Introduction
Hypertension is a risk factor in the development of cardiovascular diseases such as coronary artery disease, left ventricular hypertrophy, valvular heart disease, atrial fibrillation, stroke, and arrhythmias such as renal failure. Without timely diagnosis and appropriate treatment, it can cause illness or death (Farooq and Ray, 2015; Kjeldsen, 2018). It is estimated that 1.4 billion people worldwide suffer from hypertension, but only 14% have it under control (WHO, 2021). Angiotensin I converting enzyme plays an important physiological role in regulating blood pressure through the reninangiotensin system (RAS; Lee et al., 2004). It can convert angiotensin I to angiotensin II, and can also inactivate the vasodilator bradykinin (Yang et al., 1970; Yang et al., 1971). Therefore, inhibiting the angiotensin-converting enzyme (ACE) reduces the activity of angiotensin II, although it can also lower blood pressure by increasing bradykinin levels (Pyo and Lee, 2007).
ACE inhibitory activity is considered an effective way to treat hypertension. Synthetic ACE inhibitors such as Captopril, Alacepril, Lisinopril, and Enalapril are currently being used to treat hypertension, but they have undesirable side effects such as dysgeusia, rash, coughing, hypotension, renal failure, and hyperkalemia (Brown and Vaughan, 1998; Chakraborty and Roy, 2021; Xia et al., 2020). In contrast, naturally occurring ACE inhibitors are considered safe (Chen et al., 2022). To date, ACE inhibitors have been found in fermented milk (Rendón-Rosales et al., 2022), fish surimi (Oh et al., 2020), rabbit meat (Chen et al., 2022), and abalone viscera (Iwamoto et al., 2023), and ACE inhibitory peptides have been isolated from these foods and other sources. ACE inhibitory peptides derived from milk proteins are produced by enzymatic hydrolysis, microbial fermentation or genetic engineering (Castellano et al., 2013). In particular, many lactic acid bacteria produce ACE inhibitory peptides, such as Val-Pro-Pro- and Ile-Pro-Pro, from fermented milk (Hirota et al., 2007; Mizushima et al., 2004). Bioactive peptides derived from fermented milk by Lactobacillus helveticus have been reported to decrease the blood pressure of spontaneously hypertensive rats (SHR; Narva et al., 2004). In addition, ACE inhibitory peptides derived from the enzymatic hydrolysis of milk protein have been observed to decrease blood pressure in hypertensive mice (Ramchandran and Shah, 2011; Wang et al., 2012). The aim of this study was to investigate whether pasteurized fermented milk by Lactiplantibacillus plantarum K79, which exhibits ACE inhibitory activity, has an effect on lowering blood pressure in hypertensive rats and to investigate biomarker changes in their blood.
Materials and Methods
L. plantarum K79 was isolated from kimchi. This strain was selected after screening it for probiotic properties such as acid and bile tolerance, antibacterial activity, antibiotic tolerance and ACE inhibitory activity. The culture was maintained in an MRS broth (Difco, New York, NY, USA), and the patent accession number is KACC 81222BP.
Skim milk powder (13.5%) and 1% glucose were mixed, sterilized at 90°C for 5 min, and then cooled to 37°C. At this time, lyophilized L. plantarum K79 strain activated for 1 h was inoculated and cultured with stirring at 37°C until the pH reached 4.2. The culture was heat-treated by stirring at 85°C for 20 min, cooled to below 50°C, and then spray-dried and powdered. The powdered raw materials were suspended and used for testing.
The experiment included control groups composed of 5-wk-old SHR (SLC, Tokyo, Japan) male rats, and normal groups (NGs) composed of Wistar-Kyoto rats (WKY), which were used after 1 wk of acclimatization. The experimental animals were fed with the AIN-93G diet, and during the acclimatization period the filtered drinking water was changed every day so that it could be consumed freely. During the breeding period, the temperature was 23±1°C, humidity was 50±5%, noise was less than 60 phones, lighting time was 08:00–20:00 (12 h a day), illuminance was 150–300 Lux, ventilation was 10–12 times per hour. This experiment was conducted with the approval of the Korea Food Research Institute (KFRI-M-22024) in compliance with the regulations on animal experimentation ethics. During the breeding period, the experimental animals were fed with regular solid feed (Samtako, Osan, Korea), and their blood pressure was measured at the end of the acclimatization period. Individual identification was indicated.
Experimental group: NG (WKY): distilled water, control group (SHR): distilled water, high treatment group (HTG; SHR): 500 mg/kg/day, medium treatment group (SHR): 335 mg/kg/day, low treatment group (SHR): 170 mg/kg/day, positive control group (PCG; SHR): Enalapril, 10 mg/kg/day. Each group used in the experiment consisted of 8 animals. At this time, the oral administration method, which is the clinical application route of this formulation, was selected and each sample was directly administered into the stomach of each experimental animal.
All the experimental animals were weighed once per week before the start of administration, after the start of administration, and until the end of the test. For each breeding box, the total amount of feeding and watering on the day and the remaining amount the next day were measured once per week for 4 wk after the start of administration, and the amount of daily consumption was indicated for each group. The systolic pressure of each rat was measured with a tail cuff blood pressure meter (INDIR, Model LE5002, Panlab, Barcelona, Spain), and was maintained at 32°C for 30 min to detect the pulse of each rat’s tail artery before measurement.
At the end of the 10-wk experimental period, blood samples were collected from all the rats and immediately placed in sterile tubes. Serum was collected by centrifugation at 2,000×g for 15 min at 4°C. The serum samples were then analyzed for total cholesterol (TC), triglyceride (TG) concentration, high density lipoprotein (HDL) cholesterol, and low density lipoprotein (LDL) cholesterol by GCCL (Yongin, Korea). Also, the liver, kidneys, lungs, heart and testes of the rats were quickly isolated after sacrifice and weighed.
The angiotensinogen (AGT) protein expression, angiotensin II protein, and renin protein content in the plasma were measured using a Rat AGT ELISA kit, a Rat Angiotensin II ELISA kit, and a Rat Renin ELISA kit (MyBioSource, San Diego, CA, USA), respectively.
According to the method of Averill et al. (2003), kidney tissue was left in 4% formalin for 48 h before being transferred to 70% ethanol. Blocks of cardiac tissue were imbedded in paraffin; 5 μm sections were transferred to slides and the paraffin was removed by sequential washes with xylene, 100% ethanol, 95% ethanol, 75% ethanol, and double-distilled water. Sections of tissue were incubated with 3% hydrogen peroxide for 5 min, washed with PBS (pH 7.2), dried, and then incubated with 5% normal goat serum for 1 h at room temperature. The sections were washed with PBS and incubated overnight at 4°C with an affinity-purified rabbit polyclonal antibody to angiotensin-1 at 1:25 dilution of the antibody in 1% BSA. The Angiotensin-1 antibody was purified. The next day, the tissues were washed with PBS and incubated for 3 h at 4°C with a biotinylated anti-rabbit antibody at a dilution of 1:400 in 1% BSA. The slides were rinsed with PBS, blotted dry, and reacted immunocytochemically by avidin-biotin solution and stained brown with 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA) in Tris buffered saline (0.05 mol/L, pH 7.6 to 7.7). The reaction was stopped in PBS, and the sections were rinsed in double-distilled water before being counterstained with hematoxylin (Sigma-Aldrich). The tissue sections were dehydrated in ethanol (70% to 100%) and then Histoclear (National Diagnostics, Atlanta, GA, USA). Finally, they were mounted under coverslips with Histomount.
The results are expressed as the mean±SD per experimental group using SPSS version 23 (IBM, Armonk, NY, USA). The significance of the differences was analyzed by conducting a one-way analysis of variance (ANOVA) using Duncan’s multiple range tests. Significance was considered at p<0.05.
Results and Discussion
Looking at the change in weight according to the experimental period, there was a significant difference between the NG (WKY), which was fed with distilled water, and the hypertensive rat (SHR) group within the same period (Fig. 1). This was found to be consistent with Gattone’s (1986) report that WKY had a similar weight to SHR at birth, but that its weight became greater than that of SHR over time. However, there was no significant difference in average body weight between the treatment group and the control group, indicating that milk fermented by L. plantarum K79 used in the experiment did not have a significant effect on the weight of the rats.
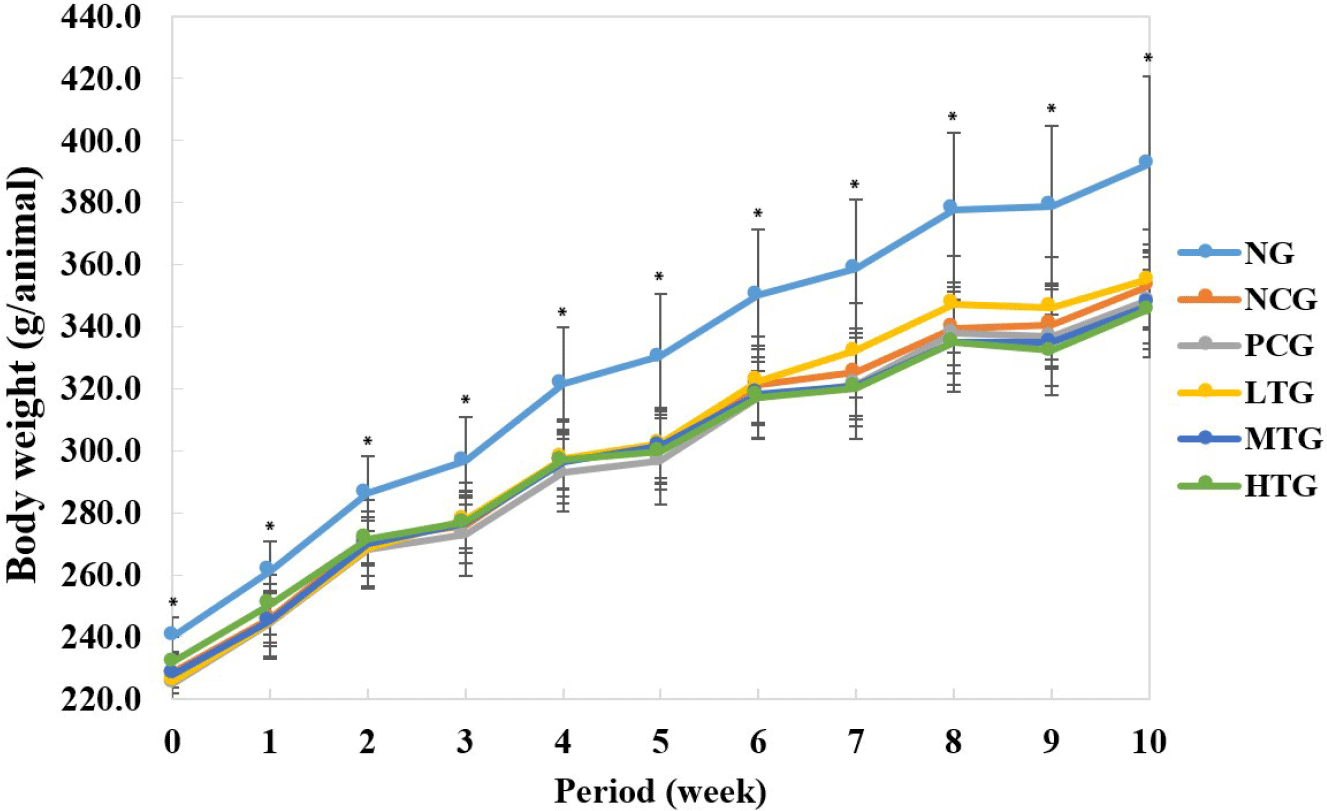
There was a slight difference in intake, but no significant change was observed, indicating that the fermented milk containing L. plantarum K79 had nothing to do with the weight gain of the rats. However, at 7 wk of feeding, the low-treatment group showed a significant increase compared to the normal and medium, high-treatment groups (Table 1).
SHR, spontaneously hypertensive rats; NG, normal group (WKY) with distilled water; NCG, negative control group (SHR) with distilled water; PCG, positive control group (SHR) with Enalapril; LTG, low treatment group (SHR); MTG, medium treatment group (SHR); HTG, high treatment group (SHR); WKY, Wistar-Kyoto rats.
The results of measuring the blood pressure according to the experimental period showed that there was a significant change in blood pressure in the NG, followed by the PCG, treatment group, and negative control group (NCG), until 10 wk. When comparing the treatment group and the NCG, the treatment group showed a tendency to lower blood pressure until 6 wk, but it was found that there was no significance (Table 2). However, after 8 wk, blood pressure was lowered more significantly in the HTG (209.9±13.3 mmHg) than in the NCG (230.8±7.3 mmHg). Ishiguro et al. (2012) reported that systolic blood pressure (SBP) and diastolic blood pressure were reduced by more than 30 mmHg and 20 mmHg, respectively, after oral administration of purified sweet potato protein hydrolyses to SHR. Tsai et al. (2006) found that the SBP of SHR decreased by 19 mmHg after 8 wk of oral administration of fermented soymilk with lactic acid bacteria. These results were similar to the results obtained in this study. According to Alshuniaber et al. (2021) and Yuan et al. (2022), this drop of blood pressure in rats is related to inflammation and could be obtained by inhibiting the ACE activity of the RAS.
NG, normal group (WKY) with distilled water; NCG, negative control group (SHR) with distilled water; PCG, positive control group (SHR) with Enalapril; LTG, low treatment group (SHR); MTG, medium treatment group (SHR); HTG, high treatment group (SHR); WKY, Wistar-Kyoto rats; SHR, spontaneously hypertensive rats.
Regarding blood lipids, TC, HDL cholesterol and LDL cholesterol were all significantly higher in the NG than in the hypertensive rat group, while TG was significantly lower in the NG (Table 3). This finding was consistent with the report of Cho et al. (2018), who observed that the SHR group had lower TC and HDL-C levels than the WKY group. Among the blood pressure rat groups, TC and LDL cholesterol were slightly higher in the medium-dose treatment group, but the levels of HDL cholesterol and TG were insignificant.
TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; NG, normal group (WKY) with distilled water; NCG, negative control group (SHR) with distilled water; PCG, positive control group (SHR) with Enalapril; LTG, low treatment group (SHR); MTG, medium treatment group (SHR); HTG, high treatment group (SHR); WKY, Wistar-Kyoto rats; SHR, spontaneously hypertensive rats.
When examining the weight of each organ of the SHR euthanized 10 wk after administration of the fermented product, the weight of the liver in the PCG was found to be significantly higher than that in the NG, while the weight of the kidneys was significantly lower in the NG than in the other groups (Table 4). There was no significant change in the weight in the lungs of all groups, and the weight of the heart was significantly higher in the NCG and the treatment group than in the PCG and the NG. The organs of the NG weigh less than those of the other groups, but the testes weigh significantly more. This was consistent with the report by Walter and Hamet (1986) that the heart, kidneys and liver weighed less in WKY than in SHR.
NG, normal group (WKY) with distilled water; NCG, negative control group (SHR) with distilled water; PCG, positive control group (SHR) with Enalapril; LTG, low treatment group (SHR); MTG, medium treatment group (SHR); HTG, high treatment group (SHR); WKY, Wistar-Kyoto rats; SHR, spontaneously hypertensive rats.
As is well known, Ang II is produced systematically. AGT, a substrate of RAS, is released by the liver and broken down in the circulation to form Ang I by renin secreted by the proximal glomerular apparatus of the kidneys. Vasodilator I (Ang I) is then readily activated, and Ang II is caused by ACE, which is expressed at high levels primarily on the surface of endothelial cells in the pulmonary circulation. When the concentration of these biomarkers increases, it affects the increase in blood pressure (Ichihara et al., 2004; Yim and Yoo, 2008). Table 5 shows changes in blood pressure-related biomarkers in the plasma of rats after 10 wk. It was confirmed that the NG, PCG, and HTG had significantly lower AGT protein expression than the NCG. Angiotensin-II showed significantly lower expression in the NG, PCG, and treated groups than in the NCG. Renin also was expressed the least in NG compared to NCG, followed by PCG and HTG. Therefore, it is judged that significantly suppressing blood pressure-related biomarker protein expression had the effect of lowering the blood pressure of the treatment group.
NG, normal group (WKY) with distilled water; NCG, negative control group (SHR) with distilled water; PCG, positive control group (SHR) with Enalapril; LTG, low treatment group (SHR); MTG, medium treatment group (SHR); HTG, high treatment group (SHR); WKY, Wistar-Kyoto rats; SHR, spontaneously hypertensive rats.
Human angiotensin is expressed in the tissues of diverse organs including those of the liver, heart, blood vessel walls, brain, and kidneys, and in adipose tissue. Although all organ systems have certain components of the RAS, the kidneys have all the components of the RAS, and also show compartmentalization and intracellular accumulation of tubular and interstitial networks (Kobori et al., 2007)
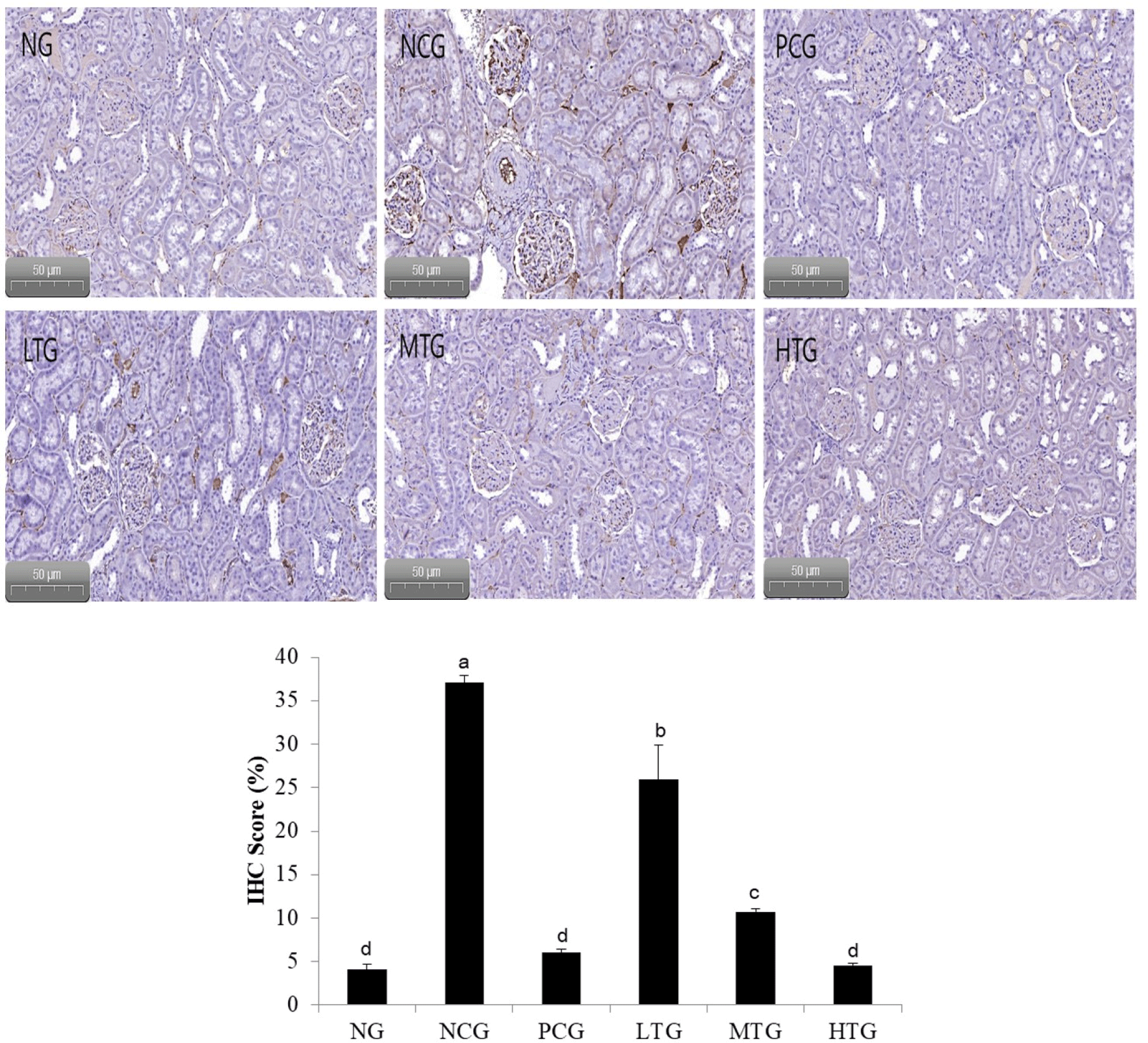
Fig. 2 shows the results of an experiment confirming the expression of angiotensin-I using immunohistochemistry. Looking at the intrarenal glomeruli, it was found that the protein expression of angiotensin-I in the hypertensive rats fed only with distilled water, i.e. the NCG, was increased to a visually observable level compared to the normal rats, the PCG, and the treatment group. Therefore, it was observed that suppressing the protein expression of angiotensin-I had an antihypertensive effect on the treated group.
Conclusion
The aim of this study was to investigate whether pasteurized milk fermented by L. plantarum K79, which exhibited ACE inhibitory activity, has the effect on lowering the blood pressure of hypertensive rats and to investigate biomarker changes in their blood. The reduction in blood pressure was significant up to 10 wk in the following order: NG, PCG, treatment group, and NCG. After 8 wk, the blood pressure of HTG was significantly lower than that of NCG. The treatment group has an effect of lowering blood pressure by significantly suppressing blood pressure-related biomarker protein expression than NG. The protein expression of angiotensin-I in the kidney of hypertensive rats fed only with distilled water, i.e. the NCG, was increased to a visually observable level compared to the other groups. Therefore, the results of this study suggest that the fermented product by L. plantarum K79 can be applied as an antihypertensive material.













