Introduction
In response to global population growth and evolving lifestyles, the imperative for sustainable food systems is now widely acknowledged (Wongprawmas et al., 2023). The environmental impacts of livestock production have raised concerns, particularly in the context of escalating animal product consumption (Meybeck and Gitz, 2017). Therefore, there is a need to meet this demand in a more environmentally friendly way by increasing the sustainability of existing protein source production and, at the same time, introducing alternative protein sources. Hybrid meat combines alternative proteins with traditional meat ingredients to mimic the functionality and nutrition qualities of meat. These products address consumer demands to balance the attributes of traditional meat and the health and environmental benefits of plant-based options while reducing overall meat consumption (Wu et al., 2024). They also serve as a transitional option for individuals shifting towards a plant-based diet (Ismail et al., 2020).
Plant-based proteins are pivotal in developing hybrid meat, influencing texture, nutritional profile, ecological benefits, and overall functionality. Plant-based proteins bind water and fat in emulsion gel manufacturing, creating an elastic texture through protein cross-linking after heat treatment. Rice protein is hypoallergenic and suitable for those allergic to soy or gluten (Pantoa et al., 2020). It enhances the texture and binding properties of hybrid meat, providing essential amino acids (EAAs) and a balanced nutritional profile (dos Santos et al., 2023). Challenges include lysine deficiency, potential flavor imbalances, and processing sensitivity, requiring careful formulation (Mariotti and Gardner, 2019). Pea protein offers a complete amino acid (AA) profile and is hypoallergenic, positively impacting soil fertility with lower environmental resource requirements (Poore and Nemecek, 2018). However, it has limitations like low to moderate protein solubility, weaker and less elastic gels compared to soy protein, pronounced taste, and anti-nutritional factors such as lectins and protease inhibitors (Sirtori et al., 2012). Soy protein is complete in EAAs and rich in nutrients like iron, calcium, and vitamins, enhancing the nutritional profile of hybrid meat (Zhang et al., 2020). Its exceptional functional properties, including notable water and fat binding capacities, improve the texture, juiciness, and overall mouthfeel of hybrid meat products (Wang et al., 2019). Although processed soy protein isolates mitigate allergenicity, residual concerns remain, and anti-nutritional factors could impede nutrient absorption (Mulalapele and Xi, 2021).
Various researchers have studied the technological and quality characteristics of hybrid meat products (Aviles et al., 2023; Scholliers et al., 2020). However, knowledge about the impact of the meat emulsion gel formed by combining animal and plant proteins on the structure of the final product is limited, and research applying various plant proteins to chicken remains scarce. Considering these factors, research aimed to analyze the quality characteristics of different isolated plant proteins when they replaced 20% of the hybrid chicken analogue. Furthermore, we evaluated the impact of various types of plant proteins on the nutritional and technological characteristics of replacing meat in processed hybrid chicken analogue (PHCA).
Materials and Methods
The chicken breast used in this study (Harim, Iksan, Korea) was purchased from a local market. Isolated rice protein (IRP) was characterized by its composition of ≥88.6% protein, 2.0% ash, 3.0% lipid, and 6.4% carbohydrate. Isolated pea protein (IPP) was characterized by its composition of ≥87% protein, 1.8% ash, 2.3% lipid, and 3% carbohydrate. Isolated soy protein (ISP) was characterized by its composition of ≥90.6% protein, 0.5% ash, 1% lipid, and 2.8% carbohydrate. These protein isolates were obtained from Almi (Oftering, Austria). Dibasic sodium phosphate (DSP) was purchased from ES Food Ingredients (Gunpo, Korea). Additionally, Microbial transglutaminase (TG) with an enzymatic activity of 100 U/g were purchased form Ajinomoto (Tokyo, Japan).
The chicken breast was prepared by removing visible fat and connective tissues and cut into 5–6 portions. Additionally, a hybrid chicken analogue was produced using salt, DSP, and TG to enhance the cohesiveness and binding properties of meat proteins. According to the blending ratio in Table 1, the cut chicken breast was processed in a food processor (FDM301SS, Kenwood, Havant, UK) for 13,000 rpm and 2 min. Salt, DSP, and TG, dissolved in 1% seasoning (chicken stock) ice water, were gradually added while processing for 13,000 rpm and 2 min. Plant-based proteins were added, and the mixture was further processed for 16,000 rpm and 2 min. The finished chicken emulsion gels were aged at 5°C for more than 15 h. The aged chicken emulsion gels, placed in a 4×4×2 cm silicone mold, were heated in an oven (Bread oven, Kumbok Stock, Seoul, Korea) at 180°C for 15 min±30 s until the sample reached an internal temperature of 72°C. All samples were allowed to stand at room temperature for 20 min before experimentation. CT, CRT, CPT, and CST stand for PHCA made with chicken breast and TG, IRP and TG, IPP and TG, and ISP and TG, respectively.
Emulsion stability was assessed by measuring the degree of phase separation after centrifugation. This procedure was implemented to evaluate the stability of the emulsion formed within the emulsion gel matrix after the aging process. Each sample was centrifuged at 438×g for 3 min, following the method described by Latreille and Paquin (1990).
Gel strength was analyzed using samples prepared in 4×4×2 cm3 silicone molds. Measurements were performed using a rheometer (CR-100, Sun Scientific, Tokyo, Japan) equipped with a 20-mm cylindrical probe in compression mode at 25°C. The test conditions were as follows: load cell, 19.61 N; distance, 4.0 mm; and table speed, 60 mm/min.
The dynamic rheological properties of the chicken emulsion gel before cooking were measured using a rotational rheometer (HR-10, TA Instruments, New Castle, DE, USA) with a 20-mm plate-plate geometry and a 1-mm gap. Measurements were performed within the linear viscoelastic region at a strain of 0.1%. A frequency sweep test was conducted within an angular frequency range of 0.1–100 rad/s at 25°C. Viscosity as a function of shear rate was determined within a shear rate range of 0.1–100 s-1 at 25°C.
Moisture content was assessed using a high-pressure drying apparatus set at 105°C, wherein 3 g of each sample was taken and dried for 5 h, and the weight of the samples before and after drying was compared (AOAC, 2000). Crude protein contents were assessed using the Kjeldahl method. Nitrogen analysis was conducted using a Nitrogen Autoanalyzer (Kjeltec 2400, Foss Analytical A/S, Hilleroed, Denmark). Crude fat contents were determined using an automatic Soxtherm fat extraction system (Soxtherm 416, Gerhardt, Königswinter, Germany).
pH was measured using a pH meter (Orion 3 Star, Thermo Fisher Scientific, Waltham, MA, USA). Before measurement, the samples were homogenized at 4,900 rpm for 30 s using a hand mixer (HR2535/00, Philips, Amsterdam, The Netherlands).
Color value (CIE LAB), including color difference, were measured using on a surface of the sample using a colorimeter (CR-400, Minolta, Tokyo, Japan) calibrated with a standard white plate. The CIE L*, CIE a*, and CIE b* values represent lightness, redness, and yellowness, respectively. The color difference of the sample before and after cooking was calculated using the following Eq. (1):
L0*: before cooking CIE L* value of the source, a0*: before cooking CIE a* value of the source, b0*: before cooking CIE b* value of the source.
The AA composition analysis was conducted using an AA analyzer (LA8080, Hitachi, Tokyo, Japan). Frozen dried powder sample (0.1 g) was hydrolyzed by adding 6 N HCl (1 mL) and nitrogen charging for 1 min, followed by hydrolysis at 110°C for 24 h. After hydrolysis, the cap was opened and dried at 80°C for 24 h. The dried sample was dissolved in 0.02 N HCl (1 mL), subjected to vortexing and sonication, filtered through a 0.45 μm filter, and used for analysis. A 20 μL sample filtrate was injected into the AA analyzer, passed through an ion exchange resin column to separate various AAs. The AAs separated from the column at high temperature reacted with ninhydrin to form colored compounds. Among the 18 AAs, proline was measured for absorbance at 440 nm wavelength, while the remaining 17 AAs were measured at 570 nm wavelength for absorbance to analyze each AA.
Spectral absorption characteristics were determined using an Fourier transform infrared spectroscopy (FT–IR) spectrometer (PerkinElmer, Waltham, MA, USA) equipped with a Quest attenuated total reflectance (ATR). ATR curves of each freeze-dried and finely ground samples were performed within the frequency range of 4,000–400 cm−1 at room temperature with a scan speed and resolution of 0.2 cm/s and 4 cm−1, respectively.
The shrinkage ratio was determined by measuring the extent of contraction in the length, width, and height using a Vernier caliper at three random points on the sample before and after cooking.
The water holding capacity was assessed by modifying the filter-paper press method (Joo, 2018). After measuring the weight of a sample of a consistent size (2×2×2 cm3), the sample was placed between two filter papers (Whatman #2). A 2 kg weight was applied for 5 min, compressing the sample, and the weight of the compressed sample was measured to express the extent of moisture exudation as a percentage.
Cooking loss, representing the percentage decrease in total mass due to heat treatment, was quantified through six repetitive measurements.
Texture profile analysis (TPA) and Warner-Bratzler shear force analysis was conducted using samples of consistent size (2×2×2 cm3) and measured on a rheometer (CR-100, Sun Scientific, Tokyo, Japan), measuring each sample five times. For TPA, hardness, springiness, cohesiveness, and chewiness were measured by a two-bite compression test at 25°C. The measurement conditions were set as follows: load cell, 98.07 N; distance, 6.0 mm; table speed, 60 mm/min; adaptor type: diameter 50 mm. For Warner-Bratzler shear force, were measured by a shear force test at 25°C. The measurement conditions were set as follows: load cell, 19.61 N; distance, 10.0 mm; table speed, 60 mm/min; adaptor type: Warner-Bratzler shear blades.
All experiments were conducted with three or more repetitions, and the results were presented as mean±SD. Analysis of variance (ANOVA) and Duncan’s multiple range test were performed using the SPSS software package (version 26, IBM, Armonk, NY, USA) with a significance level set at p<0.05.
Results and Discussion
Meat emulsion is a complex water-in-oil emulsion system in which fat functions as the dispersed phase, while the continuous phase comprises water, water-soluble proteins, muscle fibers, and connective tissues, forming an aqueous matrix system (Faridah et al., 2023). The stability of meat emulsions with desirable functional properties, such as texture modification and moisture retention, is crucial for the production of well-emulsified meat products. Evaluation of emulsion stability before cooking revealed significantly higher emulsion stability in samples containing plant proteins compared to CT, leading to the formation of stable emulsion gels (Fig. 1A). The incorporation of plant proteins during the production of meat emulsion gels enhances emulsifying capacity, resulting in denaturation and coagulation upon heating, which facilitates the entrapment of water and fat particles within the protein network through capillary forces to form elastic gels. Previous research conducted by Cîrstea Lazăr et al. (2023). has highlighted the remarkable emulsifying properties of rice, buckwheat, and soy proteins in effectively combining water and fat. Consequently, the inclusion of plant proteins demonstrates a beneficial influence on the advancement of hybrid meat products.
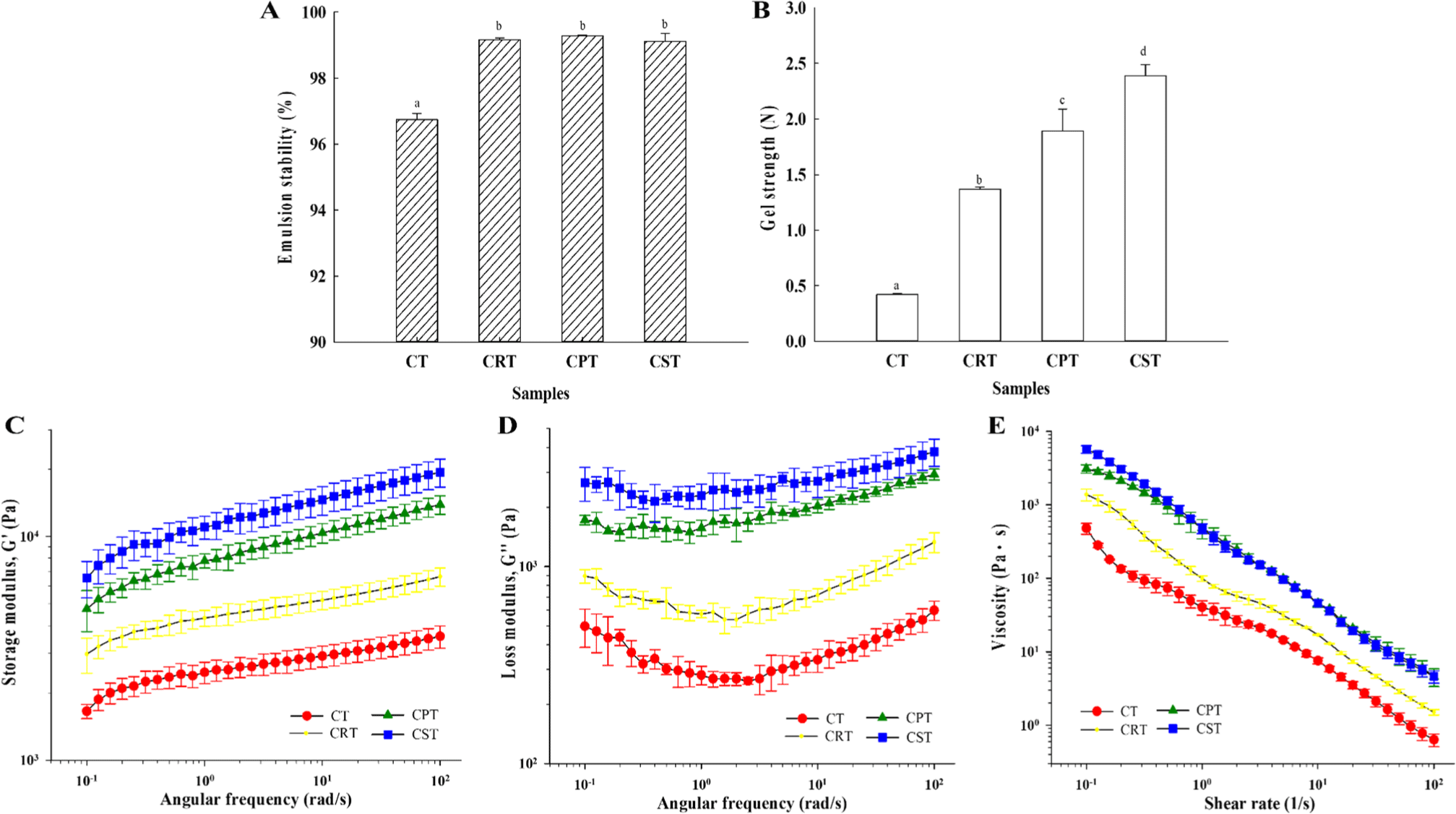
The technical and quality characteristics of meat products of significance encompass water retention and texture, primarily influenced by the properties of the protein emulsion gel (Ren et al., 2022). The evaluation of the gel strength before cooking revealed a significant increase in the order of CT<CRT<CPT<CST, demonstrating the CST is the most stable and high-strength gel properties (Fig. 1B). Plant-based proteins possess the capacity to retain water and fat, resulting in a firm texture post-heat treatment, thereby serving as a binder, filler, and functional enhancer for hybrid meat products (Chandler and McSweeney, 2022). Consequently, the incorporation of plant-based proteins can enhance the texture of hybrid meats, indicating promising production prospects. Furthermore, the formation of cross-links between polypeptide chains within protein gel networks is facilitated by various molecular forces (Sun et al., 2015). The disparity in gel strength among samples is attributed to the gelling potential of each plant-based protein.
Rheology is employed as an analytical technique to provide fundamental insights into the gelation capacity of proteins. In the results depicted in Figs. 1C and D, all samples manifest G’>G” characteristics, indicating a prevalence of elastic modulus attributes over viscous modulus (Tabilo-Munizaga and Barbosa-Cánovas, 2005). Additionally, all G’ and G” results showed a frequency-dependent correlation in the order CT<CRT<CPT<CST in the studied range (0.1–100 Hz). A higher storage modulus indicates a more robust gel structure due to the formation of a stabilized network structure or particle-particle solid interactions (Faridah et al., 2023). In conclusion, this suggests that the addition of plant-based protein produces elasticity. Plant-based proteins influenced the development of G’ values by forming aggregates within the hybrid meat batter, potentially acting as particle fillers (Lin and Barbut, 2024). This shows the same properties as the gel strength characteristics result in Fig. 1B.
All emulsion gels showed shear thinning characteristics in which viscosity decreases with shear rate, and these results indicate that the physical properties of emulsion gels are suitable for food processing in terms of shape formation (Fig. 1E). The viscosity of each sample will affect the final viscosity depending on the plant-based protein, with higher viscosity, requiring more substantial extrusion pressure during molding processing (Rao and Hsieh, 2007). Similar trends were observed with high dynamic viscosity in CPT and CST, which was mainly due to the increased degree of molecular chain entanglement due to molecular weight. The final textural properties reflect the rheological properties of meat emulsion gels.
The result confirmed a general moisture content decrease when plant-based protein substitutes for chicken (Table 2). The lowest moisture content was observed in the CRT sample. This decline is likely attributed to plant proteins inherently lower moisture content than chicken. Plant protein have the capacity to absorb water by enabling water molecules to bind to the hydrophilic components of starch and fiber, as well as to polar AAs (Ma et al., 2011). Additional water molecules may be retained if they become trapped in the protein matrix of the pulse (Soria-Hernández et al., 2015). Additionally, pea protein isolates, and rice protein isolate can lead to a reduction in the moisture content of chicken breast nuggets (Shoaib et al., 2018). The results revealed a significant increase in crude protein content compared to the CT. This increase was attributed to the addition of plant-based protein, which likely stemmed from the inherently higher protein content of plant-isolated protein in contrast to chicken breast. There is a consumer preference for meat analogs to match the protein levels found in traditional meat products (Profeta et al., 2021), and higher plant protein content offers a richer protein profile and is considered a sustainable option (Smart and Pontes, 2023). Consequently, this study suggests the potential of plant-based protein as a viable alternative to chicken breast as a protein source. There was no substantial variation across the samples in crude fat and crude ash, indicating that the fat and ash content was unaffected by the replacement of chicken breast with plant-based protein.
pH is an important criterion for determining the freshness, shelf life, and stability of meat products (Shoaib et al., 2018). The pH value is 6.25–6.60, indicating higher values for CPT and CSP tend to be significantly higher than CT (Table 2). This finding suggests that the pH levels were influenced by the alkalinity of the legume powder (average pH 6.68) was more alkaline than chicken (pH 6.52; Chandler and McSweeney, 2022). Additionally, Joo et al. (2019) noted that the pH of patties containing raw okra increased with higher amounts of okara, implying a correlation between okara content and pH levels. Since the pH of the final product can be affected by the pH of the additive itself, the pH of the sample appears to have decreased in this experiment due to the addition of plant-based protein.
Color serves as a crucial parameter for consumers in assessing quality and significantly influences consumer sensory perception. The CIE L* value tended to decline with the inclusion of plant-based protein, while the CIE a* and CIE b* values tended to increase (Table 2). Previous research has indicated that the incorporation of plant-based protein alters the color of hybrid meat to darker and more yellow in appearance (Revilla et al., 2022). These findings suggest that the color value of hybrid meat is attributed to the browning of plant-based proteins during the Maillard reaction. Given that higher CIE a* in processed meat products correlates with increased sensory preference, the addition of plant-based protein is anticipated to have a high preference among consumers (de Araújo et al., 2022). In addition, samples containing plant-based protein exhibited a notably lower overall color difference (Ngapo et al., 2010), stated that when collagen through heat, protein denaturation, and structural modifications influence the color of the final product. Hence, it is postulated that variations in plant-based protein type, protein composition, and fatty acid composition among treatments contributed to the color difference variations observed in the PHCA.
The protein quality of PHCA was evaluated based on the AA profile and EAA content (Table 3). In terms of the content of the 9 kinds of EAA, the content of PHCA was slightly lower than that of CT, the control. However, when compared to the EAA content recommended by the WHO for adults, CST showed a similar trend to CT, as can be observed at Purschke et al. (2018). This indicates that CST may be advantageous in meeting the recommended adult intake. Regarding non-EAAs, CT had a content of 38.29 mg/g, while the overall increase in non-EAAs due to the processing of plant-based protein ranged between 98.17–99.22 mg/g. Among the non-EAA, glutamic acid content was the highest in all test groups, followed by leucine and lysine content. Glutamic acid serves as a natural seasoning with umami characteristics in food taste profiles. It is notably the most abundant AA in the brain, playing a crucial role in synaptic activity, memory, and learning (Riedel et al., 2003). It may also contribute positively to the production of gamma-aminobutyric acid, a functional compound primarily produced in plants through the decarboxylation of L-glutamic acid. Moreover, pulse proteins generally lack the EAA methionine and cysteine (Boukid et al., 2021), and in this study CPT and CST contained significantly lower content than CT and CRT. Conversely, lysine, an EAA limited in grain proteins, was found to have significantly higher content in CPT and CST. These differences are attributed to the type of plant-based protein added. Therefore, substituting PHCA for plant-based protein isolate could be an excellent strategy to meet the dietary demand for non-EAAs in food formulations. In processed meat products, AAs are recognized for enhancing the flavor of meat products and altering the surface of the meat to an appealing brown color (Koh and Yu, 2015). Hence, it is crucial in hybrid meat products containing a blend of plant-based proteins to select suitable plant proteins and use appropriate quantities to maintain their organoleptic acceptability and nutritional quality.
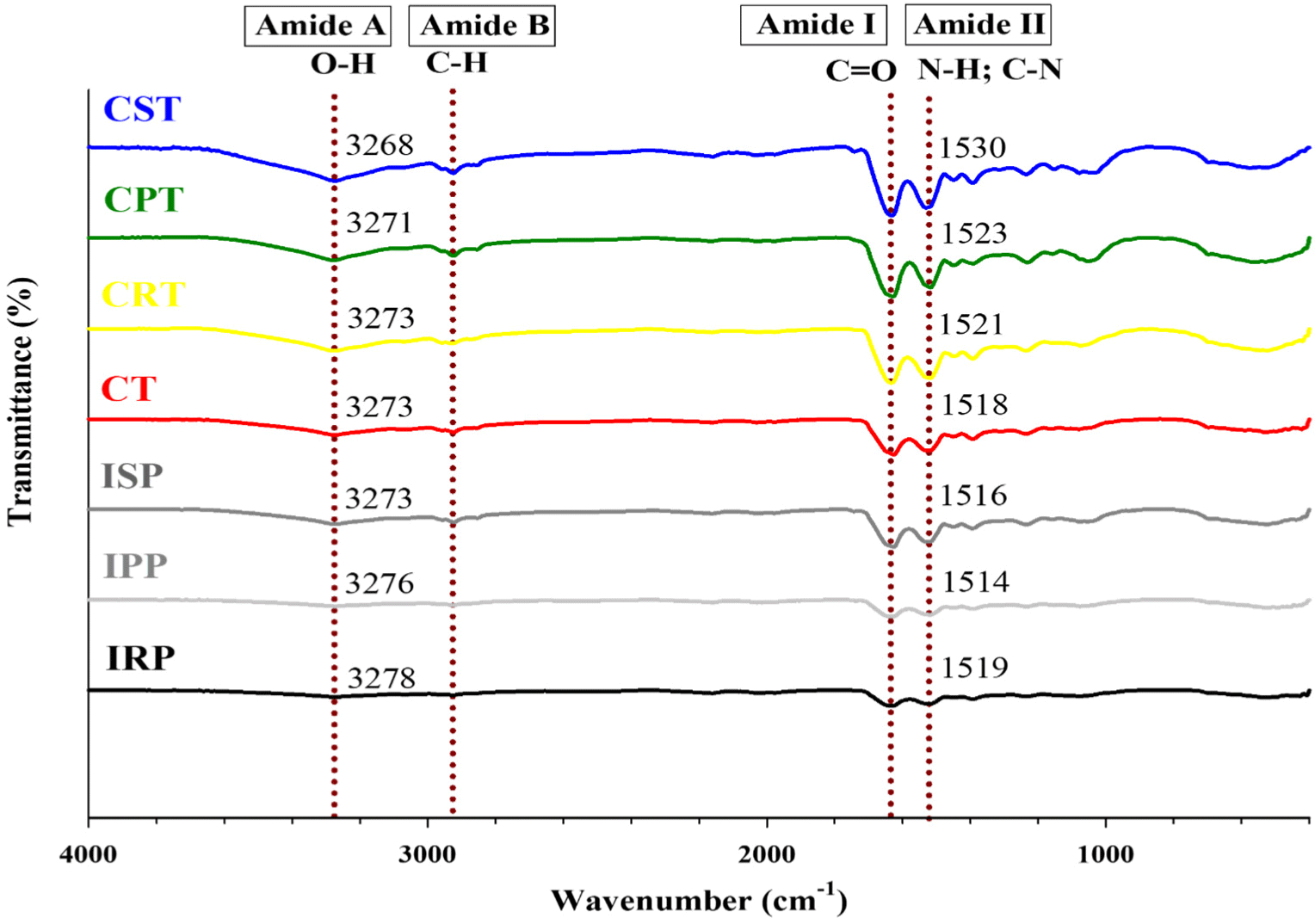
FT–IR utilizes infrared absorption wavelengths to analyze differences in molecular functional groups. To investigate the intermolecular interactions between plant-based protein isolates and hybrid chicken breast, the FT–IR spectra of the samples are depicted in Fig. 2. All samples exhibited characteristic absorption peaks at 3,270, 2,925, 1,630, and 1,520 cm–1. No new characteristic points were identified among the samples overall, with slight shifts observed in some peaks. A trend similar to the absorption peak of chicken breast reported in a previous study was confirmed (Candoğan et al., 2021). Variances in absorbance intensity were noted between peaks observed in plant-based protein isolates (IRP, IPP, ISP) and peaks in manufactured PHCA (CT, CRT, CPT, CST). This indicates an increase in the cross-linking of the intermolecular structural stability of PHCA (Kim et al., 2024). The broad amide A band that appears at about 3,270 cm–1 is the O-H stretching vibration peak and is related to hydrophobic contact and hydrogen bonding, essential for chemical bond formation (Chen et al., 2017). A blue shift toward lower frequencies was confirmed in hybrid meat, indicating stronger intermolecular interactions of hydrogen bonds between plant-based isolated proteins and chicken breast, leading to improved stability through (Wang et al., 2021). The weak amide B band that appears at 2,925 cm–1 is the -CH stretching vibration peak, a typical characteristic of saccharides (Zhang et al., 2017). It has been reported that the absorption band appearing at 1,630 cm-1 is caused by the stretching vibration of C=O and the amide I region of the protein (Zhang et al., 2017). The amide II band shown at 1,520 cm–1 contains C-N stretching and N-H bending vibrations, reflecting intermolecular or intramolecular hydrogen bonding (Li et al., 2020). The change in peak intensity indicates an increase in hydrogen bond content, reflected by a shift from 1,514–1,519 cm–1 (isolated plant proteins) to 1,518–1,530 cm–1 (PHCA).
The visual appearance of the samples revealed relatively dense structures for CRT compared to CT, whereas CPT and CST were unbalanced and slightly shrunken or bloated (Fig. 3A). This can also be confirmed in shrinkage data due to cooking. Processed meat products tend to shrink due to moisture and drip release during the cooking process, but the overall height was confirmed to have expanded in CST (Fig. 3B). In plant proteins such as soy, when heat is applied, the starch granules swell and the protein gelatinizes, improving its ability to retain water and fat (Maninder et al., 2007). This is believed to have influenced the expansion of CST.
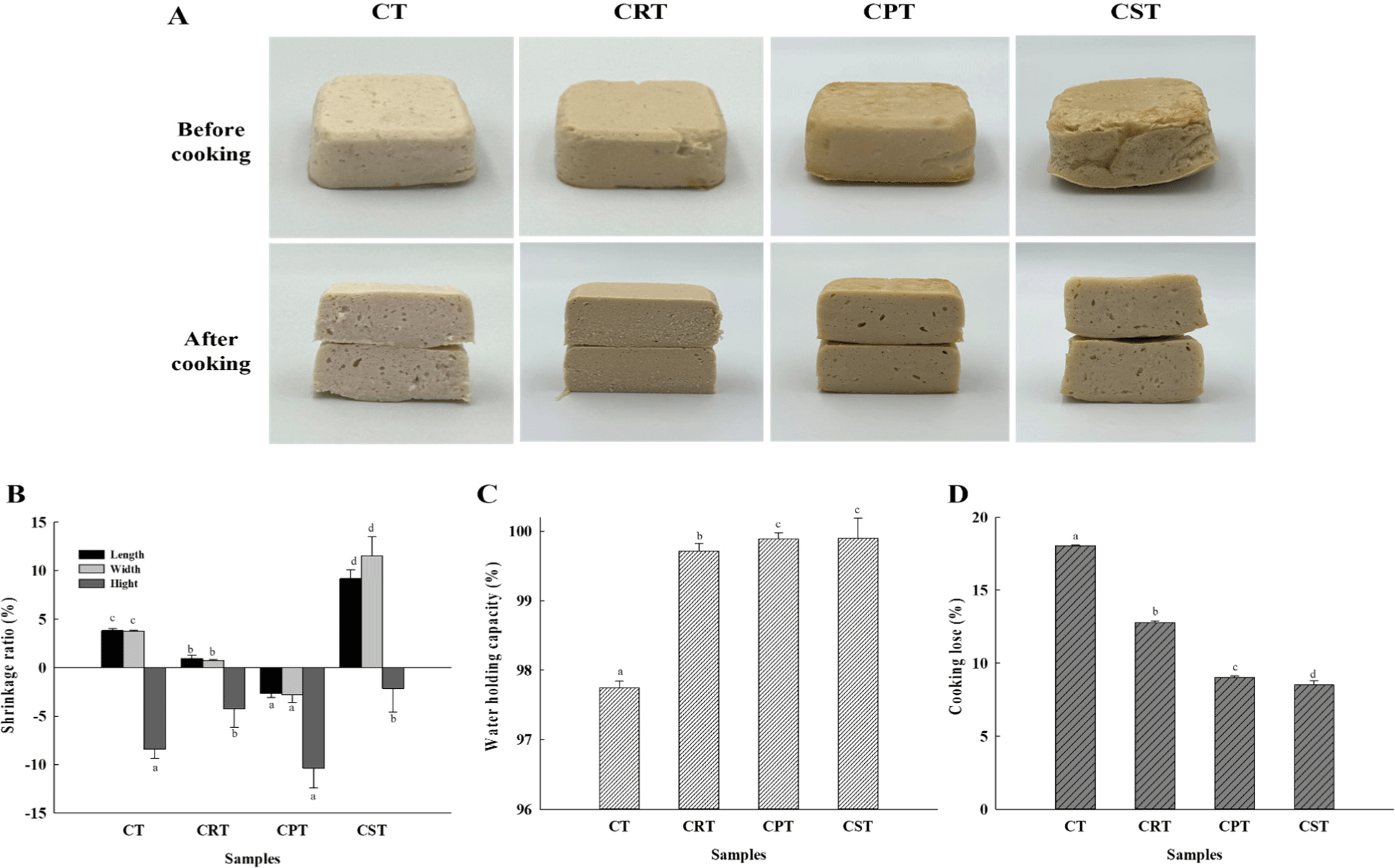
Water and fat retention capacity are among the most valuable functional properties of heat-induced protein gels. This refers to their capability to trap water, fat, and other food components within the gel structure (Sun et al., 2014). According to Fig. 3C, the overall water holding capacity in samples using plant proteins was stronger than that of CT. Previous studies have shown that incorporating plant-based proteins enhances water holding capacity by limiting moisture movement during heating through bonding water molecules and protein (García-García and Totosaus, 2008). The results were also supported by FT–IR (Fig. 2), which revealed increased intermolecular structural interactions upon the addition of plant proteins.
Cooking loss is considered a crucial indicator for assessing the quality of meat products as it reflects the amount of juice lost during meat cooking. As illustrated in Fig. 3D, CPT and CST exhibited significantly lower cooking losses (p<0.05). The incorporation of pulse proteins decreased cooking losses and potentially improved the textural characteristics of meat products by enhancing their physicochemical and binding properties. Even if the water holding capacity of the cooked sample is high, some samples may still exhibit significant cooking loss if the initial matrix does not effectively retain water during heating. This discrepancy is likely attributable to changes in the heat-induced gelation of proteins and interactions within the matrix, which may influence moisture retention differently during and after cooking. Chandler and McSweeney (2022) and Xie et al. (2022) similarly reported that the incorporation of plant-based protein could reduce the cooking loss of hybrid meat products, which is consistent with the findings observed in this study. From a technical perspective, low cooking losses are anticipated to result in economically advantageous high product yields.
The textural properties of PHCA after cooking, including hardness, cohesiveness, elasticity, chewiness, and shear force, are shown in Table 4. It has a structure very similar to real meat, with comparable moisture levels, bite resistance, and texture (Akdogan, 1999). TPA and shear force of samples after cooking exhibited significant differences depending on the type of plant-based protein, with hardness, chewiness, and shear force ranking higher in the order of CT<CPT<CRT<CST (Table 4). In particular, the characteristic enhanced physical properties of CRT can be explained through the relatively dense structure in Fig. 3A and the viscosity of the meat emulsion gel before cooking (Fig. 1E). The network of protein aggregates formed by the addition of rice protein may have altered the viscosity of the meat emulsion gel, leading to air inclusion, fat globularization, and water release (Shoaib et al., 2018). Additionally, the higher carbohydrate content in CRT, primarily from the rice protein, may have contributed to its increased hardness after cooking. This mechanism aligns with observations of carbohydrate-protein interactions in other processed food systems, where carbohydrates can significantly impact texture and hardness (Scott and Awika, 2023). Cohesiveness and springiness were found to be correlated with the viscoelasticity of the meat emulsion gel. This correlation is believed to stem from the varying intermolecular bonds formed after cooking, which differ for each plant-based protein. Soy protein, in particular, exhibits the highest intermolecular interaction force compared to other proteins. Moreover, the shear force exhibited the highest value in CST, indicating that samples with excellent chewiness can be produced when plant protein is utilized. It is recognized that differences in physical property analysis are influenced by the condition of the meat product, the type of additives used, and the fat and moisture content (Gómez et al., 2020). This study suggests that the type of plant-based protein used has an impact on PHCA. Furthermore, it is suggested that incorporating plant-based protein can enhance the physical properties of the product.
Conclusion
In this study, we developed hybrid chicken analogue supplemented with various plant-based proteins and analyzed their structural and quality characteristics. Incorporating plant-based proteins into meat enhances its nutritional and textural properties and encourages the utilization of traditional meat products to achieve the desired protein intake. The utilization of ISP and IPP in meat formulations enables the production of cost-effective and high-quality products without compromising the physicochemical attributes of meat. The inclusion of these proteins resulted in an enhancement of the protein and non-EAA content of the final product compared to CT. Furthermore, it was observed that the addition of plant-based proteins could positively impact the end product by enhancing the interaction with animal proteins. Additionally, future research could further enhance our understanding of the product formulation by comparing the hybrid chicken analogue with actual chicken meat and analyzing the protein’s thermal transition. Substituting meat with plant-based proteins up to 20% level in this study serves as a steppingstone between plant-based meat substitutes and traditional meat, making them more appealing to a wider audience by addressing nutritional concerns.













