Introduction
Chicken meat is a favorable option for consumers because of its low cost, high protein content, and low fat content (Shin et al., 2022). However, its high water activity accelerates microbial growth, leading to reduced shelf life and the deterioration of physicochemical and sensory properties (Katiyo et al., 2020; Zheng et al., 2023). In particular, chicken meat is vulnerable to contamination by foodborne pathogens, such as Listeria monocytogenes and Salmonella spp., which pose significant risks to food safety (Alves et al., 2022). These issues have driven the need for effective preservation strategies to extend the shelf life of chicken meat by maintaining its physicochemical properties and inhibiting microbial contamination.
Preservatives are widely used to inhibit microbial growth, and natural preservatives have gained increased attention owing to health concerns associated with chemical preservatives (Zheng et al., 2023). Essential oils (EOs) extracted from plants are highly valued for their antioxidant and antimicrobial properties due to their metabolites and volatile compounds (Falleh et al., 2020; Konfo et al., 2023). Mugwort (Artemisia vulgaris) has been extensively used in therapeutics and food, particularly in East Asia (Siwan et al., 2022). Mugwort essential oil (MEO), rich in bioactive compounds, such as flavonoids and terpenoids, has demonstrated significant antioxidant and antimicrobial properties (Demirbolat et al., 2022; Ekiert et al., 2020; Sharifian et al., 2013; Torres-Martínez et al., 2018), suggesting its potential for effective food preservation. For example, MEO delayed myoglobin oxidation, maintained color values, and retarded lipid oxidation by suppressing thiobarbituric acid reactive substances (TBARS) values during the storage period of chicken meat (Alirezalu et al., 2022; Yaghoubi et al., 2021). Furthermore, MEO has shown cytomembrane permeability, cell constituent leakage, and disruption of cell structures due to its diverse compounds, which contributed to its antimicrobial effects against both Gram-positive and Gram-negative pathogens (Donato et al., 2015; Xiang et al., 2018). However, previous reports have identified the disadvantages of utilizing EOs in food products, such as their strong flavor, instability due to volatility, and lipophilicity (Zhang et al., 2020; Zheng et al., 2023). Therefore, EOs require appropriate processing for use in food products.
Edible coatings have emerged as an effective method for extending the shelf life of food products by inhibiting microbial growth (Barazi et al., 2023; Kumarihami et al., 2022). Polysaccharides and proteins are commonly used in edible coatings, and their combination can enhance the properties of the coating, including its moisture barrier activity and feasibility (Hassan et al., 2018). Chitosan, a polysaccharide derived from the deacetylation of chitin, has been used in edible coatings owing to its nontoxic nature, broad-spectrum antimicrobial activity, excellent film-forming properties, and low gas permeability (Xiong et al., 2020; Zhang et al., 2018). Gelatin is known to improve film elasticity and barrier properties (Wang et al., 2021). Mixtures of edible chitosan and gelatin coatings have been studied in various food products, including chicken and beef (Cardoso et al., 2019; Safari et al., 2023). Chitosan/gelatin coatings offer decent preservative and antimicrobial effects for stored chicken, but their preservation ability can be enhanced with supplementary ingredients (Safari et al., 2023). Previous studies have found that the addition of cinnamon or oregano EO to a chitosan/gelatin edible coating decreases protein and lipid oxidation during the storage of meat products (Qiu et al., 2022; Zheng et al., 2023). Furthermore, problems associated with the sole use of EOs, such as instability and strong flavor, can be mitigated by using them in an emulsion form, such as in an edible coating (Noori et al., 2018).
Although chitosan/gelatin edible coatings have been widely applied to various foods, their effectiveness on chicken meat requires improvement due to the high susceptibility of the meat to microbial contamination. Chicken meat is particularly prone to pathogens such as L. monocytogenes and Salmonella spp., as well as to rapid bacterial growth due to its high water activity (Alves et al., 2022; Zheng et al., 2023). Also, chicken meat spoils quickly primarily due to its high polyunsaturated fatty acid content, which makes it very vulnerable to oxidation (Zheng et al., 2023). To enhance antimicrobial and antioxidant effects, incorporating natural bioactive substances such as EO is useful. MEO could be a valuable additive due to its proven ability to inhibit both Gram-positive and Gram-negative bacteria. In addition to the oxidative stability provided by the oxygen barrier function of the edible coating itself, the presence of substantial antioxidant compounds in MEO further contributes to delaying oxidative quality deterioration during storage. Therefore, we aimed to determine whether MEO enhanced the functional properties of chitosan/gelatin edible coatings, particularly in chicken breast meat. We optimized the MEO/ chitosan/gelatin ratio for chicken meat coatings and evaluated the quality characteristics and microbial safety of chicken breasts during refrigerated storage. Our study focused on exploring the effects of MEO addition to chitosan/gelatin edible coatings on chicken meat preservation and shelf life extension.
Materials and Methods
Chicken breasts were purchased from a local market. Mugwort (A. vulgaris) EO was prepared by steam distillation and supplied by Fresh Farm (Seoul, Korea). Chitosan (2,000 Da) was provided by Xi’an Best Bio-Tech (Shaanxi, China), and gelatin was purchased from Edentownfnb (Incheon, Korea). Tween 80 and glycerol were obtained from ESfood (Gunpo, Korea). Methanol, acetic acid, n-hexane, hydrochloric acid, potassium carbonate, and sulfuric acid were purchased from Duksan Science (Seoul, Korea). The antifoam was supplied by Shin-Etsu Silicone (Seoul, Korea). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
L. monocytogenes (KCCM 40307) and Salmonella enteritidis (isolated from chicken feces) were used in this study. Each strain was activated in tryptic soy broth (TSB; BD Difco, Sparks, MD, USA) containing 0.6% yeast extract (TSBYE) and TSB, respectively. Activation was performed twice at 37°C for 24 h, and the strains were streaked onto tryptic soy agar (TSA; BD Difco) for storage, and used within a month.
For identifying the compounds in MEO, samples were analyzed using an Agilent 6545XT AdvanceBio liquid chromatography-quadrupole time-of-flight (LC-QTOF; Agilent Technologies, Santa Clara, CA, USA) equipped with a Zorbax Eclipse C18 column (50×2.1 mm, 1.8 μm; Agilent Technologies, Santa Clara, CA, USA). Samples were prepared by diluting with methanol and filtered through a 0.22 μm filter. The samples were injected at a volume of 2 μL, with a flow rate and temperature set to 0.3 mL/min and 45°C, respectively. The mobile phases used for the separation were (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The gradient elution was set as follows: 0 min, 95% A; 12 min, 35% A; 30 min, 5% A; and 60 min, 95% A. Mass spectrometry (MS) conditions were as follows: collision energy, 40 eV; fragmentor, 200 V; nozzle voltage, 1,000 V; gas temperature, 325°C; gas flow rate, 11 L/min; and nebulizer pressure, 20 psi. MS and tandem mass spectrometry (MS/MS) analyses were conducted in both positive and negative ionization modes, covering an m/z range of 20–1,700. Data acquisition for MS and MS/MS was performed at scan rates of 2.0 and 1.0 scans per second for the positive and negative modes, respectively. Data acquisition was performed using MS-DIAL (Version 4.9.22), including centroiding, peak picking, and matching of spectra to imported spectral libraries. The chromatograms of both the positive and negative modes are shown in Supplementary Fig. S1. The analyzed compounds were initially sorted using a match score>0.8. The inherent compounds in MEO were selected and are listed in Supplementary Table S1.
An edible chitosan/gelatin coating solution was prepared as described previously (Zhang et al., 2020). Chitosan (1.0%, 1.5%, and 2.0%, w/v) was dissolved in 1% acetic acid solution at room temperature for 24 h, and gelatin (2.0%, 3.0%, and 4.0%, w/v) was dissolved in distilled water at 80°C for 30 min. The prepared solutions were then mixed in equal ratios with glycerol (1.0%, w/v) and Tween 80 (0.5%, w/v) as the plasticizer and emulsifier, respectively. In addition, to identify the ideal chitosan/gelatin ratio, each formulation was prepared with an equal MEO concentration of 1.0% (w/w) and blended for 3 min using a hand blender (Tefal, Mayenne, France). The formulations are referred to as follows: 1% chitosan+2% gelatin+1% MEO (C1G2M), 1% chitosan+3% gelatin+1% MEO (C1G3M), 1% chitosan+4% gelatin+1% MEO (C1G4M), 1.5% chitosan+2% gelatin+1% MEO (C1.5G2M), 1.5% chitosan+3% gelatin+1% MEO (C1.5G3M), 1.5% chitosan+4% gelatin+1% MEO (C1.5G4M), 2% chitosan+2% gelatin+1% MEO (C2G2M), 2% chitosan+3% gelatin+1% MEO (C2G3M), 2% chitosan+4% gelatin+1% MEO (C2G4M). Subsequently, MEO (0%, 0.5%, and 1.0%, w/w) was incorporated into the optimized chitosan/gelatin solution. The formulations of the MEO-supplemented groups were designated as follows: 1.5% chitosan+3% gelatin+0% MEO (C1.5G3M0); 1.5% chitosan+3% gelatin+0.5% MEO (C1.5G3M0.5); and 1.5% chitosan+3% gelatin+1% MEO (C1.5G3M1).
Viscosity of the edible coating solution was measured using a DV-E viscometer (Brookfield, Toronto, ON, Canada). The solution (35 g) was transferred to a 50 mL conical tube, and the viscosity was measured every 30 s at 50 rpm with a 63 spindle for 2 to 4 min.
The coating rate was determined as previously described (Shin et al., 2022), with some modifications. The weight of the chicken breasts was measured before and after coating, and the coating rate was calculated as follows:
The presence of MEO in the edible coating solution was confirmed by Nile red staining, which was performed as previously described (Keum et al., 2024). The solution (1 mL) was stained with Nile red (20 μL) dissolved in DMSO (0.1%, w/v) and placed on a glass slide. After covering with a coverslip, images were observed using a fluorescence microscope (Eclipse Ti2-U, Nikon, Tokyo, Japan) and captured using a Nikon Eclipse Ts2R camera (Nilon, Tokyo, Japan).
The retention rate was determined as previously described (Singh and Sheikh, 2022), with some modifications. The solution was gently shaken in n-hexane at the same ratio (w/v) and incubated in the dark for 1 h. After incubation, the amount of MEO released in the supernatant was measured at 287 nm. Retention rate was calculated using the following equation:
Where ODsupernatant represents the absorbance of MEO released in the supernatant and ODcontrol is the absorbance of non-retained MEO.
Fourier transform infrared (FT-IR) spectra of the solution were recorded using an FT-IR spectrophotometer (FT/IR-4100 type A, JASCO, Tokyo, Japan) at a resolution of 4 cm–1 over a wavenumber range of 4,000–600 cm–1.
The microstructure of the edible coating solution was observed using field emission scanning electron microscopy (FE-SEM; S-4700, Hitachi, Japan). The solution was lyophilized and the surface was examined at a magnification of 45×with an acceleration voltage of 15.0 kV.
For the storage evaluation, chicken breasts were cut into 2×2×2 cm cubes (approximately 25 g each) using a sterile knife. The coating solutions were prepared using varying concentrations of MEO (0%, 0.5%, and 1.0% w/w). Each chicken breast sample was dipped in the coating solution for 10 min and dried in a biological hood for 15 min. After drying, the coated samples were packaged into sterile polypropylene containers and stored at 4°C. Uncoated chicken breast served as a negative control, and further studies were conducted at specific storage intervals (1, 3, 6, 10, and 14 days).
To assess the antimicrobial effect of the coating solution against L. monocytogenes and S. enteritidis in chicken meat, bacterial strains were inoculated as previously described, with slight modifications (Osaili et al., 2021; Zheng et al., 2023). To eliminate surface bacteria, the chicken breasts were sterilized under ultraviolet light. Bacterial strains (1×107 CFU/mL) were inoculated (50 μL) onto the surface of the samples and allowed to dry in a biological hood for 20 min to enable absorption. A storage temperature of 4°C was used, and coating and subsequent analyses were conducted under the same conditions described above.
For microbial analysis, a sample (approximately 25 g) was homogenized 10-fold with PBS using a stomacher (WES-400, DAIHAN Scientific, Wonju, Korea). The mixtures were serially diluted in PBS and aliquoted onto Petrifilm (3M, St. Paul, MN, USA) and selective agar. The total viable count (TVC), coliforms, Escherichia coli, yeasts, and molds were determined using Petrifilm. Samples for TVC, coliforms, and E. coli were incubated at 35°C for 48, 24, and 24 h, respectively, while those for yeasts and molds were incubated at 25°C for 5 days. Oxford agar (MB cell, Seoul, Korea) with Oxford supplement (MB cell) was used for detecting L. monocytogenes and incubated at 37°C for 24 h. Xylose lysine deoxycholate agar (XLD; MB cell) was used for detecting S. enteritidis and incubated at 37°C for 24 h. After incubation, colonies were counted and expressed as Log CFU/g.
The color was determined using a CR-400 colorimeter (Konica Minolta, Osaka, Japan). Before measurement, calibration was conducted using a white plate (CIE L*=+97.27, CIE a*=+5.21, CIE b*=–3.40), and the surface of the sample was examined. The color values are represented as CIE L*, CIE a*, and CIE b*. The ΔE value (total color difference) was then calculated as follows:
Where CIE L*, CIE a*, and CIE b* represent the color values at each storage period, and CIE , CIE , and CIE represent the color values on day 1.
pH was measured using a pH meter (Orion Star A211, Thermo Fisher Scientific, Waltham, MA, USA). Before measurement, each sample (5 g) was homogenized in distilled water (20 mL) using a homogenizer (T-18-D, IKA-Werke, Staufen, Germany).
Protein degradation in chicken breast was determined as volatile basic nitrogen (VBN) content using the Conway microdiffusion method, as described previously (Lee et al., 2025). Each sample (5 g) was homogenized in distilled water (20 mL) for 1 min. The homogenate was adjusted to a volume of 50 mL using distilled water and filtered through Whatman No. 1 filter paper (Cytiva, Marlborough, MA, USA). The filtrate (1 mL) and 50% K2CO3 were loaded into the outer section of the Conway diffusion cells. In the inner section, an indicator (100 μL; 0.066% methyl red and 0.066% bromocresol green; 1:1 ratio) and 0.02 N H3BO3 (1 mL) were loaded. The cells were then incubated at 37°C for 2 h. After incubation, the inner section was titrated with a 0.02 N H2SO4 solution. The VBN content was expressed as mg percent (mg%).
Lipid oxidation in chicken breast was measured by determining the TBARS value, as previously described (Shin et al., 2023). Each sample (10 g) was homogenized in distilled water (50 mL). The homogenate was transferred to a distillation flask containing distilled water (47.5 mL), a 4 N HCl aqueous solution (2.5 mL), and an antifoam agent (1 mL) for distillation. After distillation, the distillate was mixed with 0.02 M thiobarbituric acid reagent at a 1:1 ratio. The mixture was heated at 95°C for 30 min in a water bath. Following heating, the reactants were cooled for 10 min in an ice bath and the absorbance was measured at 532 nm. The TBARS value is expressed as milligrams of malondialdehyde per kilogram of sample (mg MDA/kg).
Shear force was examined using a texture analyzer (TA.XT plusC, Stable Micro Systems, Surrey, UK) equipped with a Warner Bratzler shear blade. Each raw chicken breast sample was cut into a 2×1×1 cm cube, and the test conditions were as follows: distance (32.0 mm) and speed (2.0 mm/s). The maximum force was considered as the shear force.
Drip loss was measured using the method described by Zheng et al. (2023). After storage, the samples were gently wiped with a tissue to remove surface moisture. The drip loss was then expressed as follows:
Each experiment was conducted at least three times, and the data are presented as the mean±SD. Statistical analyses were performed using SPSS PASW (version 22.0, SPSS, Chicago, IL, USA). Data were analyzed using one- and two-way analysis of variance (ANOVA). Significant differences were identified using Duncan’s multiple range test (p<0.05).
Results and Discussion
The viscosity of the edible base-coating solution was measured to optimize the chitosan/gelatin ratio. To identify the ideal chitosan/gelatin ratio, each formulation was prepared with an equal MEO concentration of 1% (w/w). Viscosity significantly increased with higher concentrations of chitosan or gelatin (p<0.05; Fig. 1A). This increase in the viscosity of the chitosan/ gelatin polymer systems is attributed to ionic and hydrogen bonding within the polymers (Amiri et al., 2018). Additionally, the degree of polymer chain entanglement and their interactions in solution influence the viscosity (Keum et al., 2025; Voo et al., 2015). Consequently, the C2G4M group, which exhibited the highest viscosity (p<0.05), likely exhibited the strongest chemical interactions (Fig. 1A).
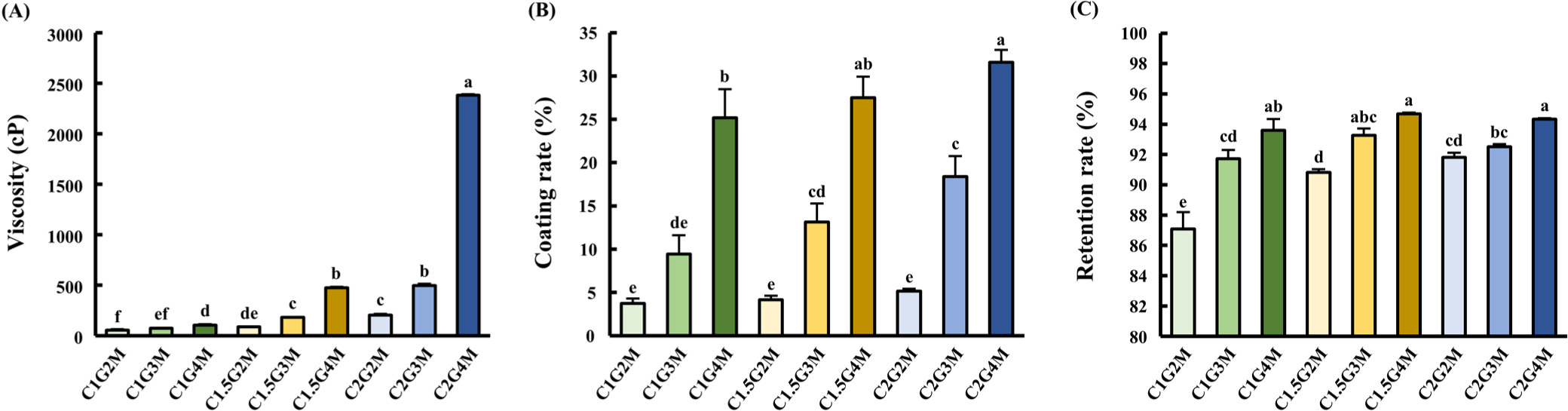
The coating rate followed the same pattern as the viscosity (Fig. 1B), showing a significant increase (p<0.05) with greater amounts of both chitosan and gelatin. The higher viscosity resulting from the entanglement of chitosan and gelatin likely improved the coating rate by preventing detachment from the chicken breast. This aligns with prior research demonstrating that high viscosity facilitates the formation of thicker coating layers (Chang et al., 2023). However, the increase in the coating rate appeared to be more strongly driven by the gelatin content, as evidenced by the significantly higher values observed in the G4 group (C1G4M, C1.5G4M, and C2G4M) than in the other groups (p<0.05). This is attributed to the sol-gel transition of gelatin (Dressler et al., 2011). The melted gelatin applied during preparation solidified upon cooling, forming a well-adhered gel that minimized flow and led to a higher coating rate in the groups with high gelatin concentrations.
The retention rate of MEO within edible coatings was evaluated (Fig. 1C). This was determined by measuring the amount of MEO released into the hexane during gentle shaking. All samples except C1G2M demonstrated retention rates exceeding 90% (Fig. 1C). The lower efficiency of C1G2M is also reflected in the FT-IR spectra (Supplementary Fig. S2), showing the weakest peak in the 1,060–1,090 cm–1 range, characteristic of plant-based EO (Topala and Tataru, 2016). The G4 groups and C1.5G3M exhibited the highest MEO content (p<0.05). High viscosity, which is known to hinder the diffusion of entrapped substances (Funami, 2011), suggests that an increased material concentration enhances entrapment by increasing the viscosity (Sinha et al., 2004). Furthermore, the chitosan/gelatin complex layer is known to slow the release of EOs (Singh and Sheikh, 2022). Therefore, higher chitosan and gelatin levels led to an increased viscosity of the coating solution, resulting in high retention rate by limiting MEO diffusion and improving its entrapment within the complex layer.
Nile red staining confirmed MEO was consistently and evenly dispersed at the microscale throughout all coating samples, with no significant differences observed (Supplementary Fig. S3). Given that micro-sized EO droplets in emulsions have demonstrated superior antimicrobial activity compared to nano-sized droplets (Kim et al., 2025), the observed MEO droplet size and distribution in our coatings suggest their suitability as edible coatings for chicken breasts. Subsequently, FE-SEM analysis revealed the microstructures of freeze-dried edible coatings. The C2 groups (C2G2M, C2G3M, and C2G4M) displayed porous structures (Fig. 2) that became more prominent with increasing polymer content. The hydrophilic natures of chitosan and gelatin facilitate network formation via hydrogen bonding, leading to porous structures (Ji Yin et al., 2000; Wang et al., 2021). Thus, the observed porosity in C2 groups likely stems from extensive hydrogen bonding at high chitosan and gelatin concentrations. The pore size significantly affects the retention of retained materials during storage, and smaller pores can impede their release from hydrogel systems (Zhang et al., 2015). Considering the MEO retention based on pore size, the larger pores of the C2 groups suggest that they may not be ideal for edible coatings requiring prolonged storage.

To effectively preserve chicken meat, the coating should maximize the antimicrobial and antioxidant agent levels, while minimizing the overall concentration. Thus, retention rate was the primary criterion for selecting the optimal chitosan/gelatin ratio for edible coatings. The G4 (C1G4M, C1.5 G4M, and C2G4M) and C1.5G3M groups exhibited significantly higher retention rates (p<0.05). However, the G4 group also showed a significantly higher coating rate (p<0.05) and larger pore size than the C1.5G3M. Given that consumers prefer thin, glossy, and transparent edible coatings (Galus and Kadzińska, 2015), the thick coating of G4 groups is less desirable. For extending the shelf life of chicken meat, C1.5G3 was the optimal edible coating, attributed to its low viscosity, medium coating rate, high MEO retention, and small pore size. Consequently, further research focused on edible coatings optimized with 1.5% chitosan and 3% gelatin.
Microbial growth and visual appearance of chicken breast were assessed during 4°C storage. Chicken breast samples were coated with chitosan/gelatin solutions containing 0%, 0.5%, or 1.0% (v/v) MEO and an uncoated control. On day 1, CON showed significantly higher TVC at 3.52 Log CFU/g compared to all other samples (p<0.05), followed by the C1.5G3M0 group at 2.75 Log CFU/g (Table 1, Fig. 3). Increasing the MEO concentration significantly reduced the TVC (p<0.05). Although TVC gradually increased in all samples during storage (p<0.05), CON consistently exhibited the highest counts, followed by C1.5G3M0, C1.5G3M0.5, and C1.5G3M1. A TVC exceeding 7 Log CFU/g is a spoilage indicator (Shin et al., 2022). In our study, the CON spoiled by day 10, whereas the coated samples remained below this threshold, indicating the effectiveness of the edible coating in delaying spoilage. Addition of MEO to the chitosan/gelatin coating solution helped maintain the initial quality of the chicken breast, as evidenced by its significantly lower TVC compared to other samples throughout the storage period (p<0.05). These results were confirmed by the visual appearance of the chicken breasts during storage (Fig. 3). Distinct slime formation was observed on the surface of chicken breasts in the CON group on day 14. Slime formation occurs because exopolysaccharides are secreted by spoilage bacteria when the bacterial counts exceed 8 Log CFU/g (Katiyo et al., 2020). On day 14, the CON group exhibited a significantly higher TVC (8.07 Log CFU/g; p<0.05), leading to prominent slime formation. Similarly, minor slime formation was noted on day 10 in CON and on day 14 in C1.5G3M0, as both showed higher TVC than the other samples at these time points (p<0.05).
A–E Different capital letters indicate significant differences between storage periods within the same treatment group (p<0.05).
a–d Different lowercase letters indicate significant differences between the treatments during the same storage period (p<0.05).
TVC, total viable count; CON, uncoated chicken breast; C1.5G3M0, chitosan/gelatin (1.5%/3%) edible coating-coated chicken breast without mugwort essential oil; C1.5G3M0.5, chitosan/gelatin (1.5%/3%) edible coating-coated chicken breast with 0.5% mugwort essential oil; C1.5G3M1, chitosan/gelatin (1.5%/3%) edible coating-coated chicken breast with 1% mugwort essential oil; ND, non-detection.
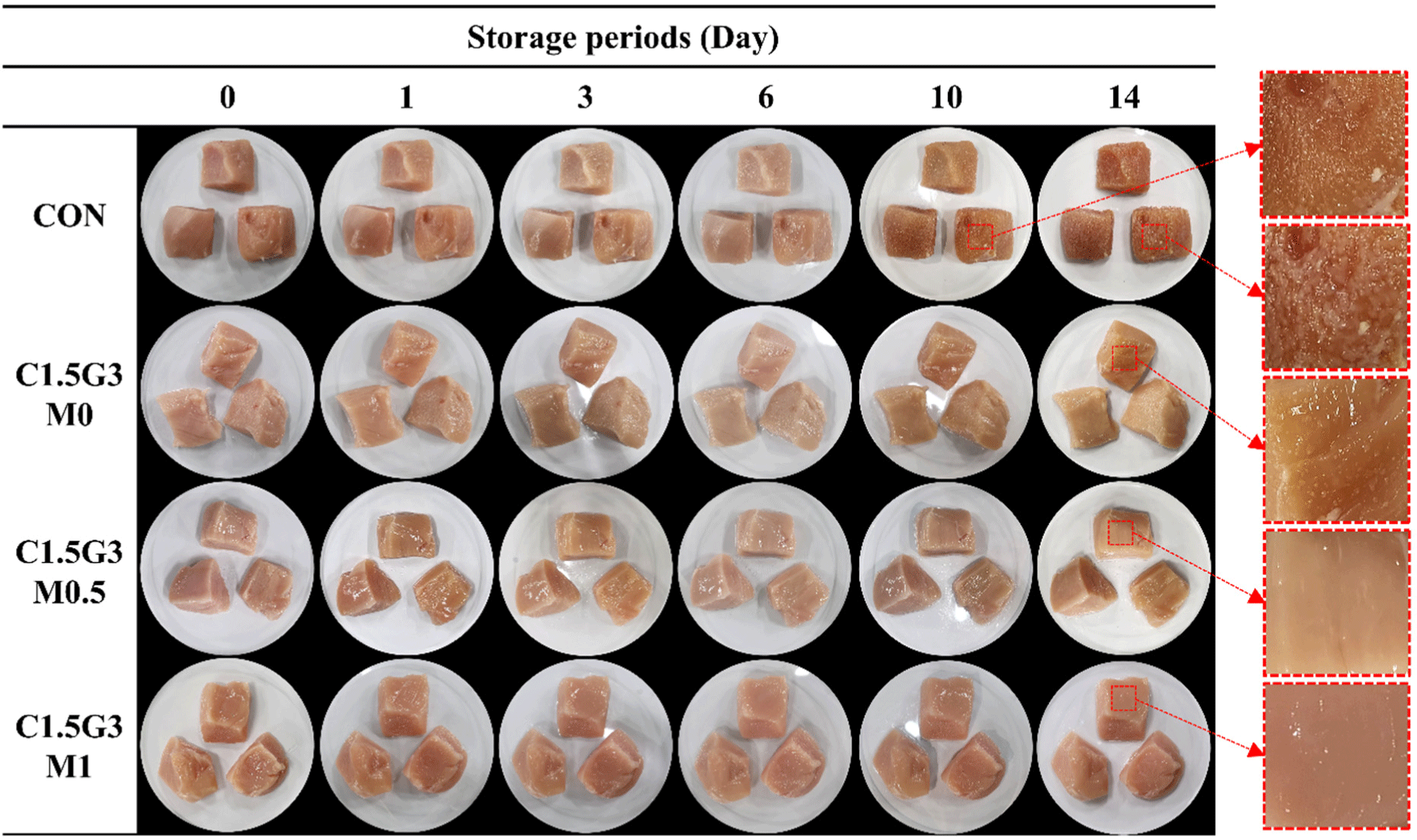
Regarding the other parameters, E. coli was not detected in any of the samples throughout the storage period. Coliforms were undetectable in all samples until day 3. However, on day 6, 2.96 Log CFU/g was detected in the CON group, followed by a gradual and significant increase throughout the storage period (p<0.05). The coated samples exhibited detectable coliforms starting on day 10, with the MEO-added groups showing significantly inhibited coliform growth (p<0.05). The growth trends of yeasts and molds were similar to those of aerobic bacteria and coliforms. CON consistently showed the highest values across the storage period (p<0.05), whereas the coated samples, particularly those with C1.5G3M0.5 and C1.5G3M1, significantly reduced the growth of yeasts and molds (p<0.05). Furthermore, given their frequent occurrence in chicken breast, we tested two pathogenic bacteria, L. monocytogenes and S. enteritidis, which were chosen to represent Gram-positive and Gram-negative types, respectively (Maragkoudakis et al., 2009). Similar to other microorganisms, the L. monocytogenes and S. enteritidis counts of the coated samples were lower than those of the CON throughout the storage period (p<0.05; Table 1). Furthermore, on day 14, S. enteritidis counts were significantly lower in all coated samples, suggesting strong anti-pathogenic effects of the chitosan/gelatin coating (Table 1).
Our edible coating effectively reduced the growth of microorganisms. The ingredients used for the edible base coating were chitosan and gelatin. Chitosan is well known for its broad-spectrum antimicrobial activity (Zheng et al., 2023). The proposed antibacterial mechanism of chitosan is based on electrostatic interactions between chitosan molecules and microbial cell membranes, which possess opposite charges (Xiong et al., 2020). These interactions alter membrane permeability, ultimately causing breakdown of the cell membrane (Kumarihami et al., 2022). Additionally, as food contamination typically begins with microbial growth on the food surface, the film-forming ability of chitosan coatings can inhibit microbial growth by preventing the transport of nutrients into microbial cells (Katiyo et al., 2020; Zhang et al., 2020). In addition to chitosan, MEO can also act as an effective antimicrobial agent. In general, EO target common bacterial cells by crossing the cytoplasmic membrane and mitochondria because of their hydrophobicity and lipophilicity (Falleh et al., 2020). This process disrupts the integrity of polysaccharides, fatty acids, and phospholipids, leading to bacterial cell-wall degradation and subsequent cell death (Burt, 2004). Our study revealed that MEO was composed of various potent antimicrobial compounds (Supplementary Table S1). The primary components of MEO are terpenoids and flavonoids, which exhibit antimicrobial properties (Álvarez-Martínez et al., 2021; Angane et al., 2022; Cushnie and Lamb, 2011). Other compounds, such as benzoic acid, 1,2-diols, and shogaols, have also demonstrated antimicrobial activity (Khatiwora et al., 2012; Koshak et al., 2024; Zhang et al., 2024). Additionally, the coated samples exhibited higher antimicrobial effects against S. enteritidis than L. monocytogenes. This could be attributed to the higher resistance of L. monocytogenes to the hydrophobic substances of EO (Osaili et al., 2021), as well as structural distinctions between gram-positive and gram-negative bacteria, including the peptidoglycan layer on the cell wall (Fabio et al., 2025). However, the addition of MEO did not inhibit the growth of L. monocytogenes or S. enteritidis. This finding is consistent with a previous study reporting that EO had no significant effect in inhibiting L. monocytogenes in chicken meat, likely due to interactions with food matrix components such as proteins, carbohydrates, and pH, which modulate the efficacy of EO in food systems (Shekarforoush et al., 2015). Overall, the edible coating effectively inhibited microbial growth in chicken breasts during storage, and the incorporation of MEO further enhanced these antibacterial properties owing to its potent bioactive compounds. Subsequent studies will aim to evaluate the antimicrobial activity of MEO against diverse pathogens and understand its underlying antibacterial mechanisms.
The meat color of the chicken breast samples was analyzed during storage. On day 1, the edible coating did not significantly affect the CIE a* and CIE b* values of chicken breasts (p>0.05; Table 2). However, the CIE L* values of C1.5G3M0 and C1.5G3M0.5 were significantly higher than those of the CON group (p<0.05; Table 2). This could be attributed to the glossy edible coating, which increased light scattering on the surface. These data are consistent with previous studies showing that chitosan-based edible coatings increase the CIE L* values of meat products (Galus and Kadzińska, 2015; Shin et al., 2022). During storage, the CON group exhibited a decrease in CIE L* and CIE a* values, while the CIE b* value increased. Similar trends in color change were observed for the coated samples. On day 14, the CON group exhibited the lowest CIE L* and CIE a* values and the highest CIE b* value (p<0.05). The MEO (0.5% and 1.0%)-treated samples showed a lower reduction in the CIE a* value and an increase in the CIE b* value compared to the CON and C1.5G3M0. The decrease in CIE a* and increase in CIE b* values suggested myoglobin oxidation to metmyoglobin (Xiong et al., 2020), indicating that CON experienced the most oxidation during storage compared to the coated samples. Minimizing the color changes in meat products relies on delaying myoglobin oxidation. Edible coatings are likely to delay this process through multiple mechanisms. First, it acts as an oxygen barrier by forming a thin layer on the chicken breast surface, which is a common strategy for preventing food oxidation (Hassan et al., 2018; Ribeiro et al., 2024). Second, the incorporated MEO exhibited antioxidant activity. Our LC-MS/MS data (Supplementary Table S1) revealed the presence of numerous flavonoids, terpenes, and terpenoids in MEO, which likely contributed to delayed oxidation (Falleh et al., 2020; Konfo et al., 2023).
A–D Different capital letters indicate significant differences between storage periods within the same treatment group (p<0.05).
a–c Different lowercase letters indicate significant differences between the treatments during the same storage period (p<0.05).
CON, uncoated chicken breast; C1.5G3M0, chitosan/gelatin (1.5%/3%) edible coating-coated chicken breast without mugwort essential oil; C1.5G3M0.5, chitosan/gelatin (1.5%/3%) edible coating-coated chicken breast with 0.5% mugwort essential oil; C1.5G3M1, chitosan/gelatin (1.5%/3%) edible coating-coated chicken breast with 1% mugwort essential oil.
These results are further supported by the ΔE data, which measures color changes in chicken breast over time. According to a previous study, ΔE values can be classified as follows: not noticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), well visible (3.0–6.0), and great difference (6.0–12.0; Zheng et al., 2023). Following these classification scales, the MEO-coated samples did not show well-visible changes (ΔE<3.0) until day 14. In contrast, C1.5G3M0 exhibited well visible changes on days 10 and 14, with ΔE values of approximately 3.72 and 4.04, respectively. CON exceeded a ΔE value of 6.0 on day 14, showing a significant difference compared to all other chicken breast samples (p<0.05). Based on our data, the edible coating reduced myoglobin oxidation in chicken breast and helped maintain its initial color state during storage. Furthermore, the incorporation of MEO into edible coatings appeared to delay color changes owing to its potent antioxidant capacity.
pH is a significant indicator of meat freshness (Xiong et al., 2020). As shown in Fig. 4A, pH values ranged from 5.48–5.90 during the first six days in all samples. Given that chicken breast has a typical 3–5 day refrigerated shelf life, our samples maintained a near-initial pH until day 6. Despite a significant pH increase in all chicken breast samples by day 10 compared to day 6 (p<0.05; Fig. 4A), the MEO-treated samples exhibited significantly lower pH values than CON and C1.5G3M0 (p<0.05). This demonstrated that MEO effectively limited the increase in pH during storage. Generally, the pH of raw chicken breast meat is influenced by microbial proliferation (Zhang et al., 2018). Microbial growth increases the pH due to the accumulation of volatile bases, such as ammonia and trimethylamine (Xiong et al., 2020). The antimicrobial properties of chitosan and MEO suppressed microbial growth throughout storage (Table 1), resulting in lower pH levels than those of the control. Moreover, increasing the MEO concentration (0.5% and 1.0%) further improved the reduction in pH compared to the coating without MEO. The pH increase from day 1 to day 14 was 1.56 (CON), 1.20 (C1.5G3M0), 0.82 (C1.5G3M0.5), and 0.52 (C1.5G3M1), respectively, indicating that the MEO-supplemented edible coatings effectively limited the rise in pH.
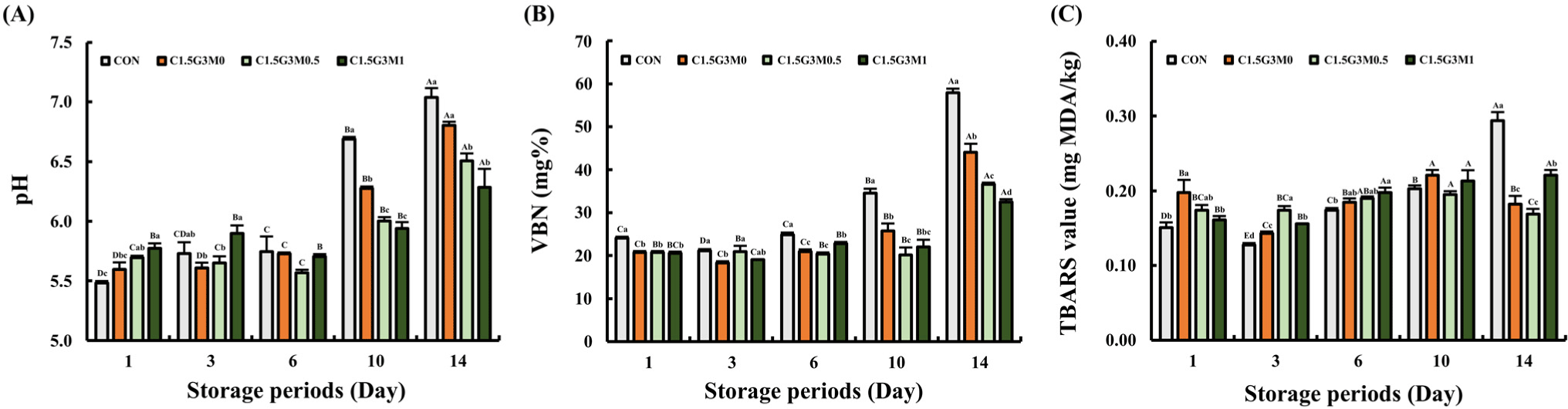
VBN content, a key indicator of protein breakdown (Han et al., 2024), was significantly lower (p<0.05) in all coated chicken breast samples than in the CON throughout storage (Fig. 4B). While the CON consistently showed the highest VBN levels, the coated samples exhibited only a rapid increase on day 14. Notably, the MEO-treated samples maintained stable VBN levels between days 1 and 10 (p>0.05), indicating that the MEO coating effectively delayed protein degradation for 10 days. This inhibition of VBN production, likely owing to the antimicrobial properties of the coatings and MEO, aligns with the observed pH trends. VBN contributes to pH elevation through the formation of basic compounds resulting from microbial and enzymatic protein degradation (Zheng et al., 2023).
Malondialdehyde, measured using the TBARS assay, is a secondary lipid oxidation product that contributes to rancidity and reduces meat marketability (Xiong et al., 2020). Up to day 10, all samples showed low TBARS values (0.12–0.21 mg MDA/kg; Fig. 4C). Although TBARS in the CON significantly increased to 0.29 mg MDA/kg by day 14 (p<0.05), all coated samples maintained lower levels. The protective effects of edible coatings against lipid oxidation are likely due to their ability to limit oxygen exposure (Xiong et al., 2020; Zhang et al., 2018). Notably, the overall TBARS values were low (max. 0.29 mg MDA/kg) compared to other reports (Jang et al., 2024; Zhang et al., 2020), which may be attributed to the inherent resistance of chicken breast to lipid oxidation owing to its endogenous antioxidants (Min et al., 2008) and low fat content (Shin et al., 2022). Interestingly, C1.5G3M1 showed higher TBARS values than C1.5G3M0.5 (p<0.05). Due to the potential for excessive antioxidants to act as pro-oxidants (Rivaroli et al., 2016), C1.5G3M1 may have experienced increased oxidation during storage. To achieve the best antioxidant effects, the TBARS data suggest that EO should be used at an appropriate concentration, such as that found in C1.5G3M0.5. Overall, considering the pH, VBN, and TBARS results, the edible coatings effectively preserved the initial quality of chicken breast, and MEO further enhanced this preservation.
The initial shear force of chicken breasts was similar across all samples on day 1 (p>0.05; Fig. 5A). However, by day 14, the CON group showed a significant decrease in shear force (p<0.05), which was indicative of textural degradation. Although C1.5G3M0 exhibited a decreasing trend, the MEO-treated samples effectively maintained their initial shear force values (p>0.05; Fig. 5A). This stabilizing effect of MEO against texture loss is attributed to its ability, along with chitosan, to inhibit protein deterioration caused by enzymatic and bacterial activity (Shin et al., 2022), which leads to autolysis and proteolysis (Jung et al., 2022; Zheng et al., 2023). The antimicrobial and antioxidant properties of these compounds likely suppress their degradation. The consistent shear force values in the MEO-treated samples suggested that MEO enhanced the maintenance of chicken breast texture during storage.
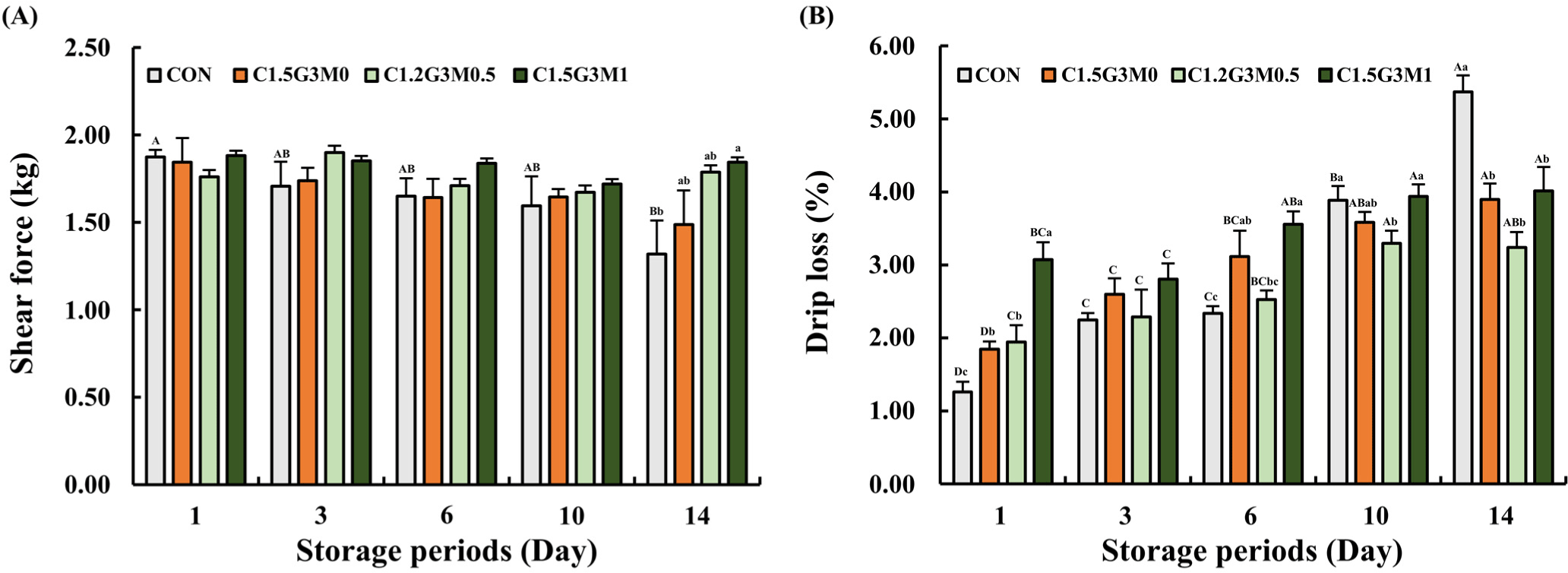
Drip loss was examined to evaluate the water-holding capacity of the chicken breasts during storage. As shown in Fig. 5B, all coated samples showed a higher drip loss than CON on day 1 (p<0.05). However, as time progressed, drip loss in the CON group increased significantly from 1.26% to 5.37%, reaching its highest value on day 14 (p<0.05). In contrast, the application of an edible coating to chicken breast resulted in lower drip loss compared to CON on day 14. Interestingly, a higher drip loss was observed in the coated samples until day 6, which might be attributed to the release of moisture from edible coating itself rather than from the chicken breast. Several factors have been proposed to explain the drip loss in meat during storage. Microbial growth during storage can lead to degradation of meat proteins through enzymatic activity (Shin et al., 2022), while loose muscle structures due to protein denaturation can cause drip loss during storage (Hong et al., 2015). In addition to protein denaturation, myofibrillar lattice spacing and drip channel development may also contribute to drip loss (Hughes et al., 2014; Zheng et al., 2023). As storage progressed, the increased microbial growth in the CON group likely contributed to protein degradation, resulting in significantly higher drip loss compared to the coated samples on day 14 (p<0.05). Conversely, the coated samples effectively inhibited protein degradation by suppressing microbial growth through their antimicrobial properties, leading to reduced drip loss. As shown in Fig. 5A, the lower shear force observed in CON on day 14 may have contributed to higher drop loss, as the weakened protein structure could not effectively retain water during storage. Thus, our findings suggest that edible coatings can maintain initial textural properties and decrease drip loss. Interestingly, although the meat quality of C1.5G3M1 was well-maintained during storage with minimal changes, it showed a higher drip loss than that of C1.5G3M0, which was inconsistent with our previous results. It can be estimated that excessive MEO content in the edible coating had a negative effect. As the MEO content increased, the hydrophobicity increased, and the solubility of the coating decreased, despite the presence of the emulsifier (Tween 80). Previous studies have shown that the addition of lipids can reduce mechanical integrity and flexibility (Ribeiro et al., 2024). Therefore, excessive MEO content (1%) may have hindered proper adhesion to the chicken breast surface, reducing the stability compared to C1.5G3M0.5. Overall, C1.5G3M0.5 was found to be the most suitable for maintaining the initial textural properties of chicken breast, as well as other storage properties.
Conclusion
In this study, we successfully optimized a chitosan/gelatin edible coating for its processability and ability to retain MEO. Applying this coating to chicken breast effectively controlled the growth of various spoilage and pathogenic microorganisms (TVC, coliforms, yeasts, molds, L. monocytogenes, S. enteritidis) and helped preserve key physicochemical properties (color, pH, VBN, TBARS, shear force, and drip loss) during storage compared to the control. The incorporation of MEO further enhanced both the antimicrobial activity and the maintenance of the initial quality. Consequently, this optimized edible coating, particularly with MEO, offers a promising approach for extending the shelf life of chicken breast through its combined antimicrobial and antioxidant actions. In particular, C1.5G3M0.5 exhibited superior preservative performance compared to C1.5G3M1, including lower TBARS and drip loss values, suggesting that 0.5% MEO is the optimal concentration for preservative use. Future research should focus on the mechanisms underlying the antimicrobial effects of MEO to explore its potential to enhance the safety of other meat products.













