Introduction
Pork fat and meat are generally used as ingredients for sausages, Frankfurt sausages, canned meat, and other foods to improve flavor and texture (Hsieh and Gajewski, 2016). Food manufacturers use pork fat tissue as an ingredient to increase weight and taste because it is cheap and readily available (Aida et al., 2005). Meat mixed with pork fat tissue or other meat is a general method used in food industries to gain economic benefit. In 2013, after equine DNA was found in frozen beef hamburger patties sold in several supermarkets in Ireland and the UK, a full investigation found pork DNA (O’Mahony, 2013). In addition, pork DNA was also detected in chocolate, which had received JAKIM (Jabatan Kemajuan Islam Malaysia) halal certification in Malaysia in 2014 (Jaques, 2015). In Korea, beef jerky labeled as 100% beef has been found to contain 45% pork (Han et al., 2020). In particular, pork is much cheaper than beef in Korea, so beef hamburger patties and beef tteok-galbi mixed with pork meat or fat are often sold as pure beef products (Heo et al., 2014).
Pork fat and meat are not harmful to health, but meat and non-meat products containing pork fat and flesh can cause severe problems for Muslims, Jews, and vegetarians (Al-Teinaz, 2020). In addition, food fraud using pork fat tissue is challenging to detect because it resembles other animal fats once mixed with ground meat and food (Kim et al., 2017). Therefore, reliable and sensitive analytical methods are required to detect and identify pork fat and meat that can be mixed in food. Various analytical methods, including immunoassay, molecular techniques, and chromatographic methods, have been well-developed for detecting porcine meat (Hsieh and Ofori, 2014; Zvereva et al., 2015). A few methods, such as electronic nose, gas chromatography, and Fourier transform infrared spectroscopy, have been developed for pork fat and lard detection (Man et al., 2005; Nurjuliana et al., 2011; Rohman et al., 2011). However, immunoassays for detecting pork fat tissue have not yet been reported.
We previously reported the antigenicity of thermal stable-soluble proteins (TSSPs) in pork fat and meat, and the protein profiles from the pork fat and meat extracts by non-heating treatments (Kim et al., 2016; Kim et al., 2017). Additionally, we reported the development of monoclonal antibodies specific to TSSP from pork fat tissues (Kim et al., 2017). This study reports an indirect enzyme-linked immunosorbent assay (iELISA) based on the previously reported monoclonal antibody (mAb) to detect mixed pork fat tissue in heat-processed beef meatballs. This study, we investigated the adsorption rate of proteins to various 96-well microtiter microplates to improve the sensitivity of the iELISA and applied it to detect pork fat tissue mixed in beef meatballs heat-processed such as autoclaving, steaming, roasting, and frying.
Materials and Methods
Fats (pork, beef, chicken, duck, sheep horse, and goat), meats (pork, beef, chicken, duck, turkey, sheep, horse, and goat), egg, and soybean were purchased from local supermarkets and farms (Jinju, Korea). Bovine serum albumin (BSA) and ABTS [2,2′-azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid)] were obtained from Sigma-Aldrich (St. Louis, MO, USA). Tris [2-Amino-2-(hydroxymethyl)-1,3-propanediol] was purchased from Roche (Indianapolis, IN, USA). Goat anti-mouse IgG (H+L) peroxidase conjugate was obtained from Thermo Fisher Scientific (Rockford, IL, USA). The filter paper (Whatman No. 4) was purchased from Whatman (Buckinghamshire, UK). 96-well Nunc-Immuno MaxiSorp® (Thermo Fisher Scientific), 96-well ELISA microplate, MICROLON® 600 and 96-well single-break strip ELISA plates, MICROLON® 600 (Greiner Bio-One, Kremsmüenter, Austria), 96-well ELISA plate (Jet Biofill, Guangzhou, China), and 96-well immunoplate strip (SPL Life Sciences, Pocheon, Korea) were used to investigate the adsorption rate of proteins depending on different microtiter plates. The 12-channel microplate washer and Spark 10M multimode microplate reader were obtained from TECAN Trading AG (Switzerland, Männedorf).
Our previous report presented the monoclonal hybridoma PF 2B8-31 which produces a mAb specific to TSSP in porcine adipose tissue (Kim et al., 2017). For mass production of mAb, the hybridoma cell was grown in 10% FBS/DMEM, and 1×107 cell/mL of the hybridoma was intraperitoneally injected into BALB/c mice that had been pretreated with an intraperitoneal injection of 0.5 mL pristane. After 1 week, ascites fluid was obtained from the mice and purified by saturated ammonium sulfate precipitation followed by protein A affinity chromatography. The purified mAb was lyophilized and stored at –20°C before use. All animal treatments were performed with approval from the Institutional Animal Care and Use Committee (IACUC) at the researcher’s institution in Gyeongsang National University, Jinju, Korea (GNU-221103-M0153-01).
This study used raw and cooked fat and meat samples. Pure fats were prepared by trimming off visible meat and connective tissues, and lean meats were obtained by trimming off visible fat and connective tissues. In order to prepare cooked samples, pure fat and meats were placed in a glass beaker and then double-heated in boiling water for 15 min. Fat and meat without any processing were used as raw samples. For TSSP extraction, 10 g of cooked and raw fat and meat were mixed with 20 mL of 0.025 M Tris-buffered saline (TBS, pH 7.4) and homogenized for 5 min using a homogenizer (D-500, Wiggen Hauser, Berlin, Germany). The homogenized samples were heated at 100°C for 15 min, cooled to room temperature, and centrifuged at 3,220×g at 4°C for 15 min. The supernatant containing TSSP was filtered through Whatman No. 1 filter paper. The fat and meat samples were extracted at 4°C for 1 h, prepared as described above, and used as control tests. The total soluble protein in the filtrates was quantified using a Quick StartTM Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
An iELISA based on PF 2B8-31 mAb for the detection of TSSP in pork fat tissue was optimized by checking the incubation temperature (4°C and 37°C), time (1 h and overnight) and buffers for the coating and blocking steps, as well as the dilution times of PF 2B8-31 mAb and a second antibody and the time for color development. First, five buffers [0.05 M phosphate-buffered saline (PBS, pH 7.4), 0.1 M carbonate buffer (pH 9.6), 0.5 M sodium chloride (NaCl, pH 6.5), 0.025 M TBS (pH 7.4), and 0.02 M Tris-hydrochloride (Tris-HCl, pH 7.4)] were tested as a dilution buffer for the extracts, which are used as tested samples. Ten grams of each pork fat tissue and beef meat were extracted by the method described above and used as 100% pork fat and 100% beef meat solutions, respectively. The standard pork fat tissue solutions (100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, and 0%, w/w) were prepared by diluting 100% pork fat with 100% beef meat solution. The wells of the microplate were coated using the extracts of pork fat tissue (100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, and 0%, w/w) and incubated at 4°C overnight or 37°C for 1 h. Two different blocking buffers, skim milk and BSA (0.5%−2%), were used to investigate blocking effects on the residual surface of the wells coated with extracts. The purified mAb 2B8-31 diluted with PBS (1:1,000, 1:2,000, 1:4,000, and 1:6,000) and horseradish peroxidase-conjugated goat anti-mouse IgG diluted with PBS (1:2,000, 1:4,000, 1:6,000, and 1:8,000) were tested to optimize the iELISA. Finally, a step of color development was performed at 37°C for 10−30 min. After performing the iELISA, absorbance was measured at 405 nm, and each condition showing the highest sensitivity was chosen as an optimal condition for the iELISA. Additionally, the test sample exhibiting an absorbance of 0.2 less, corresponding to the absorbance of the negative sample+5 SD of the absorbance of the negative sample, was judged to be negative in iELISA (Kim et al., 2023).
The sensitivity of the PF 2B8-31 mAb based-iELISA was measured by analyzing the standard pork fat tissue solutions (100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, and 0%, w/w). Fats (pork, beef, chicken, duck, sheep horse, and goat), meats (pork, beef, chicken, duck, turkey, sheep, horse, and goat), egg yolk, and egg white were also extracted with the same method previously described and analyzed to investigate the specificity of the iELISA.
In addition, five kinds of 96-well microplates (96-well Nunc-Immuno MaxiSorp®, 96-well ELISA microplate, 96-well single-break strip ELISA plate, 96-well ELISA plate, 96-well immunoplate strip) were used to determine the rate of protein adsorption to the wells, which can affect the sensitivity of the iELISA. The sample solution (100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, 0.01%, and 0% w/w) was coated on each well, reacted at 37°C for 1 h, and washed 3 times with PBST. After blocking each well with 1% BSA (200 μL), the blocking step was carried out at 37°C for 1 h, and the wells were washed 4 times with PBST. A 1,000-fold diluted PF 2B8-31 mAb solution was added to each well, reacted at 37°C for 1 h, and washed 5 times with PBST. A secondary antibody (horseradish peroxidase-conjugated goat anti-mouse IgG) diluted 1:5,000 in PBS was put into each well, reacted at 37°C for 1 h, and washed 6 times with PBST. Then the color development was performed by adding a substrate solution (100 μL) [3 mg of 2,2’ -azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) and 7 μL of 30% hydrogen peroxide in 10 mL of citrate buffer, pH 4.0] to the wells and incubating at 37°C for 30 min. The protein adsorption ratio of each microtiter plate well was evaluated through absorbance measurement at 405 nm. A 96-well microtiter microplate well showing a constant and high protein adsorption rate was selected to enhance the sensitivity of the PF 2B8-31 mAb based-iELISA.
Beef meatballs were prepared using a slightly modified method using reference (Huang et al., 2005). The beef and pork fat were separately cut into small pieces and ground using a commercial meat grinder. Based on 100 g, pork fat to beef meat ratios were 100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, and 0% (w/w), and beef meatballs were prepared by well mixing pork fats in beef meat and weighted into 10 g.
The beef meatballs (10 g) prepared were processed by autoclaving, steaming, roasting, and frying with different processing times and used to validate whether the pork fat tissue could be detected by the iELISA based on PF 2B8-31 mAb (Li et al., 2020; Mandli et al., 2018). Ten grams of the processed samples were put into a glass flask, crushed using a glass rod, and mixed with 20 mL of 0.025 M TBS (pH 7.4). The mixtures were vortexed for 30 sec and heated in boiling water for 15 min. The samples were centrifuged at 4°C for 15 min at 3,220×g, the supernatants were filtered through a filter paper (Whatman No. 4), and the filtrates were subjected to the iELISA.
All data was performed in triplicate and analyzed using SigmaPlot 10.0.1 for Windows (Systat Software, Palo Alto, CA, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA). The analysis of variance (ANOVA) with the Tukey test, equality of variances, and descriptive statistics functions of SPSS 27.0 software (IBM, Chicago, IL, USA) were used to clarify significant differences among the groups.
Results and Discussion
For efficient extraction of TSSP from fat and meat samples, raw and processed fats and meats were extracted at 4°C for 1 h or 100°C for 15 min. The concentration of total soluble protein in the extracts is presented in Table 1. When raw fat and meat were extracted, the total protein concentrations of the extracts through the cold extraction (4°C for 1 h) ranged from 3.2 to 17.2 mg/mL, which were much higher than those (0.3–1.9 mg/mL) obtained of the extract through the hot extraction (100°C for 15 min). Meanwhile, in the case of cooked fat and meat, the total protein concentrations of the extracts through the cold extraction ranged from 0.04 to 0.8 mg/mL, which were much lower than the total protein concentration (0.1–1.0 mg/mL) obtained through the hot extraction method. Notably, the total soluble protein concentration of the processed fat and meat extracts by hot extraction was much higher than that of the extracts treated with cold extraction. The hot extraction may have increased the protein extraction efficiency because the fat and meat tissue are expanded by heat to form a space, and the extraction buffer penetrates this space to increase the contact area (Kim et al., 2023). This result means that hot extraction is more effective than cold extraction for extracting TSSP to be analyzed from processed fat and meat. Therefore, the hot extraction method (100°C for 15 min) was applied to the samples used in the subsequent experiments.
1) Prepare 10 g of fat and meat (cooked and raw), homogenize and mix with 20 mL of 0.025 M TBS (pH 7.4).
2) Cold extraction: samples were extracted at 4°C for 1 h, hot extraction: samples were extracted at 100°C for 15 min.
The PF 2B8-31 mAb based-iELISA was optimized by key experimental factors, such as coating, blocking, primary antibody, and secondary antibody steps. The standard pork fat tissue solutions (0%, 10%, 30%, and 100%, w/w) were prepared and used as representative samples to optimize the iELISA. This study, used 5 kinds of buffers [0.05 M PBS (pH 7.4), 0.1 M carbonate buffer (pH 9.6), 0.5 M NaCl (pH 6.5), 0.025 M TBS (pH 7.4), and 0.02 M Tris-HCl (pH 7.4)] as extraction buffers to compare the extraction efficiency of TSSP from pork fat tissues. The samples (5 g) were homogenized with 10 mL of the buffers in a glass tube and extracted for 15 min in boiling water. After cooling at room temperature, the samples underwent centrifugation and filtration according to the previously mentioned. The filtered samples were used for coating the 96-well microplates and analyzed by iELISA. Supplementary Fig. S1 shows the extraction effect and antigenicity changes of pork fat TSSP extracted from beef meatballs by the 5 extraction buffers. In the 100% pork fat tissue sample, 0.5 M NaCl and 0.025 M TBS showed the highest and most similar absorbance, but in the case of 30% and 10% (w/w) pork fat tissue in beef meat, the absorbance in 0.025 M TBS buffer was significantly higher than 0.5 M NaCl. Although 0.05 M PBS and 0.02 M Tris-HCl showed lower absorbance values in 100% pork fat tissue than 0.5 M NaCl, both buffers showed higher absorbance values in 30% and 10% (w/w) pork fat tissue in beef meat than 0.5 M NaCl. However, 0.025 M TBS was the most effective buffer for the extraction effect and antigenicity improvement for pork fat TSSP. In this study, heat treatment was performed in boiling water for 15 min to extract pork fat TSSP. The result demonstrated that the extraction effect and antigenicity of pork fat TSSP differed by buffers and heat treatment. The results also showed a tendency similar to those reported (Fowler et al., 2012), indicating that the immunoreactivity of proteins could be recovered by heating in buffers at high temperatures (Fowler et al., 2011).
The optimized conditions of PF 2B8-31 mAb based-iELISA were as follows: 0.025 M TBS (pH 7.4) as an extraction and coating buffer, 0.5% skim milk as a blocking solution, PF 2B8-31 mAb diluted 1:2,000 (0.05 μg/100 μL/well) in PBS as a primary antibody, and horseradish peroxidase-conjugated goat anti-mouse IgG diluted 1:5,000 (0.04 μg/100 μL/well) in PBS as a secondary antibody. The incubation temperature and time for all steps of the optimized PF 2B8-31 mAb based-iELISA were the same as in Optimization of an indirect enzyme-linked immunosorbent assay (iELISA) for the analysis of thermal stable-soluble protein (TSSP) in pork fat tissue.
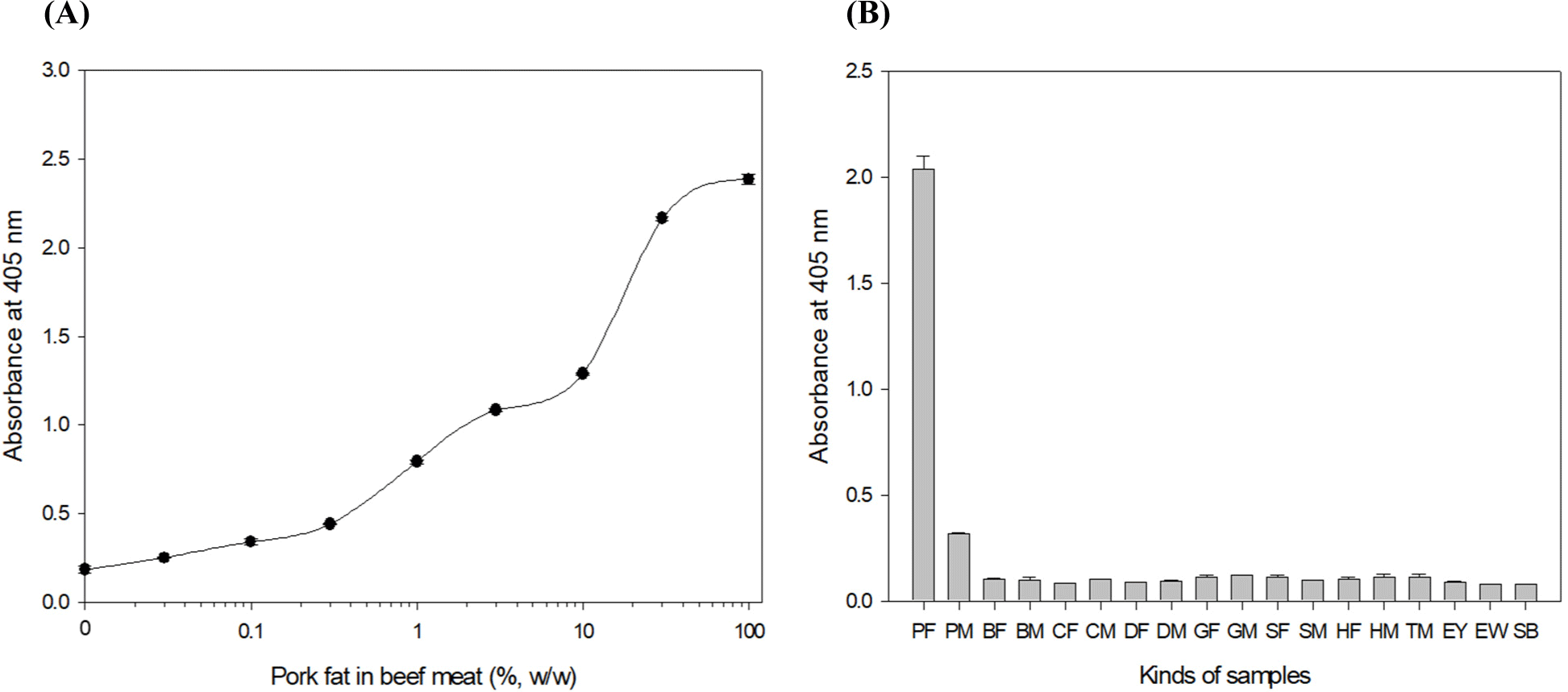
The sensitivity of the optimized ELISA was verified by analyzing extracts of various concentrations (100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, and 0%, w/w) of pork fat in beef meat. Fig. 1A shows that the iELISA can detect 0.1% (w/w) pork fat in beef meat samples. Table 2 compares the sensitivities and target to complete tests in the iELISA and 3 kinds of commercialized kits [Porcine trace rapid test Kit (7FoodPillars), XEMATest pork fat/blood (XEMATest), ELISA-TEKTM cooked meat pork species Kit (R-Biopharm AG)]. The sensitivities of the three commercialized kits were reported to be 0.5%–2% (w/w). Given the results above, the developed iELISA has been confirmed to be more sensitive than the current commercialized kits. Eighteen foods, including pork fat and meat, other meats (beef, chicken, duck, turkey, sheep, horse, and goat) and fats (beef, chicken, duck, sheep, horse, and goat), egg yolk, egg white, and soybeans were tested by the iELISA (Fig. 1B). The iELISA analysis obtained the highest OD value (2.0) from the pork fat tissue sample, and around a 0.3 OD value was shown in the pork meat sample. However, most OD values were lower than 0.2 for the other foods, indicating no cross-reaction with other foods (Hendrickson et al., 2021).
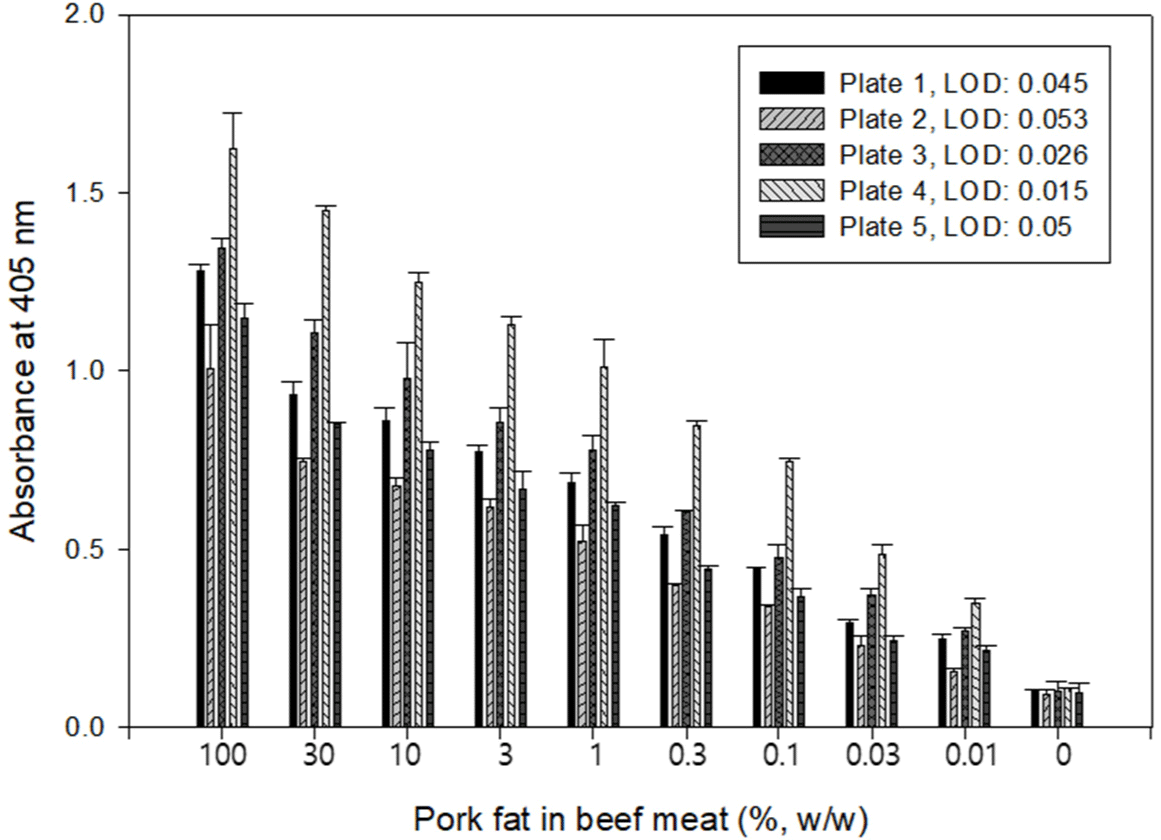
This study, developed an iELISA based on PF 2B8-31 mAb specific to TSSP in pork fat tissue. A 96-well microplate showing high absorbance, which means high protein adsorption from extracts, was chosen to develop the iELISA. Fig. 2 shows the absorbance values of iELISA performed with 100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, 0.01%, and 0% (w/w) pork fat in beef meat. Plate 4 showed the highest absorbance at concentrations of 10%, 1%, and 0.1% pork fat extracts and low absorbance in the 0% sample. This result means that plate 4 possesses a high protein adsorption rate for the target protein in the extracts and provides the highest sensitivity (LOD: 0.015%, w/w). This result also demonstrated that selecting an appropriate 96-well microplate for sample types is critical in optimizing an iELISA. Therefore, plate 4 was chosen to improve the sensitivity of PF 2B8-31 mAb based-iELISA to detect pork fat tissue.
In order to measure the stability of protein adsorption on 96-well microplate 4, the extracts with concentrations of 1%, 0.1%, 0.01%, and 0% (w/w) pork fat in beef meat were coated 10 times on the wells and analyzed within a day (intra-assay). In addition, the same sample was coated and tested once a day for 10 days (inter-assay; Chunsheng et al., 2018). Table 3 shows the absorbance values obtained by the PF 2B8-31 mAb based-iELISA performed daily and for 10 days with 1% to 0% (w/w) pork fat extracts. Each experiment showed similar absorbance values indicating that a certain amount of the target protein in the extract was adsorbed to the well, even if the target protein was present in the food matrix. However, since all plates tested showed similar performance on the iELISA based on PF 2B8-31 mAb, it could not be concluded that one 96-well microplate is superior to the others. Thus, iELISA with commercial 96-well microplates exhibiting high and uniform protein adsorption can be available for sensitively detecting or identifying pig adipose tissue.
Sandwich ELISA formats have been usually used to detect large macromolecules, such as bacteria and proteins, and have superior sensitivity and reliability compared to the iELISA. In sandwich ELISA, capture and detector antibodies are used, requiring more time and cost to be developed. Utuk et al. (2012) reported that iELISA, which uses a single antibody, is also reproducible and cheaper than sandwich ELISA. The iELISA developed in this study also has high reproductivity and sensitivity compared to commercial kits based on sandwich assay format. Therefore, the iELISA can be used to analyze foods that contain pork fat but are unlabeled.
In the food and livestock industries, heat treatments such as cooking and pasteurization are essential to process products and ensure safety. Heat treatment can denature and insolubilize most soluble proteins, and target proteins that are not heat-stable soluble proteins may become undetectable in an immunoassay. Therefore, in order to evaluate the effectiveness of the iELISA developed in this study, beef meatballs containing pork fat tissue (100%, 30%, 10%, 3%, 1%, 0.3%, 0.1%, 0.03%, and 0%, w/w) with or without heat-treatment by autoclaving, steaming, roasting, and frying were tested (Stachniuk et al., 2021).
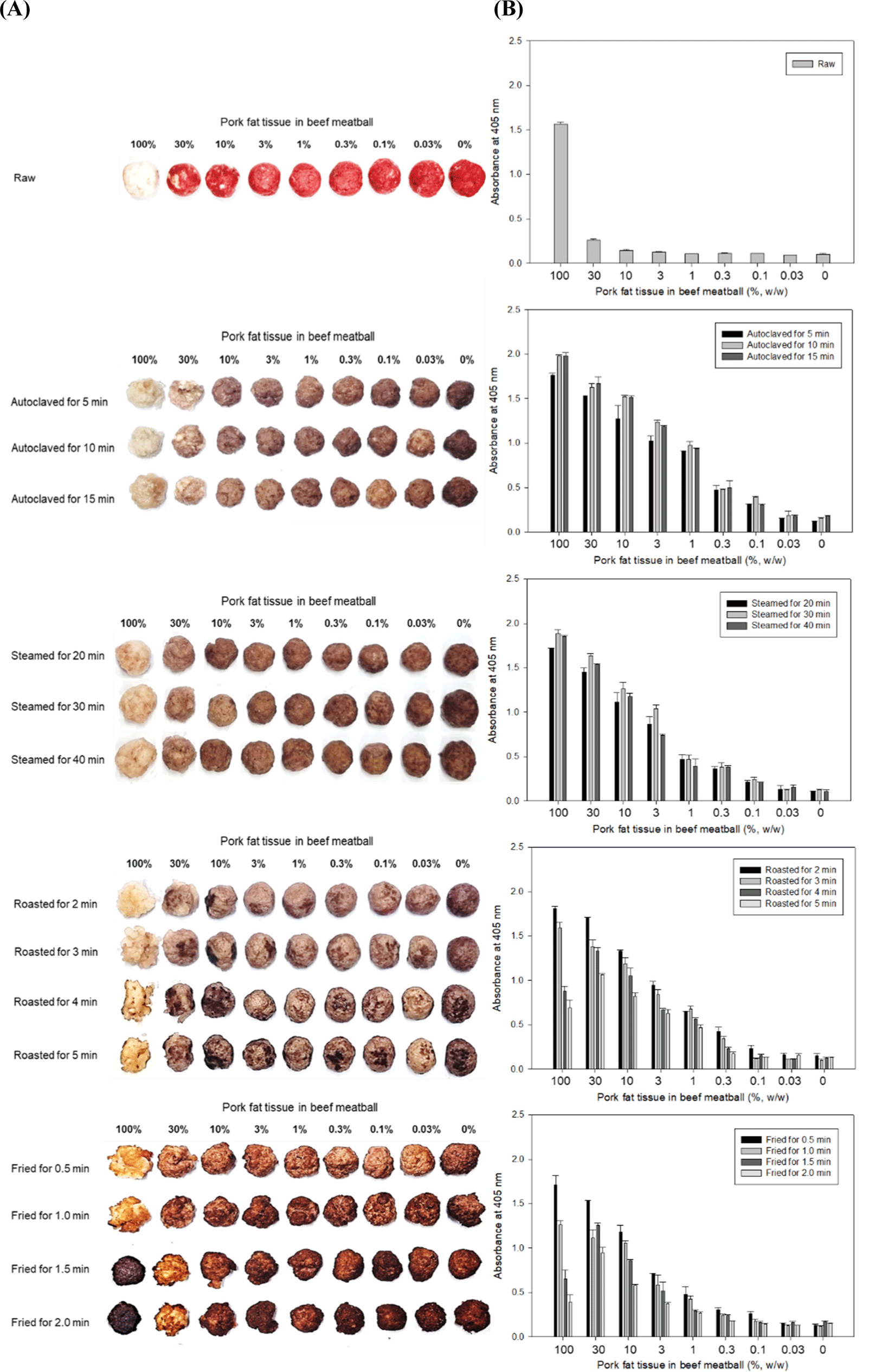
Fig. 3 shows the shapes of beef meatballs containing different amounts of pork fat tissue after heat treatments and the PF 2B8-31 mAb based-iELISA results for the beef samples. Samples autoclaved for 5 to 15 min and samples steamed for 20 to 40 min showed similar absorbance values at all concentrations of pork fat tissue in the beef meatballs in the PF 2B8-31 mAb based-iELISA. The ELISA can detect 0.1% (w/w) pork fat tissue in autoclaved and steamed beef meatballs. The iELISA showed an absorbance decrease in the lean pork fat (100%) samples roasted and fried as the heating time increased. However, the absorbance of the 30% (w/w) pork fat tissue in the roasted and fried beef meatballs did not decrease rapidly as the heating time increased, and the samples roasted for 4 and 5 min and fried for 1.5 and 2 min even showed higher absorbance values than those of the 100% pork fat samples that were roasted and fried. The ELISA can detect 0.1% (w/w) pork fat tissue in beef meatballs roasted for 2 min and fried for 30 sec, but could only detect 0.3% (w/w) pork fat tissue in beef meatballs roasted for 3 to 5 min and fried for 1 to 2 min. It was determined that 0.3% (w/w) pork fat tissue could be detected in the roasted and fried samples by the iELISA because it was not possible to recognize how long the processed meat products sold in the markets had been roasted or fried. Compared to autoclaving and steaming, roasting and frying are processing methods in which heat is directly transferred to the sample, so pure pork fat samples have better heat transfer due to the oil converted from lard by the heat. In this state, even TSSPs may be denatured or burned. On the other hand, the absorbance of 100% pork fat raw was higher than 1.5, whereas the absorbance of the 30% (w/w) or lower pork fat and in the raw beef meatballs raw decreased rapidly. Therefore, the iELISA can detect more than 3% (w/w) pork fat tissue in raw beef meatballs.
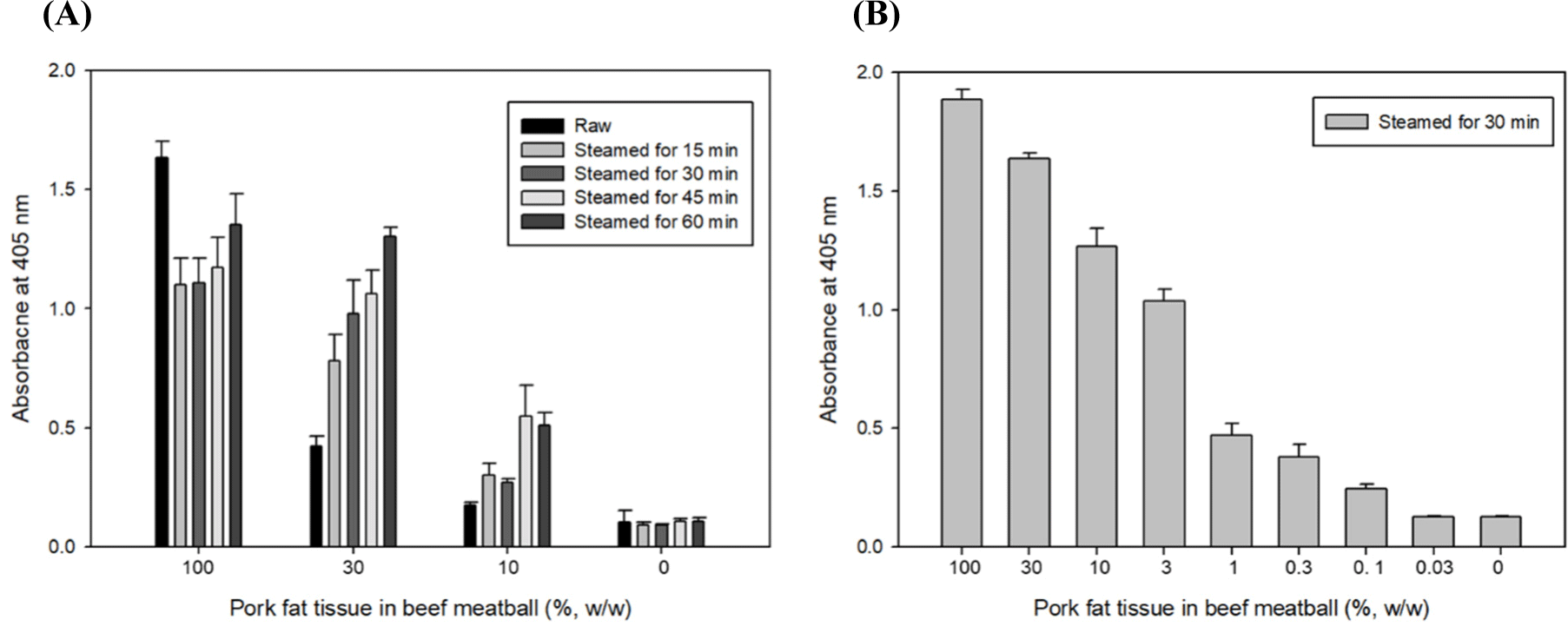
As an additional experiment, raw samples were steamed for different amounts of time (15, 30, 45, and 60 min) as a pretreatment and analyzed by ELISA. The 30% (w/w) pork fat tissue in beef meat steamed for less than 30 min showed lower absorbance than those steamed for more than 30 min (Fig. 4A). We supposed the phenomenon that thermally unstable-soluble protein present in beef meat was not sufficiently denatured in the insoluble type and existed in the soluble type even through the extraction process by the heating extraction method in boiling water for 15 min, and the extracted thermally unstable-soluble proteins interfered the interaction of TSSP and PF 2B8-31 mAb (Park et al., 2014). As shown in Fig. 4B, it was possible to measure 0.1% (w/w) of pork fat tissue in beef meatballs by ELISA in the raw samples steamed for more than 30 min. From the above results, the optimized iELISA was highly sensitive and successfully detected 0.1 pork fat tissue mixed in raw, steamed, and autoclaved beef meatballs and 0.3% (w/w) pork fat tissue mixed in roasted and fried beef meatballs.
Conclusion
TSSP in pork fat tissue was effectively extracted from heat-processed beef meatballs by hot extraction in boiling water for 15 min. The iELISA based on PF 2B8-31 mAb for detecting pork fat tissue in heat-processed beef meatballs was developed and optimized with an appropriate 96-well microplate. It was found that selecting a 96-well microtiter microplate with high and uniform protein adsorption can be an important factor in improving the sensitivity of an iELISA. The iELISA can sensitively detect 0.015% (w/w) pork fat in beef meatballs and could detect 0.1% and 0.3% (w/w) pork fat mixed in raw, steamed, and autoclaved beef meatballs and roasted and fried beef meatballs, respectively. In conclusion, the iELISA based on PF 2B8-31 mAb is therefore expected to be a useful analytical tool for screening and quantification of pork fat tissue in edible meat products.













