Introduction
Korean native cattle were traditionally used as working animals until the 1960s. However, with advancements in farming technology, cattle have begun to be raised for beef production (Lee et al., 2019b). Four breeds of Hanwoo cattle exist: brown Hanwoo, brindle Hanwoo (Chikso), black Hanwoo, and Jeju black cattle. Jeju black cattle are a minor breed that reside exclusively on Jeju Island (Han et al., 2011). Due to their limited growth rate and shorter stature compared to Hanwoo or Wagyu cattle, it is essential to enhance their growth and reproductive performance. This has led to the development of synthetic breeds such as Jeju crossbred cattle (JCC), which are crossbred with Hanwoo and Jeju Black cattle to produce beef with improved reproductive performance and favorable qualities (Cho et al., 2024). According to recent research, the meat quality and muscle fiber characteristics of JCC were distinct from those of Hanwoo (Cho et al., 2024). JCC had higher moisture and crude ash contents, CIE a*-value, cooking loss, and shear force, but lower crude fat and protein contents than Hanwoo. The muscle fiber size (cross-sectional area) of JCC was larger than that of Hanwoo across all muscle types. Type IIX was the dominant muscle fiber type in JCC; however, Hanwoo had the highest proportion of type I fibers compared to the other types (IIA, IIAX, and IIX) in the psoas major. However, information on the physicochemical and flavor characteristics of JCC remains limited.
Meat quality is crucial for both consumers and the meat industry, with tenderness and juiciness being the most important factors. Aging process is a common method used to overcome rigor mortis and to enhance the tenderness and flavor of beef. Especially, wet aging is a method seal the meat in a vacuum packaging bag and stored with a managed regime of environment. Unlikely with simple refrigeration storage with vacuum packaging, it improves tenderness, flavor, and juiciness [i.e., water-holding capacity (WHC)] and is increasingly used to enhance low-marbled products (Kim et al., 2020).
During aging, meat tenderness improves due to the proteolysis of myofibrillar proteins and the degradation of structural proteins by endogenous proteases (Kim et al., 2019). This process leads to the formation of beef flavor precursors such as small peptides, free amino acids, and reducing sugars, which enhance the flavor of beef during cooking through the Maillard reaction and Strecker degradation (Bischof et al., 2021). Wet aging imparts an intense sour, metallic, and bloody flavor to beef and offers greater safety and yield than dry aging. Additionally, meat undergoes drastic and irreversible physicochemical and metabolic changes during aging (Muroya et al., 2020). Therefore, metabolomic studies combined with analyses of flavor-associated precursors and sensory evaluations could help to identify candidate biomarkers linked to the eating quality of meat. However, very limited research has been conducted on the physicochemical and flavor characteristics of beef loins from JCC during wet aging.
This study profiles the physicochemical and flavor characteristics of beef loins from JCC during wet aging, using chemometric analyses that included volatile organic compounds (VOCs), metabolites, and electronic tongue (E-tongue) measurements. Furthermore, proper aging time was investigated at the point of the physicochemical and flavor characteristics.
Materials and Methods
Beef loin (m. longissimus thoracis) was obtained from three JCC (Jeju Black cattle×Hanwoo; JCC) aged 33±1 months, with a quality grade of 1+ or 1, and an average carcass weight of 459±60 kg, 48 h postmortem. After removing the outer edges, visual fat, and connective tissue, the central portion was used to obtain 10 standardized cubes (approximately 7×7×5 cm, weighing approximately 250±25 g each) from each of the three animals, totaling 30 loin cubes. Two cubes were allocated for each aging time point: one for the quality characteristic analysis and the other for the shear force analysis. Beef samples were sealed in low-density polyethylene/nylon bags [2 mL O2·(m2)–1·24 h–1 at 0°C, 0.09 mm thickness; Sunkyung, Seoul, Korea] and vacuum-packaged using an HFV-600L machine (Hankook Fujee Machinery, Hwaseong, Korea). The wet aging process was conducted at 4°C for 28 days, with samples taken at 7-day intervals (n=3 for each wet aging period). On each experimental day, the beef samples were analyzed for color, ground and used for physicochemical quality assessment. The remaining samples were immediately vacuum-packaged and stored at –70°C until further analysis.
The meat color was measured using a colorimeter (CM-5, Konica Minolta Censing, Osaka, Japan) after allowing the samples to bloom for 30 min. The colorimeter was pre-calibrated with a standard white plate, and measurements were conducted under the conditions of a D65 illuminant, a 10° standard observer, and using a plate with a 30 mm diameter. The lightness, redness, and yellowness of meat were expressed as Commission Internationale d’Eclairage CIE L*, CIE a*, and CIE b* values, respectively. Six measurements were taken for each sample and the average was used as a single replicate. Drip loss was determined by calculating the percentage of weight loss compared to the original wet weight of the beef samples.
The pH of beef samples was measured using a method described by Lee et al. (2021b). Three grams of each sample were homogenized with 27 mL of deionized distilled water using a homogenizer (T25 digital ULTRA-TURRAX, Ika Works, Staufen, Germany) at 9,600 rpm for 1 min. The resulting homogenates were centrifuged at 2,265×g for 10 min using a Continent 512R centrifuge (Hanil, Daejeon, Korea). The supernatants were then filtered through Whatman No. 4 filter paper (Whatman PLC, Maidstone, UK) and the pH of each sample was measured using a pre-calibrated pH meter (Seven2Go, Mettler-Toledo, Schwerzenbach, Switzerland) with buffer solutions (pH 4.01, 7.0, and 9.21).
Each beef sample (5 g) was placed in a centrifugation tube with filter paper (Whatman No. 4) and centrifuged at 252×g for 10 min (Continent 512R). After centrifugation, the WHC was calculated as the remaining moisture in the meat sample based on the moisture content of the original meat sample as described by Kim et al. (2019). The water content of the beef samples was determined by drying 1 g of the samples at 110°C for 16 h.
The shear force value and cooking loss of beef samples were measured according to the method described by Kim et al. (2023), with some modifications. Samples with an average weight of 100 g were placed in polyethylene bags and cooked in a water bath at 85°C until the internal temperature of the samples reached 72°C. After cooking, the samples were cooled to 4°C. Five cylinders (1.27 cm diameter) were taken using a cork borer parallel to the muscle fiber. A texture analyzer (TA1, AMETEK Lloyd Instruments, Fareham, UK) with a crosshead speed of 200 mm/min, a test speed of 60 mm/min, and a trigger load of 0.1 N was used. The cooking loss of beef samples was defined as the percentage of weight loss before and after cooking.
VOC analysis was carried out using solid-phase microextraction and gas chromatography-tandem mass spectrometry, as described by Lee et al. (2023). Ground beef samples (5 g) were placed in a 20-mL headspace vial and sealed with a PTFE-faced silicone septum. The samples were preincubated at 40°C for 10 min. VOCs in the headspace of the vial were extracted using a polydimethylsiloxane/divinylbenzene fiber (Supelco, Bellefonte, PA, USA) at 40°C for 30 min. The extracted VOCs were injected and desorbed by GC for 2 min at a split ratio of 1:10. The VOCs were separated using a DB-Wax column (60 m×0.25 mm i.d., and 0.50 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). The GC conditions were as follows. Helium was used as the carrier gas at a flow rate of 1.5 mL/min. The initial oven temperature was held at 40°C for 2 min, increased at a rate of 4°C/min, held at 150°C for 10 min, then increased at a rate of 4°C/min, held at 200°C for 5 min, and finally increased at a rate of 10°C/min and held at 230°C for 5 min. Mass spectra were obtained using a triple quadrupole mass spectrometer (TSQ 8000, Thermo Fisher Scientific, Waltham, MA, USA) in electron impact (EI) mode with a scan range from 35 to 550 m/z in full-scan mode at a 0.2-s scan interval. The VOCs in the beef samples were identified by comparing their mass spectra with those from the National Institute of Standards and Technology (NIST) mass spectral library (version 2.0 g).
The metabolomic profiles of beef samples was analyzed using nuclear magnetic resonance (NMR), as following the procedure described by Lee et al. (2023). To extract polar metabolites, 5 g of beef samples were homogenized with 20 mL of 0.6 M perchloric acid using a T25 digital ULTRA-TURRAX. The homogenates were centrifuged at 2,265×g for 20 min using Continent 512R. The pH of the collected supernatants was adjusted to 7.0, and the samples were centrifuged again at the same speed for 20 min. The samples were then filtered through Whatman No. 1 filter paper and lyophilized using a freezer dryer 18 (Labco, Kansas City, MO, USA). The lyophilized samples were dissolved in 1 mL of 20 mM deuterium oxide-based phosphate buffer (pH 7.0) containing 1 mM 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid (TSP) as an internal standard. The reconstituted samples were incubated at 37°C for 10 min and centrifuged at 2,265×g for another 20 min. Final centrifugation was performed at 17,000×g for 10 min using a HM-150IV (Hanil, Gimpo, Korea), after which the supernatants were transferred to NMR tubes and analyzed immediately. One-dimensional 1H NMR and 1H-13C heteronuclear single quantum coherence (HSQC) spectra of the samples were recorded on a Bruker 850 MHz cryo-NMR spectrometer (Bruker Biospin Rheinstetten, Germany). The spectra were manually corrected using Chenomx NMR Suite 7.1 (Chenomx, Edmonton, AB, Canada). Peak identification was performed by comparing the HSQC spectra of the samples with data from the Human Metabolome Database (www.hmdb.ca) using Topspin 4.0.8 (Bruker Biospin, Ettlingen, Germany). The identified peaks were quantified using the Chenomx NMR Suite 7.1, with quantification referenced to TSP resonance.
E-tongue analysis was performed as described by Lee et al. (2023). Ground beef samples (5 g) were homogenized in 100 mL of deionized distilled water at 10,000 rpm for 30 s (T25 digital ULTRA-TURRAX). The homogenized samples were then centrifuged at 2,265×g for 10 min (Continent 512R) and the supernatants were filtered through Whatman No. 4 filter paper. The taste attributes of the samples were analyzed using E-tongue (Astree; Alpha MOS, Toulous, France). Taste screening was performed using Alpha Soft (Alpha MOS) to compare the relative taste intensities of samples. Taste intensity was evaluated using seven types of sensors: AHS (sour), CTS (salty), NMS (umami), ANS (sweet), SCS (bitterness), PKS (universal taste intensity), and CPS (universal taste intensity).
All experiments were conducted in triplicate and statistical analyses were performed using one-way analysis of variance (ANOVA) with a linear model (SAS 9.4, SAS Institute, Cary, NC, USA). Significant differences between treatments were determined using Tukey’s multiple comparison test (p<0.05). Results are expressed as mean values with SEM. For multivariate analysis, principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA) were conducted using MetaboAnalyst 6.0 (www.metaboanalyst.ca). The data were normalized using log transformation and auto-scaling. The pattern of results from beef samples across different aging times was visualized using a heat map. The model exhibited R2>0 and Q2<0, indicating that it was not overfitting and reliable. Variables with variable importance in projection (VIP) values greater than 1 were considered to have a high contribution to the model.
Results and Discussion
The physicochemical qualities of the wet-aged JCC loins are shown in Table 1. The initial pH of the aged JCC was 5.7, and no significant differences were observed throughout the aging process. Similarly, there were no significant changes in drip loss, cooking loss, CIE L*-value, or water content during aging. These results may have been potentially influenced by temperature fluctuations during aging or the oxygen permeability of packaging materials. However, the CIE a*- and CIE b*-values of the aged JCC loin at days 21 and 28 were lower than the unaged JCC loin (p<0.05). The values of WHC and shear force exhibited sharp changes from day 0 to day 7, and continued to change gradually throughout the aging period. Specifically, the WHC of the JCC loin aged for 21 days increased, while the shear force at day 21 decreased compared to that of the unaged JCC loin (p<0.05). Aging is commonly employed to improve the tenderness, flavor, and juiciness of meat. This improvement is primarily achieved through the proteolysis and degradation of myofibrillar proteins, especially structural proteins (Kim et al., 2019). This degradation helps prevent water loss, thereby increasing WHC (Go et al., 2023). Previous studies have indicated that wet aging, which involves storing meat in a sealed barrier package, such as a vacuum-packaged container at refrigerated temperatures, is a commonly used technique for up to 7 days (Kim et al., 2020). In this study, the JCC loin was aged in a vacuum-packed container for up to 28 days, and significant physicochemical changes were observed as early as day 7.
A total of 33 VOCs were detected in the JCC loin during the aging period (Table 2). The VOCs were classified into six groups: alcohols (n=12), aldehydes (n=4), esters (n=3), carboxylic acids (n=8), furan (n=1), and ketones (n=2). The intensity of eight VOCs increased, while that of 11 VOCs decreased during the aging period (p<0.05). Several VOCs dramatically changed between day 0 and days 7 or 14, followed by minor fluctuations during the rest of the aging period. Notably, octanal and heptanoic acid methyl ester were only detected on day 0, contributing flavors such as lemon, citrus, soap, orange peel, fat, waxy, fatty, aldehydic, green, berry, floral, fruit, green, orris, sweet, and waxy (Table 3). The 2-pentylfuran was present in the aged JCC loin from day 0 to day 14; however, it disappeared by day 21 (p<0.05). This compound is a product of lipid oxidation, which may diminish as lipid degradation progresses during extended aging (Barker et al., 2023). Conversely, compounds such as 2-pheynlethanol, 3-methylbutan-1-ol, 2-methylbutanal, 3-methylbutanal, and 7-aminoheptanoic acid were first detected in the JCC loin after 14 days of aging. By day 28, the total intensity of the VOCs increased significantly, driven by a substantial increase in butane-2,3-diol, acetic acid, 2-hydroxybutan-2-one, and butane-2,3-dione. During aging, the production of free amino acids through various degradation processes contributes to the generation of various VOCs in the meat. For instance, 2-methylbutanal can be formed through the Strecker degradation of isoleucine, a reaction that occurs because of the higher concentration of isoleucine resulting from increased microbial activity during aging (Lee et al., 2019a). Additionally, 2-phenylethanol, 3-methylbutanal, and 7-aminoheptanoic acid are derived from the amino acid metabolic decomposition pathway (Zheng et al., 2024). A previous study also reported an increase in the concentrations of leucine, isoleucine, valine, alanine, lactic acid, creatine, carnitine, and acetic acid in beef with extended aging (Kodani et al., 2017).
As the aging time increases, the composition of VOCs in the JCC loin becomes more complex, significantly influencing the development of a rich meat flavor. Notably, changes in the VOCs composition were evident on day 7 of the early aging period (Fig. 1). The prominent VOCs detected in the initial JCC loins were alcohols, carboxylic acids, and ketones. Alcohols decreased by day 7 and then increased until day 28, which is consistent with previous results (Lee et al., 2021a). Alcohols are typically produced through the microbial fermentation of unsaturated fatty acids (Mancinelli et al., 2021). Conversely, carboxylic acid decreased significantly by day 28. These acids are recognized as key compounds that can serve as substrates for ester formation, which greatly affects the aroma profile of beef products (Lee et al., 2021a). Ketones, however, appeared to increase after aging commenced and maintained consistent levels without significant fluctuations throughout the remainder of the aging period. Esters, aldehydes, and furans all showed a decreasing trend during the aging period, whereas hydrocarbons increased until day 7 or 14 before declining again. Esters are produced by esterification reactions between alcohols and acids and are closely related to microbial esterase activity (Ozkara et al., 2019). Aldehydes, known for their significant role in beef aroma, contribute to sweet, floral, salty, and cheesy notes because of their low odor thresholds (Domínguez et al., 2019). Furans and hydrocarbons, which are odor-active volatiles, are formed by oxidation of fatty acids (Ozkara et al., 2019).
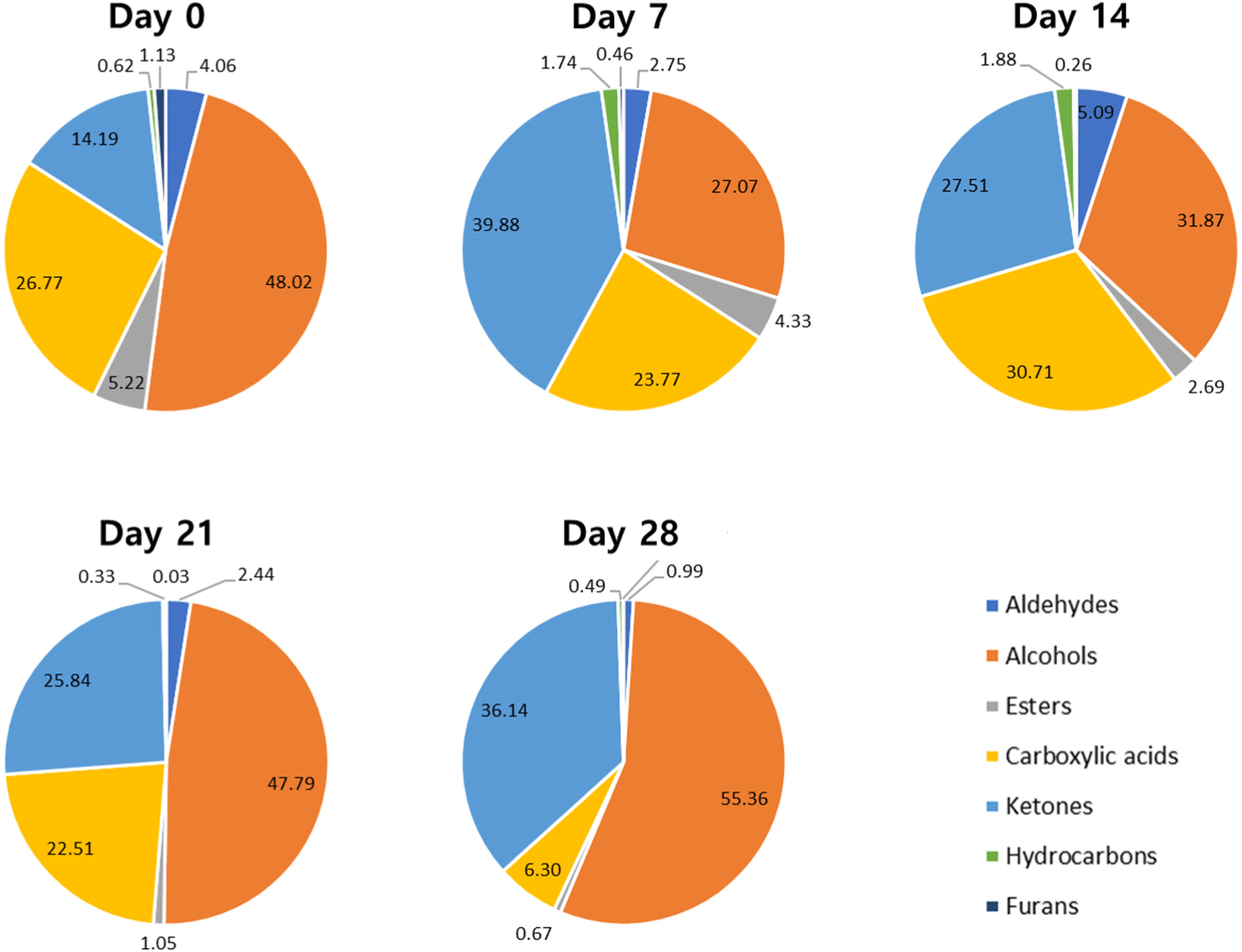
When examining the overall pattern of VOCs content changes in the aged JCC loin, the top 10 VOCs showed no consistent trends, while 11 and 12 VOCs exhibited increasing and decreasing trends during the aging process, respectively (Fig. 2A). PLS-DA analysis effectively separated the aged JCC loins based on aging time, accounting for 71.1% of the total data variance (Fig. 2B). The value of component 1 increased with aging time, indicating that VOC changes significantly occurred as aging time increased. A total of 20 VOCs contributed to the separation of each aged JCC loin, with VIP scores greater than 1.0 (Fig. 2C). Notably, 3-methylbutan-1-ol, 2-phenylethanol, 2-methylbutanal, and butane-2,3-diol were particularly influential in distinguishing between the samples, with their intensities increasing after 14 days of aging. Among these alcohols, 3-methylbutan-1-ol is associated with odors such as alcohol, banana, burnt, fruity, fusel, malt, oil, and whiskey. The 2-pheynlethanol contributes to bitter, floral, honey, lilac, rose, dried rose, and spice odors, while butane-2,3-diol is linked to buttery, creamy, fruity, and onion-like odors. Additionally, 2-methylbutanal is known for its aldehydic, chocolate, ethereal, fatty, malt, peach, and sour odors (Table 3). However, 3-methylbutan-1-ol and butane-2,3-diol have been identified as negative indicators of vacuum-packed beef during storage (Mansur et al., 2019). Consequently, aging JCC loin for more than 14 days may negatively impact its flavor, suggesting that careful management of the aging time is critical to maintaining desirable flavor profiles.
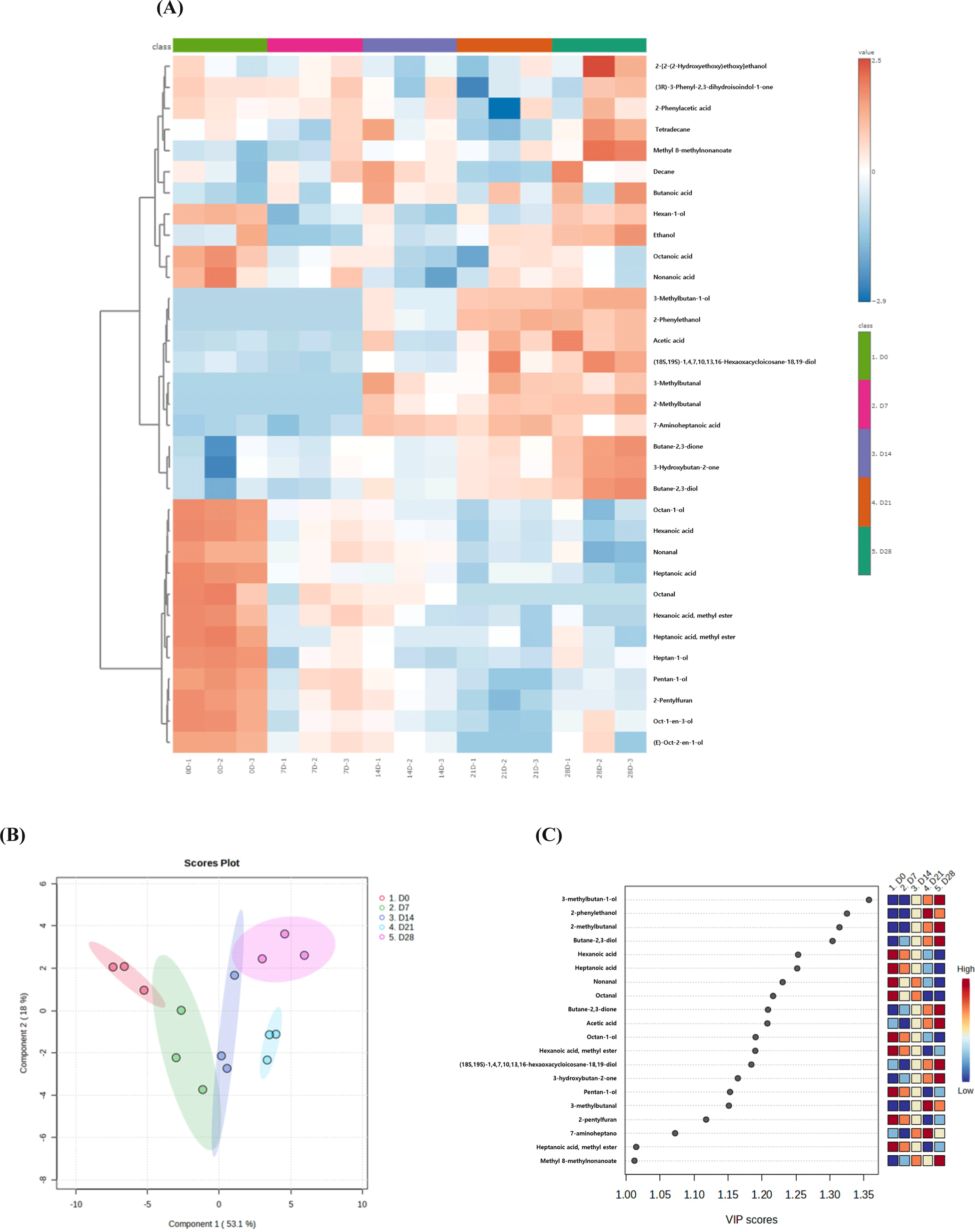
Muscle metabolites play a crucial role in shaping the physiological characteristics and meat quality traits of muscles, which are the primary determinants of their phenotypic features (Muroya et al., 2020). These metabolites include key low-molecular-weight hydrophilic and hydrophobic compounds, which are important nutrients and functional and flavor-associated compounds in meat. In this study, 26 metabolites were identified in the JCC loin during the aging process (Table 4). Most of these metabolites belong to the amino acid and peptide groups. During aging, 10 metabolites, namely asparagine, glutamine, isoleucine, leucine, methionine, tyrosine, valine, phenylalanine, hypoxanthine, and ethanol, increased (p<0.05), which is consistent with the findings of a previous study (Bischof et al., 2021). Postmortem aging typically leads to significant alterations in the levels of flavor precursors, including small peptides, free amino acids, and free fatty acids. These changes are driven by the action of endogenous enzymes such as proteolytic and lipolytic enzymes (Ha et al., 2019). The amino acids identified may serve as precursors to flavor compounds in cooked beef, each contributing distinct taste characteristics: asparagine and glutamine are associated with sweetness; isoleucine, leucine, phenylalanine, tyrosine, and valine contribute a combination of bitter and slightly sweet flavors; whereas methionine is linked to umami taste (Xu et al., 2023). Additionally, ethanol is frequently produced during vacuum packaging due to the fermentation activity of lactic acid bacteria (Toomik et al., 2023).
Meanwhile, only the IMP content of the aged JCC loin decreased as aging time increased (p<0.05). In particular, IMP content decreased dramatically between days 7 and 14, whereas hypoxanthine content increased significantly from day 0 to day 7 (p<0.05), consistent with previous studies (Bischof et al., 2021). IMP is a product of postmortem metabolism of adenosine triphosphate, which continuously degrades into inosine and hypoxanthine. These compounds are crucial for the formation and development of meat flavors.
Based on the change in pattern of metabolites, two subgroups were identified: one with a trend towards decreasing, and the others with trends increasing during aging (Fig. 3A). The metabolite data of aged JCC loin were effectively separated by aging time (Fig. 3B). Component 1 was strongly influential in this separation, accounting for 47.3% of variation. The pattern shifted from left to right along component 1, supported by increasing levels of valine, phenylalanine, isoleucine, asparagine, and leucine, and a decreasing level of IMP, with VIP score>1.2 (Fig. 3C). Therefore, the metabolites in the loin of JCC were primarily influenced by changes in amino acid levels during the aging process.
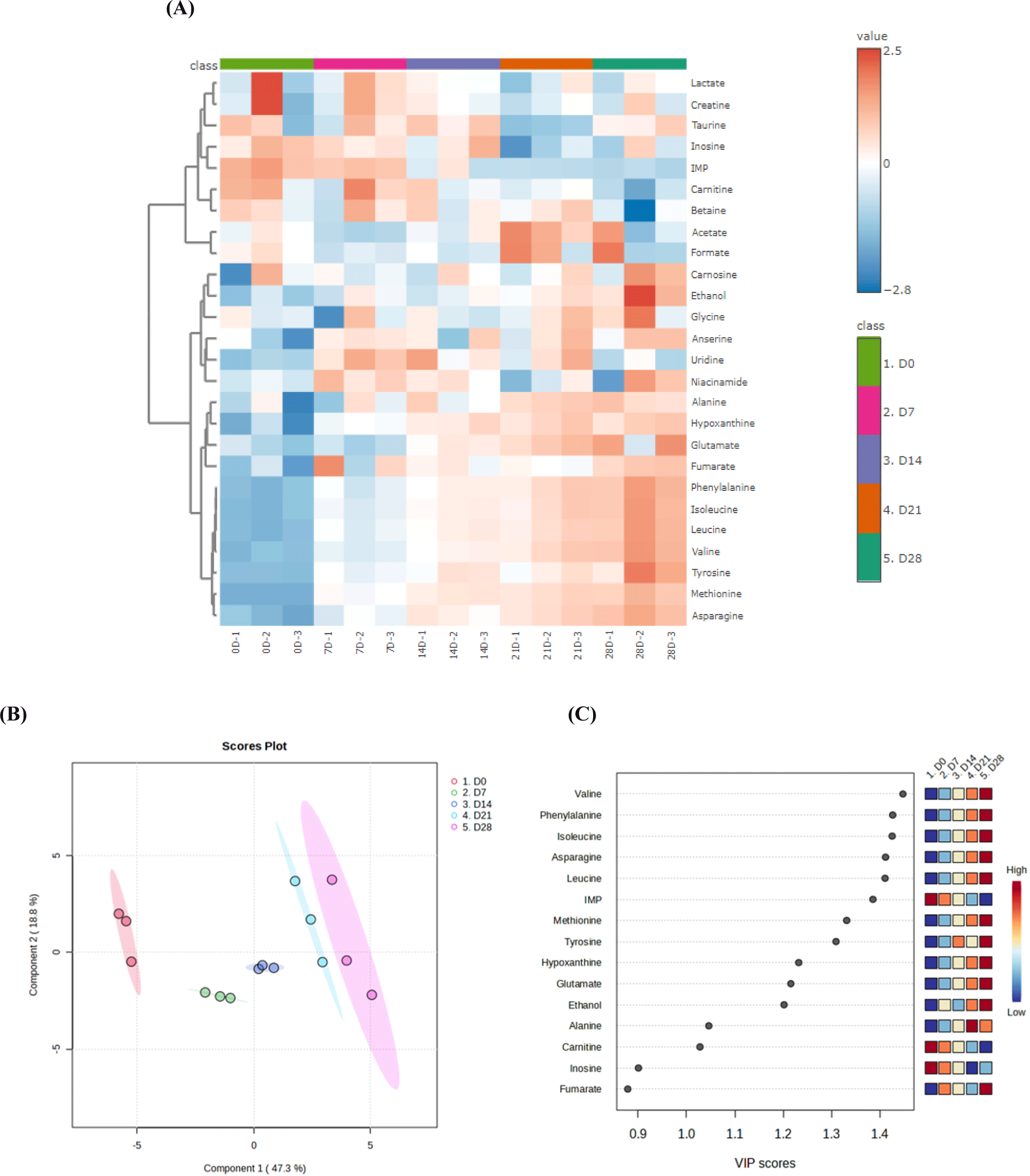
The overall taste profile of the aged JCC loin was analyzed using an E-tongue (Fig. 4). The radar plot for unaged JCC loin was distinctly different from those of other aged JCC loins (Fig. 4A). The taste patterns of JCC loin aged from day 7 to day 21 were similar, while the pattern for JCC loin aged to day 28 showed a clear separation. Except for NMS (umami), the scores of the taste sensors were higher in aged JCC loin than in unaged JCC loin, suggesting that aging enhances the overall taste profile of JCC loin. On day 0, the JCC loin had the highest IMP content compared to the aged JCC loin (p<0.05; Table 4). IMP, along with aspartic acid, cysteine, methionine, and glutamic acid, contributes to the characteristic umami flavor (Dashdorj et al., 2016). This may explain why NMS (umami) was highest in JCC loin at day 0. In contrast, the aged JCC loin at day 7 exhibited the highest SCS (bitterness), AHS (sourness), CTS (saltiness), NMS (umami), CPS (universal), and ANS (sweetness) among all aged JCC loins. The metabolite analysis showed that aged JCC loin at day 7 had relatively high contents of lactate, anserine, creatine, fumarate, uridine, and niacinamide (Fig. 3A). Lactate is associated with a sour taste, while creatine and anserine contribute to the flavor and juiciness of beef (Bischof et al., 2021). Fumarate, uridine, and niacinamide each contribute to the flavor of beef in various ways. Fumarate provides a slight sour taste (Shi et al., 2022), while uridine and niacinamide add complexity to the flavor and enhance the nutritional content of the meat (Chen et al., 2024). These compounds play important roles in shaping the overall taste profile of aged JCC loin at day 7.
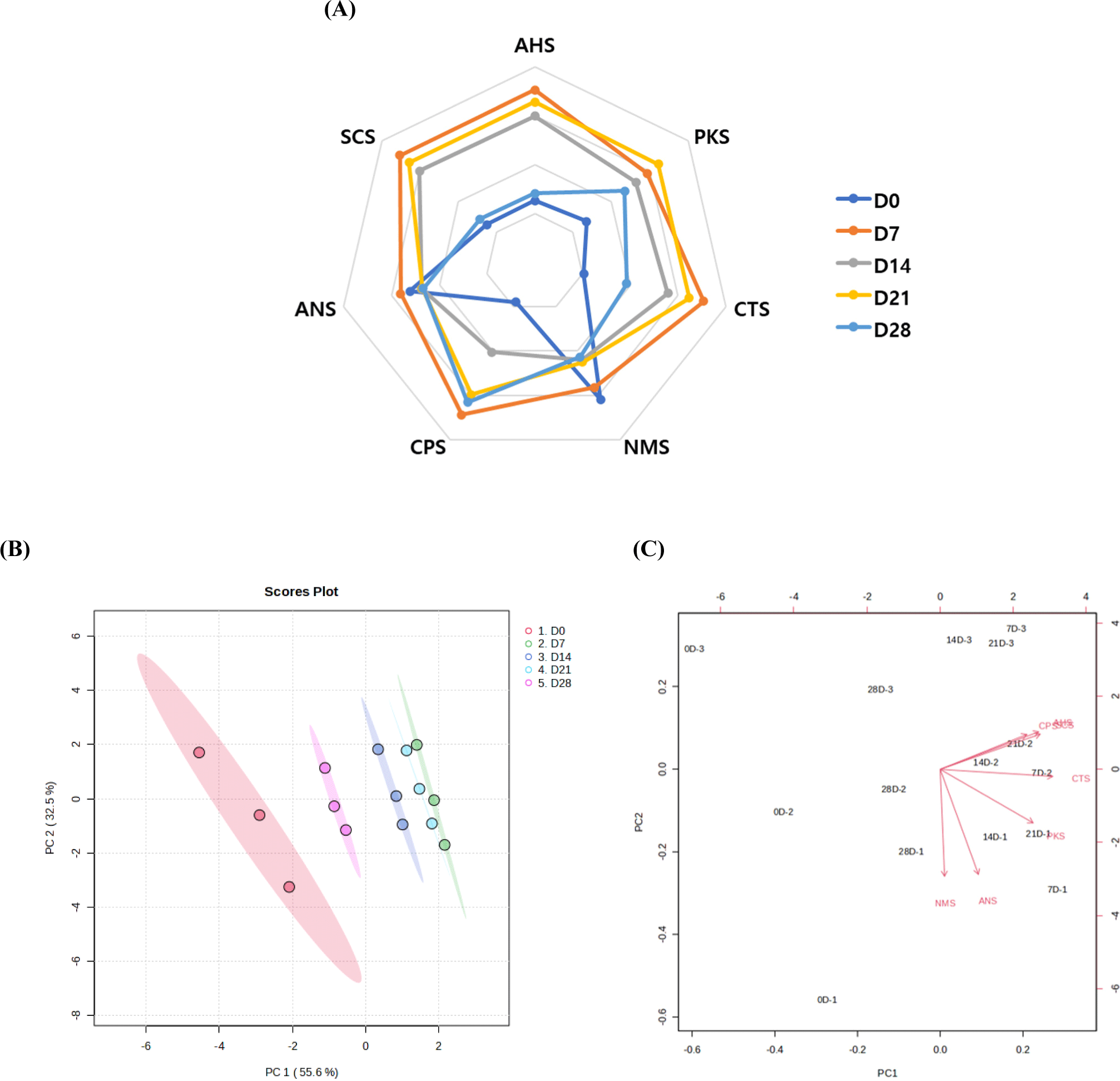
The PCA analysis was conducted to illustrate the variances among aged JCC loins based on individual E-tongue results (Fig. 4B). Principal Component 1 (PC1) and Principal Component 2 (PC2) accounted for 55.6% and 32.5% of the total variation, respectively. The groups of aged JCC loin at day 7 and day 14 were closely located on the positive side of PC1, indicating similarities in their taste profiles. In the PCA biplot (Fig. 4C), a larger number of taste sensors were associated with JCC loins aged for 7, 14, and 21 days. In the contrast, the JCC loin from day 0 and day 28 were positioned in the same quadrant, however, with fewer taste sensors values, indicating a distinct different in their taste profiles compared to the intermediate aging periods.
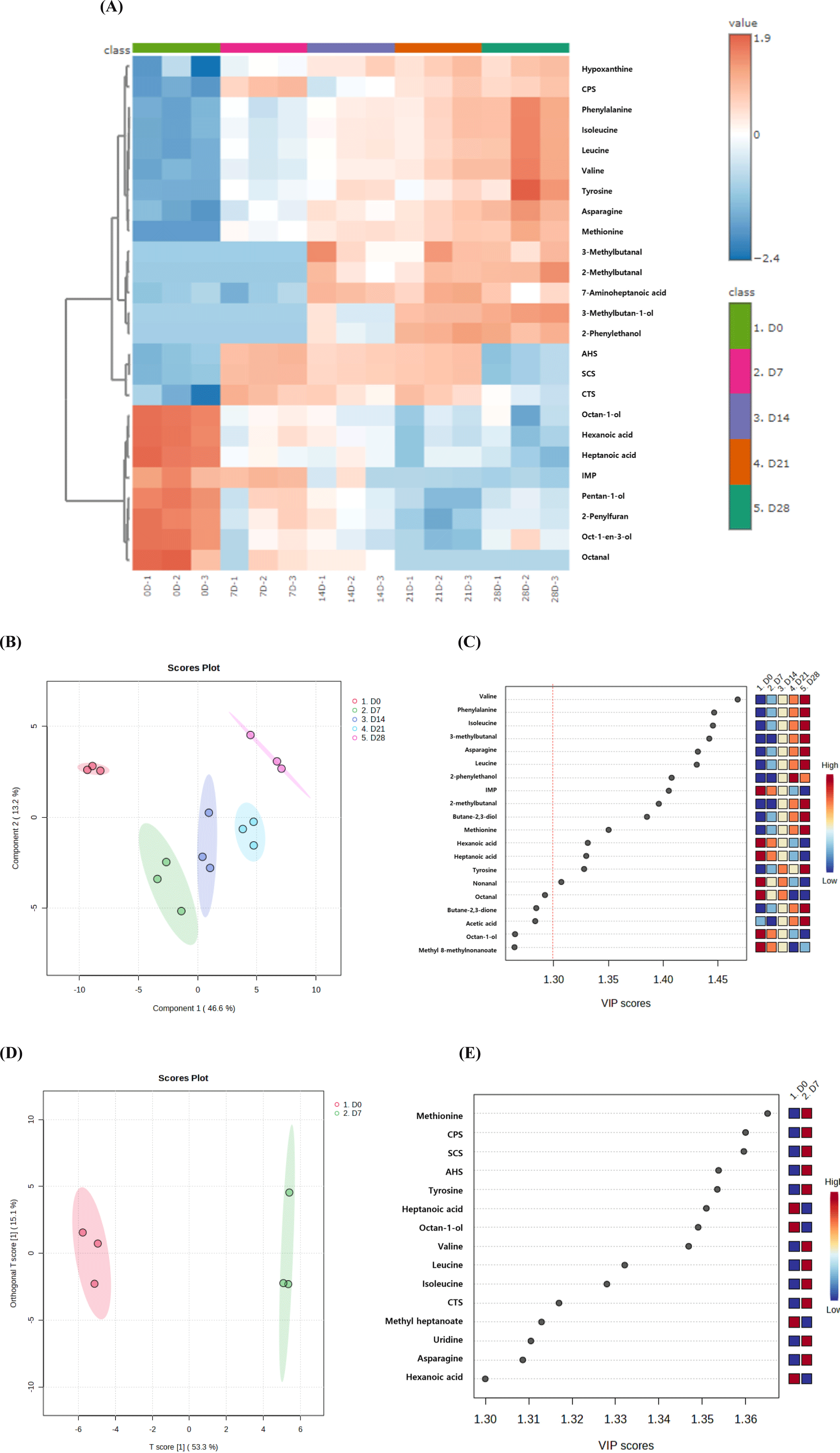
The top 25 flavor-related compounds (VOCs, E-tongue, and metabolites) that contributed to the separation of JCC loin samples during aging are shown in Fig. 5A. These compounds were divided into three subgroups: 14 compounds increased during aging, 8 compounds decreased, and the remaining 3 compounds showed both increasing and decreasing trends. Each JCC loin sample was distinctly classified according to aging time using PLS-DA (Fig. 5B). The aged JCC loin at day 7 was notably distant from the unaged JCC loin (day 0) and was grouped with the aged JCC at days 14 and 21, consistent with the E-tongue results (Fig. 4). Component 1 in the PLS-DA model was primarily influenced by several key metabolites and VOCs, such as valine, phenylalanine, isoleucine, 3-methylbutan-1-ol, asparagine, and leucine, which were present in high concentrations during aging (Fig. 5C). This suggests that the 7-day mark is acritical point in the aging process where significant changes in flavor occur in JCC loin. The OPLS-DA model between unaged JCC loin (day 0) and aged JCC loin at day 7 showed a clear separation with high accuracy (R2=0.972) and predictive power (Q2=0.908) values for the predictive component (Fig. 5D). The unaged JCC loin samples were clustered on the left, while the samples aged for 7 days clustered on the right. The highest VIP score indicated that methionine was the most influential variable in distinguishing between JCC loin at days 0 and 7 (Fig. 5E). Methionine is associated with an umami taste. Additionally, some fatty acids and alcohols (heptanoic acid, octan-1-ol, hexanoic acid) showed high concentrations (indicated in red on the heatmap) in unaged JCC loin, which is related to lipid metabolism. In contrast, certain amino acids (tyrosine, valine, leucine, isoleucine) and E-tongue sensor values (CPS, SCS, and AHS) were elevated (red in heatmap) in aged JCC loin at day 7, suggesting changes in amino acid metabolism and taste.
Conclusion
During the aging period, the physicochemical and sensory characteristics of JCC loin underwent changes: tenderness increased and flavor profile became more enriched. Notably, JCC loin aged for 7 days exhibited a distinct flavor pattern compared to unaged control, characterized by high concentrations of metabolites such as fatty acids and alcohols (heptanoic acid, octan-1-ol, hexanoic acid) as well as some amino acids (methionine, tyrosine, valine, leucine, isoleucine). However, after 14 days of aging, off-odor VOCs, such as 3-methylbutan-1-ol and butane-2,3-diol, began to emerge, potentially adversely affecting flavor. Additionally, aged JCC loin at day 7 showed the highest E-tongue sensor values among aged JCC loins, suggesting that day 7 is a critical point for flavor development in JCC loin during aging. Understanding the mechanisms responsible for the JCC beef aging process is important for researchers and the meat industry. This might give rise to the possibility of predicting and standardizing the quality of JCC beef enabling the industry to generate beef of repeatable high quality. However, further sensory evaluations involving consumers are necessary to directly assess the flavor characteristics of JCC loin subjected to wet aging. Also, further studies could explore the effect of different aging techniques or packaging methods on JCC loin quality.













