the control. However, T1 and T0 treatments became less effective in maintaining TVBN levels below the 25 mg/100 g threshold after 6 d. TVBN increases in control coinciding with an objectionable flavor, highlighting its correlation with spoilage biomarkers like pH (Bekhit et al., 2021).
Meat color is a critical determinant of quality and shelf life, influencing consumer acceptance (Wongphan et al., 2024). Table 7 shows that T1-treated meat exhibited a lighter color (CIE L*=51.77) compared to the control (CIE L*=41.43) at zero time. This trend continued, with T1 maintaining significantly higher CIE L* values than the control and T0 after 3 d at 8°C. The lighter color observed in T1-treated meat aligns with findings from other studies using ginger extracts in camel meat (Abdel-Naeem et al., 2022). Extended storage reduced the CIE L* values in the control group, likely due to increased TBARS and TVBN levels (Tables 5 and 6) as well as bacterial proliferation (Fig. 2).
| Day/treatment1) | C | T0 | T1 |
|---|---|---|---|
| CIE L* | |||
| 0 | 41.43±0.73ABc | 47.85±0.47Bb | 51.77±0.75Aa |
| 3 | 40.89±0.66Bc | 50.96±0.58Ab | 52.61±0.59Aa |
| 6 | 37.38±0.16Cc | 49.30±0.49Aa | 47.59±0.45Ba |
| 9 | 38.21±0.11Cb | 48.77±0.07Aa | 48.86±0.17Ba |
| 12 | 44.37±3.80Ab | 46.67±0.21Ba | 47.11±0.21Ba |
| CIE a* | |||
| 0 | 15.53±0.87Ba | 13.75±0.27Bb | 11.93±0.20Cc |
| 3 | 17.18±0.16Aa | 15.27±0.16Ab | 12.73±0.17Bc |
| 6 | 14.94±0.43Ba | 9.98±0.10Db | 10.01±0.09Cb |
| 9 | 15.12±0.10Ba | 11.33±0.05Cb | 14.22±0.08Aa |
| 12 | 8.56±0.26Cb | 9.69±0.12Db | 12.28±0.04Ba |
| CIE b* | |||
| 0 | 15.56±0.66Aa | 15.03±0.23Bb | 14.68±0.06Bb |
| 3 | 15.89±0.28[19]b | 17.22±0.23Aa | 16.02±0.02Ab |
| 6 | 14.64±0.24Ba | 12.27±0.22Dc | 13.48±0.06Cb |
| 9 | 14.12±0.16Bb | 14.24±0.15Cb | 16.75±0.12Aa |
| 12 | 11.65±0.13Cc | 12.90±0.14Db | 13.94±0.08Ca |
Regarding CIE a*, T1-treated meat showed a significant decrease compared to the control and T0 (Table 7). Natural antioxidants in T1 contributed to reduced CIE a*, like findings with ginger-treated camel meat (Abdel-Naeem et al., 2022). CIE a* reduction is often associated with lipid and pigment oxidation, leading to metmyoglobin formation (Maqsood et al., 2015).
Table 7 shows CIE b* values during storage. The control exhibited a steady decline in CIE b*, while T1-treated meat showed fluctuations. Papaya peels, which contain β-carotene, may have contributed to CIE b* values in treated meat (Nieto Calvache et al., 2016; Vinha et al., 2024). Table 8 presents ΔE* in camel meat stored for 12 d at 8°C. ΔE* is considered as a small difference (ΔE*<1.5), distinctive (1.5<ΔE*<3), or very distinctive (ΔE*>3; Ferysiuk et al., 2020).
| Days | C1) | T0 | T1 |
|---|---|---|---|
| 3 | 1.78Db | 4.10Ba | 1.77Cb |
| 6 | 4.19Bb | 4.89Aa | 4.75Aa |
| 9 | 3.55Cb | 2.71Ac | 4.24Ba |
| 12 | 8.52Aa | 4.73Ab | 4.73Ab |
Conclusion
Camel meat, like other red meats, is highly susceptible to quality deterioration during storage, with lipid oxidation and protein degradation being the primary contributors to compromised quality and safety. Natural products derived from food processing waste, such as papaya fruit peels (PE), are rich in phytochemicals with antioxidant and other bioactive properties. This study demonstrated the in situ lipid antioxidation capability of PE, as evidenced by the TBARS assay in fresh camel meat stored at abusive temperatures (8°C). The combination of PE with acetic acid and gum Arabic positively influenced key quality parameters, including TBARS, TVBN, and bacterial load in camel meat stored for a maximum of 3 d at 8°C. Abusive storage temperatures (>4°C) for fresh meat are prevalent in certain regions, both locally and internationally. Therefore, the inclusion of PE in common acid-based marinades (as part of a hurdle technology approach) holds promise as a quality preservative for fresh camel meat. This approach may also be extended to other meat types under recommended refrigeration systems, offering an innovative solution to enhance food safety and shelf life of products.













