Introduction
An increase in the number of food-borne illness outbreaks caused by food-borne pathogens, including Listeria monocytogenes, Staphylococcus aureus, pathogenic Escherichia coli, Clostridium perfringens, Campylobacter spp., and Vibrio spp., has become a significant public health challenge, resulting in a substantial economic damage to many countries (Yu et al., 2021). The global consumption of meat proteins is projected to rise by 11% by 2031 compared to the average of the base period of 2019–2021, primarily driven by income and population growth (OECD and FAO, 2022). Among all commercial foods, meat is one of the most perishable, and its shelf-life is influenced by multiple factors including microbial growth, enzymatic activity, oxidation processes, package type, and the product environment, particularly at the point of sale (Cenci-Goga et al., 2020). Sustainable food production and consumers’ increasing demand for fresh, nutritious, and safe food had led to exploration of novel processing and preservative interventions to extend the shelf life of food products (Xinyu et al., 2020).
Over the past two decades, non-thermal processing technologies, including high-pressure processing, ultrasound, pulsed electric field, ultraviolet light, high-intensity pulsed light, gamma irradiation, and cold plasma have gained significant interest in the meat industry for ensuring microbiological safety (Laroque et al., 2022). Plasma, the fourth state of matter, is an ionized gas generated by applying an electric current to a neutral gas (Lee et al., 2017) and has emerged as a promising technology for various applications, including non-thermal food pasteurization (Lee et al., 2017; Misra and Jo, 2017). Plasma contains reactive oxygen species (ROS), reactive nitrogen species (RNS), ultraviolet radiation (UV), free radicals, and charged particles (Laroque et al., 2022).
The application of cold plasma technology in a wide variety of food, both natural and processed, has gained significant importance in recent years. Its appealing qualities lie in its low temperature and high efficacy (Misra et al., 2015). Regarding foods and food-related materials, cold plasma treatment offers numerous options for food preparation, including surface decontamination, surface property modification, and mass transfer augmentation (Pankaj, 2015). The application of non-thermal plasma in different food categories has shown promising results. For instance, it has been applied to vegetables (Mahnot et al., 2020; Prasad et al., 2017; Shah et al., 2019), fruits (Misra et al., 2014; Pathak et al., 2020; Won et al., 2017; Wu et al., 2021a), meat (Bauer et al., 2017; Jayasena et al., 2015; Zhuang et al., 2019), seafood (Chen et al., 2019; De Souza Silva et al., 2019; Olatunde et al., 2019), dairy (Kim et al., 2015; Lee et al., 2012a; Lee et al., 2012b; Yong et al., 2015a; Yong et al., 2015b), grains (Lee et al., 2016b; Selcuk et al., 2008), and juices (Pankaj et al., 2017b; Rodríguez et al., 2017; Xu et al., 2017), demonstrating effective microbial inactivation, extended shelf life, reduced spoilage losses, and improved nutritional, functional, and sensory properties of food products (Nwabor et al., 2022; Starek et al., 2019). Moreover, cold plasma technology has shown successful surface sterilization of packaging materials and functional modification to achieve desired qualities (Scholtz et al., 2015).
Different plasma sources, including plasma jet, corona discharge, radiofrequency, dielectric barrier discharge (DBD), and microwave (Peng et al., 2020) are being tested for their antimicrobial efficacy in meat, such as beef, pork, lamb, and chicken and their products. Researches indicate that plasma treatments have a greater potential for the inactivation of foodborne pathogens, making them a valuable tool in microbial control (Kim et al., 2016a). Cold plasma, in particular, offers advantages such as cost-effectiveness, versatility, environmental friendliness, and minimal generation of hazardous substances during the sterilization process (Chen et al., 2020; Lee et al., 2017; Pankaj et al., 2018). It has also shown to increase the bioactivities of naturally occurring bioactive components with health benefits (Beyrer et al., 2020). Additionally, plasma technology has been recognized for its ability to protect packaged food from pathogenic microorganisms and improve food quality parameters (Jadhav and Annapure, 2021). Nevertheless, the initial installation cost, process-specific equipment requirements, need of highly trained personal, and safety measures could be listed as disadvantages of using this technology (Chen et al., 2020). Despite these challenges, this review presents a comprehensive analysis of the current knowledge on cold plasma technology and its potential applications in meat and meat products processing industry as a non-thermal pasteurization method and a novel innovative curing method.
Plasma Technology
Plasma the fourth state of matter is partially or fully ionized gas composed of many different species including positive and negative ions, electrons, free radicals, gas atoms, molecules in the ground or excited state, neutral particles, and electromagnetic radiation quanta as visible light and UV photons (Akhtar et al., 2022; Nehra et al., 2008; Nwabor et al., 2022). Plasma can be created by applying energy across neutral gases in a variety of ways, such as thermal, electrical, optical (UV light), magnetic, irradiation, and microwave fields. The system may run on a mixture of noble gases, such as helium, argon, or neon, or it may use a basic gas like air or nitrogen (Pankaj et al., 2018). Mixtures of gases such as He/O2, He/N2, N2/N2O, N2/O2, Ar/O2, and He/O2/H2O have also been used in various plasma operations (Guo et al., 2015).
Types of Plasma
Plasma can be classified based the thermal equilibrium and the pressure conditions. Based on the thermal equilibrium, plasma technology is divided into high-temperature (thermal equilibrium state: 106 to 108K) and low-temperature plasma. The latter can be further subdivided into thermal plasma (quasi-equilibrium plasma; local thermal equilibrium state: 4,000 to 20,000 K) and non-thermal plasma (non-equilibrium plasma/cold plasma; non-equilibrium state: 300 to 1,000 K; Lee et al., 2017; Nehra et al., 2008; Pankaj et al., 2018). Non-thermal plasma (cold plasma) has confirmed its effectiveness for use in heat sensitive foods including meat and meat products compared to high temperature and thermal plasmas (Akhtar et al., 2022; Lee et al., 2017; Misra et al., 2016).
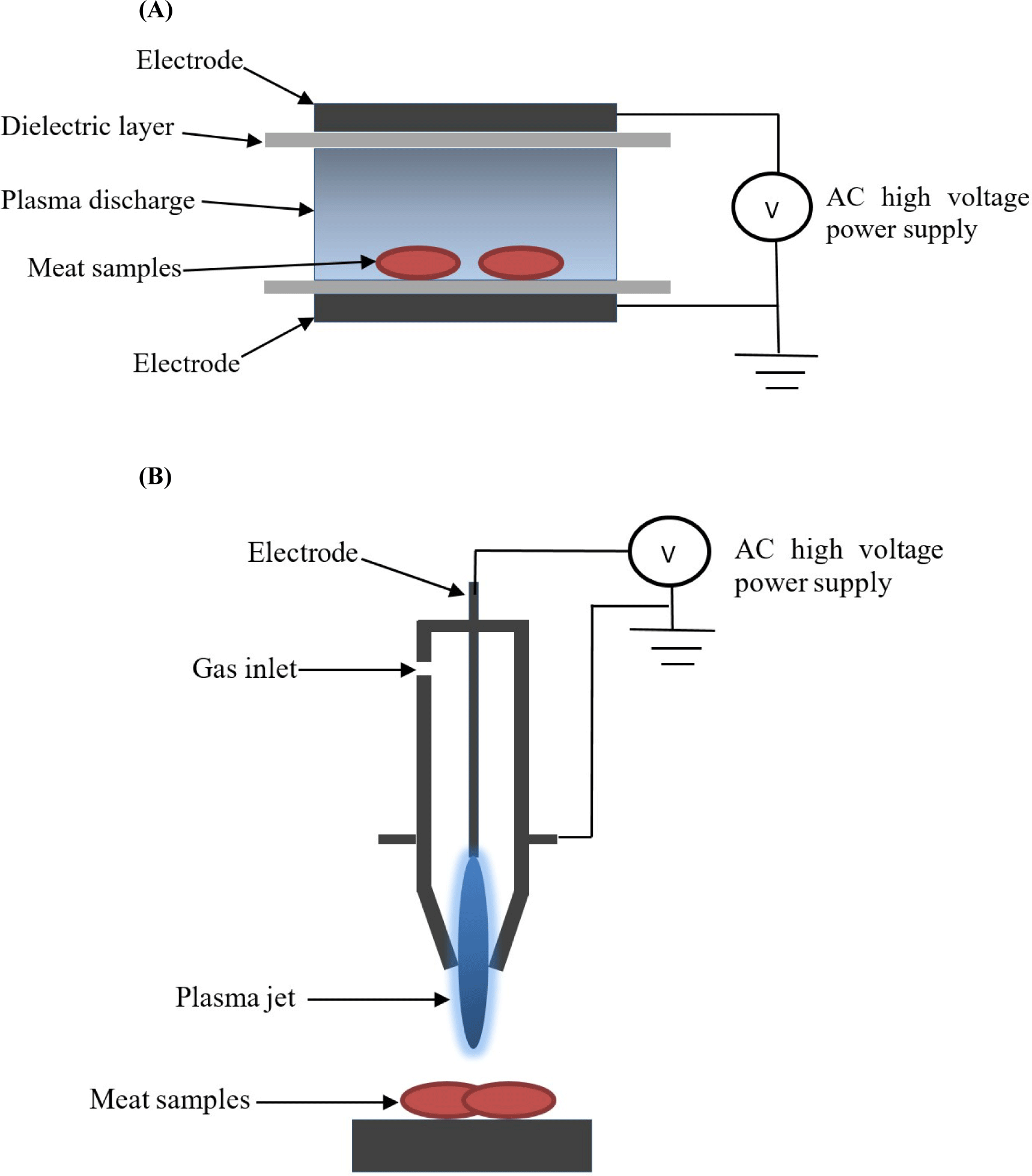
According to the pressure conditions, plasma could further be subdivided into high-pressure, atmospheric pressure and low-pressure plasma (Pankaj et al., 2018). However, the requirement for a vacuum system for plasma generation at low pressure condition limited its usage and opened new avenues for plasma generation at atmospheric pressure (Lee et al., 2017; Nehra et al., 2008). Atmospheric pressure cold plasma (30°C–60°C) can be generated using several electrical discharges such as corona discharge, DBD, gliding arc discharge, plasma needle, and plasma jets (Akhtar et al., 2022; Misra et al., 2011) with various discharge gases such as oxygen, nitrogen, helium, argon and ambient air (Lee et al., 2017; Nehra et al., 2008). However, DBD and plasma jet are considered as the most commonly used cold plasma devices (Fig. 1) in food industry including meat processing industry due to their uncomplicated designs and flexibility to be altered to meet a variety of treatment needs (Akhtar et al., 2022; Pankaj et al., 2018). Specificities for each cold plasma source suitable for food application are available in detail in the review published by Laroque et al. (2022). Besides its application in food industry, cold plasma technology has been applied in a number of manufacturing industries including medical devices, textiles, automotive, aerospace, electronics, and packaging materials (Bermudez-Aguirre, 2020; Laroque et al., 2022; Olatunde et al., 2019; Table 1).
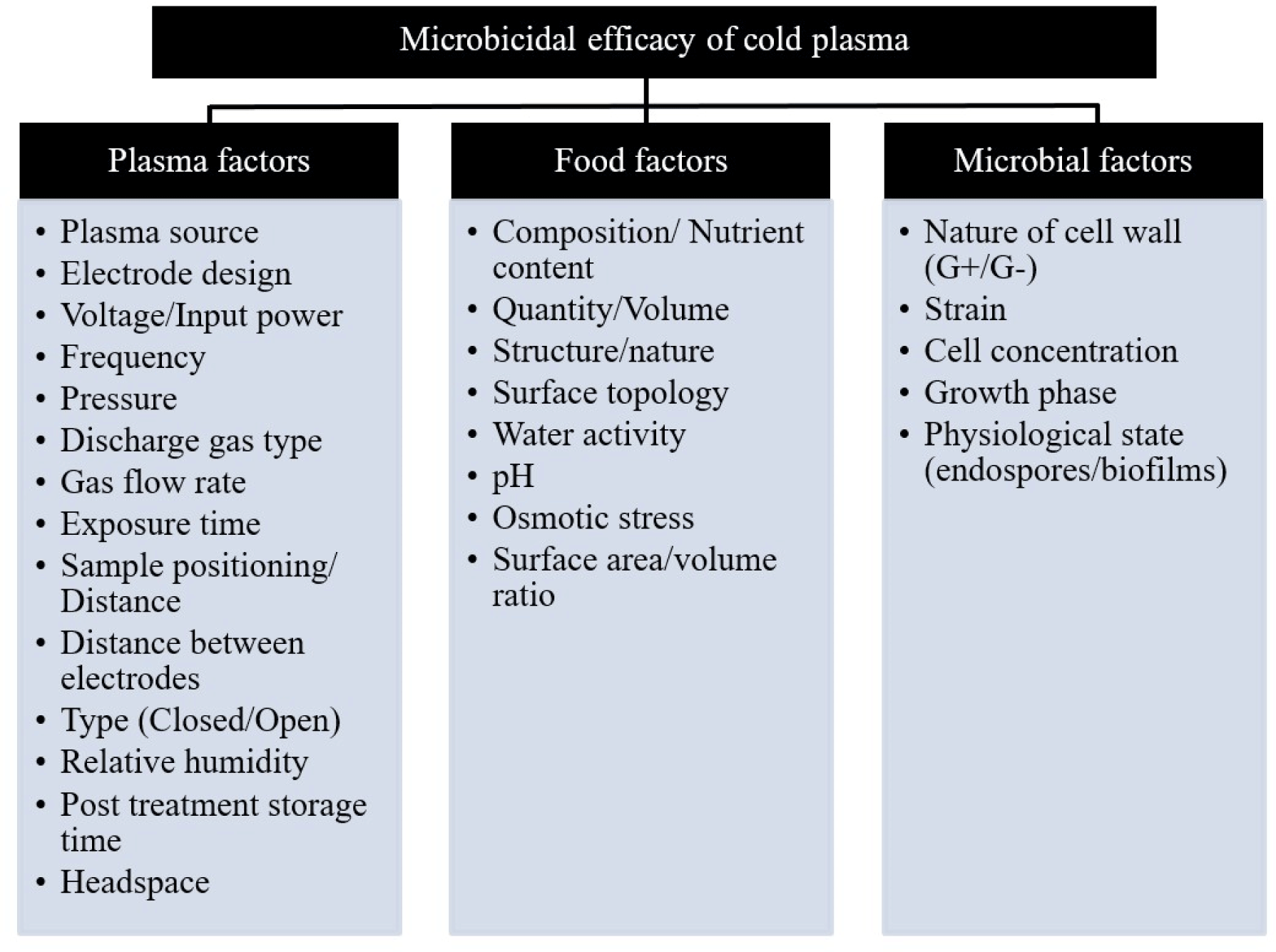
Factors Affecting the Efficacy of Cold Plasma
The microbicidal efficacy of cold plasma, as depicted in Fig. 2, is influenced by three main categories of factors: microbial factors, food factors, and plasma operational parameters. Therefore, a comprehensive consideration of these factors is necessary to achieve enhanced antimicrobial efficacy in food systems. The working parameters and instrumental settings of cold plasma treatment, as illustrated in Fig. 2, play a crucial role in determining the concentration of reactive species, discharge characteristics, gas speciation, and overall efficiency of the cold plasma process (Pankaj et al., 2018). For instance, the effectiveness of cold plasm-mediated inhibition of L. monocytogenes, E. coli, and Salmonella Typhimurium in bacon (Kim et al., 2011) and L. monocytogenes in chicken breast (Lee et al., 2011) was affected by the type of gas used; a mixture of helium and oxygen and a mixture of nitrogen and oxygen were more effective in reducing the microbial counts than helium and nitrogen alone, respectively. Furthermore, studies by Kim et al. (2011) and Laroussi and Leipold (2004) confirmed that an increase in input power resulted in a greater microbicidal effect. In-package (closed) plasma treatment offers advantages by preventing subsequent contamination of food systems and providing continuous pasteurization effect against microorganisms even after plasma treatment (Yong et al., 2014; Yong et al., 2017a) as compared to an open plasma system.
The formation of biofilm on food contact surfaces is a leading cause of food contamination, foodborne disease outbreaks, and recalls of finished food products. In recent years, food processors have been exploring modern green technologies as alternatives to conventional antimicrobial chemical sanitizers for the decontamination of food processing lines and facilities (Nwabor et al., 2022). Scientific studies have demonstrated that cold plasma treatment effectively disrupts and inactivates the biofilms formed by various microorganisms, including Pseudomonas aeruginosa (Ziuzina et al., 2014a), Candida albicans (He et al., 2020), Aspergillus flavus (Los et al., 2020), E. coli (Los et al., 2017; Ziuzina et al., 2015a; Ziuzina et al., 2015b), Bacillus subtilis, Lactobacillus spp. (Los et al., 2017), L. monocytogenes, and S. aureus (Ziuzina et al., 2015a). However, the anti-biofilm effectiveness of cold plasma is also influenced by several factors, such as gas composition (single gas/gas mixture), attachment surface (biotic surface, abiotic surface, roughness, hydrophilicity, hydrophobicity), type of biofilm (mono-species or mixed-species), processing parameters (power, voltage, frequency, flow rate), types of bacteria (Gram-positive/Gram-negative), individual variations in cellular properties, age of biofilm, biofilms thickness, and storage conditions (Nwabor et al., 2022; Zhu et al., 2020).
Application of Cold Plasma in Meat Industry
Meat processing has always played the leading role of developing and implementing novel technologies in the food industry. To ensure a sanitary manufacturing environment, various technologies aimed at enhancing food safety are employed in meat processing. Due to its high nutritional value and perishable nature, meat is susceptible to microbial contamination, which poses risks to both quality and public health. Previous studies have shown that this challenge can be effectively addressed by utilizing cold plasma treatment as a non-thermal pasteurization method for meat and meat products.
A broad range of microorganisms could be effectively inactivated by cold plasma processing which generates reactive species lethal to cells (Nicol et al., 2020; Yoo et al., 2021). The oxygen in the air forms the ROS, which tend to react with other oxygen molecules leading to the formation of singlet oxygen, hydroxyl radical, superoxide anion, hydrogen peroxide, and ozone during the plasma generation (Han et al., 2016; Oehmigen et al., 2010; Park et al., 2018). Ozone has been shown to possess a greater microbicidal properties owing to its relatively long lifetime (Han et al., 2016; Laroussi and Leipold, 2004; Ziuzina et al., 2014b). Moreover, cold plasma generation results in RNS, including peroxynitrite, nitric oxide, and nitrite (Burlica et al., 2006; Laroussi and Leipold, 2004).
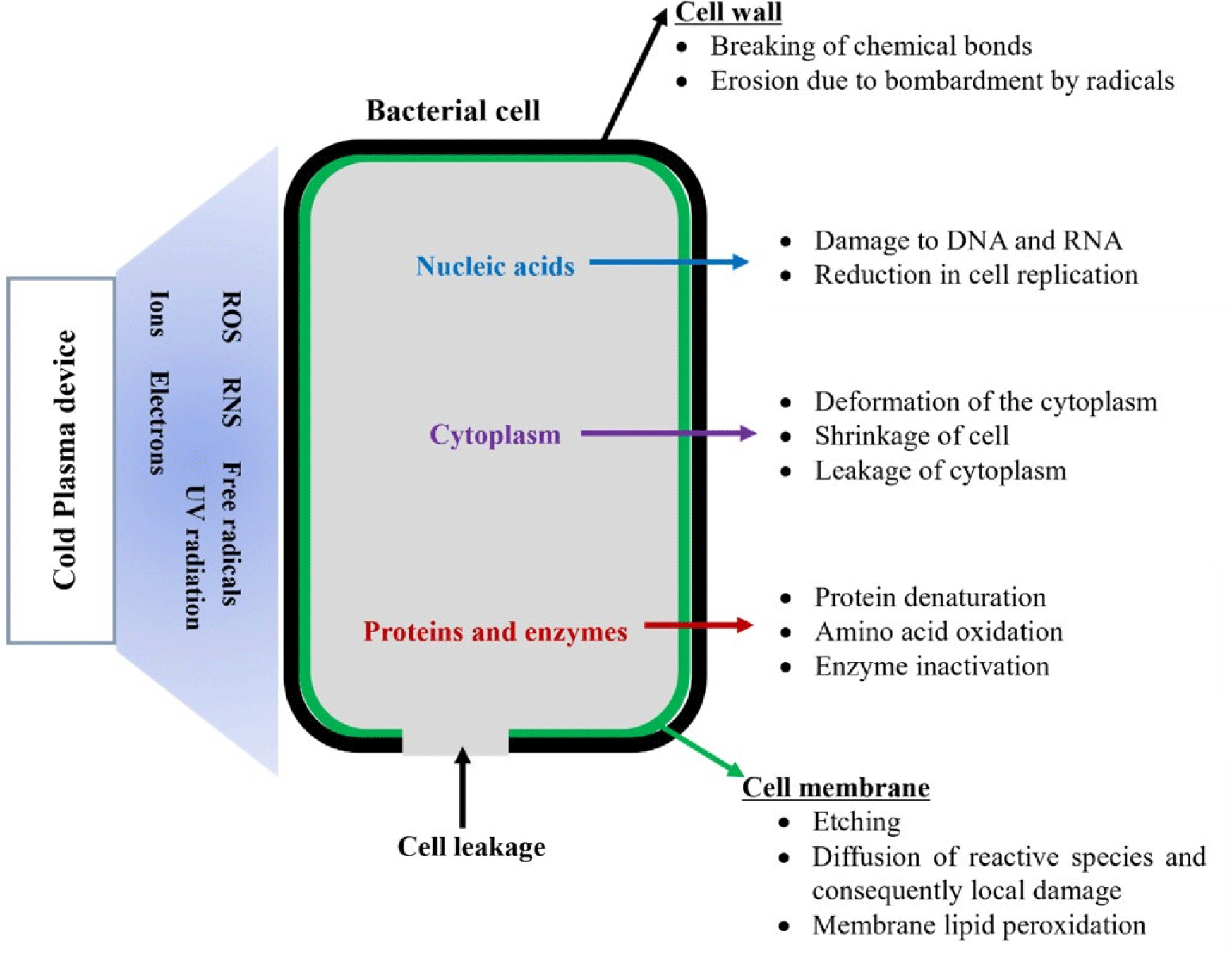
Gavahian et al. (2019) thoroughly reviewed the mechanism of inactivation of microorganisms by plasma and highlighted that the plasma-induced reactive species primarily disrupt the bacterial cell wall membrane. Free radicals present in plasma can be adsorbed on the surface of microorganisms and diffused into the cell membrane, causing damage to proteins and nucleic acids (Fernández and Thompson, 2012). Distinct microbicidal mechanisms on Gram-positive and Gram-negative bacteria have been suggested (Fig. 3). Han et al. (2016) proposed that the microbicidal effects of cold plasma treatment on Gram-positive bacteria is mainly due to oxidative damage to intracellular components, particularly DNA without cell leakage. In Gram-negative bacteria, the irreversible destruction of the cell wall via oxidative damage leads to leakage of intracellular compounds such as protein, DNA, and lipids, resulting in microbial inactivation (Han et al., 2016). Further details on microbial inactivation mechanisms can be found in other references (Akhtar et al., 2022; Nasiru et al., 2021; Nwabor et al., 2022).
Many studies have revealed the significant impact of cold plasma technology on microbial decontamination in meat and meat products. Table 2 shows the microbicidal effects of cold plasma generated using different plasma sources on common microorganisms found in chicken, pork, beef, lamb and processed meat products such as bacon, ham, and jerky. The results clearly indicated that cold plasma technology can achieve substantial log reductions in tested microbes. For instance, a reduction of 0.43 to 6.52 Log CFU/g in L. monocytogenes counts has been reported in inoculated meat and meat products following cold plasma treatment (Bauer et al., 2017; Choi et al., 2016; Cui et al., 2017; Jayasena et al., 2015; Kim et al., 2011, Kim et al., 2013; Lee et al., 2011, Lee et al., 2016a; Yong et al., 2017a). In addition, studies conducted to improve the safety of meat and meat products found a 0.34 to 7.50 Log CFU/g reduction in E. coli (Bauer et al., 2017; Choi et al., 2016; Jayasena et al., 2015; Kim et al., 2011; Kim et al., 2013; Lee et al., 2016a; Stratakos and Grant, 2018; Yong et al., 2017a), and a 0.98 to 5.30 Log CFU/g reduction in S. Typhimurium counts (Chaplot et al., 2019; Jayasena et al., 2015; Kang et al., 2022; Kim et al., 2011; Lee et al., 2016a; Yong et al., 2017a) after cold plasma treatment. The treatment of chicken breast and Bresaola with cold atmospheric gas plasmas showed a 3.30 and 1.60 Log CFU/g reduction in Listeria innocua levels, respectively (Noriega et al., 2011; Rød et al., 2012). Moreover, a 1.33 to 4.00 Log CFU/g reduction in S. aureus counts in chicken, beef, and beef jerky (Bauer et al., 2017; Kim et al., 2014b; Royintarat et al., 2020; Sahebkar et al., 2020) and a 0.78 to 2.55 Log CFU/g reduction in Campylobacter jejuni counts in chicken skin and breast were reported upon cold plasma treatment (Dirks et al., 2012; Rossow et al., 2018).
| Meat/meat product | Plasma source | Processing parameters | Microorganism | Microbial reduction (Log10) | Reference |
|---|---|---|---|---|---|
| Beef | Atmospheric pressure plasma jet | Air, 600 W, 1 min, plasma activated water | Total viable counts Fungi and yeast | 1.62 1.76 |
Xinyu et al. (2020) |
| Beef slices | Plasma activated lactic acid (PALA) | 19.2 kV, 80 s, PALA 0.2% | Salmonella Enteritidis | 3.52 | Qian et al. (2019) |
| Beef | DBD plasma | 20 MHz, 6 kV, 5 min | Escherichia coli | 1.82 | Stratakos and Grant (2018) |
| Beef loin | DBD plasma | 9 kHz, 29.9 W | Staphylococcus aureus Listeria monocytogenes E. coli | ≥2 ≥2 ≥2 |
Bauer et al. (2017) |
| Beef loin | Flexible thin-layer DBD plasma | N2/O2, 100 W, 10 min | L. monocytogenes E. coli Salmonella Typhimurium | 1.90 2.57 2.58 |
Jayasena et al. (2015) |
| Beef | Low pressure plasma | He, Ar, 20 kPa, 10 min | Psychrotropes Yeast and mold | 1.48 (He)/1.32 (Ar) 0.98 (He)/0.50 (Ar) |
Ulbin-Figlewicz et al. (2015b) |
| Chicken breast and drumstick | Encapsulated atmospheric DBD plasma treated 0.8% acetic acid | Air, 2.2 kHz, 8.4 kV, 30 min | S. Typhimurium | 0.98 (Breast) 1.19 (Drumstick) |
Kang et al. (2022) |
| Chicken breast fillets | Atmospheric cold plasma (ACP) | Ar, 32 kHz, 10 min | S. aureus E. coli | −3 −4 (treatment with essential oil) |
Sahebkar et al. (2020) |
| Chicken breast | In package DBD-ACP | Air, 100 kV, 233 W, 60 Hz, 5 min, 24 d storage | Mesophiles Psychrophiles Enterobacteriaceae |
1.5 1.4 0.5 |
Moutiq et al. (2020) |
| Chicken breast | DBD plasma | 14.5 W, 10 min | Salmonella | 3.7 | Aboubakr et al. (2020) |
| Chicken meat and skin | Plasma activated water and ultrasound | 1.5 MHz, 6.8 kV, 40 Hz, 60 min, 40°C | E. coli S. aureus | 1.12/0.86 1.33/0.83 |
Royintarat et al. (2020) |
| Chicken breast | In package DBD-ACP | Air, 70 kV, 5 min, 5-d storage | Psychrophiles Campylobacter jejuni S. Typhimurium |
1.00 0.93 0.65 |
Zhuang et al. (2019) |
| Chicken meat | ACP and peracetic acid (PAA) | 0 to 30 kV, 3.5 kHz, 4°C, PAA (100–200 ppm), 60 min | S. Typhimurium | 3.8–5.3 | Chaplot et al. (2019) |
| Chicken skin and breast | Atmospheric pressure plasma jet | Ar or air, 1 MHz, 2–3 kV, 180 s, distance from nozzle to sample 5, 8, 12 mm | C. jejuni | 0.78–2.55 | Rossow et al. (2018) |
| Chicken breasts | Flexible thin-layer DBD plasma | Air, 100 W, 15 kHz, 10 min | Total aerobic bacteria L. monocytogenes E. coli S. Typhimurium |
3.36 2.14 2.73 2.71 |
Lee et al. (2016a) |
| Chicken breast fillet | DBD plasma | 5% N2+30% CO2+65% O2, 80 kV, 180 s | Mesophiles Psychrophiles Pseudomonas spp. |
1.0 0.5 0.9 |
Wang et al. (2016) |
| Skinless chicken breast | DBD plasma | Air, 30 kV, 0.5 kHz, 3 min |
Salmonella enterica
C. jejuni |
2.54 2.45 |
Dirks et al. (2012) |
| Chicken breast and skin | Cold atmospheric plasma (CAP) pen | He+O2, 6.5–16 kV, 23–38.5 kHz | Listeria innocua | 1 (8 min treatment on skin) >3 (4 min treatment on breast) |
Noriega et al. (2011) |
| Cooked chicken breast | Atmospheric pressure plasma jet | He, N2, O2, 2 kV, 50 kHz, 2 min | L. monocytogenes | 1.37–4.73 | Lee et al. (2011) |
| Lamb meat | DBD plasma | 80 kV, 50 Hz, 5 min | Brochothrix thermosphacta | 2.0 | Patange et al. (2017) |
| Pork loin | DBD plasma | CO2+N2+O2, 85 kV, 60 s | Total viable aerobic count | 0.4 (20% CO2+40% N2+40% O2) 0.8 (20% CO2+20% N2+60% O2) |
Huang et al. (2019) |
| Pork loin | Cold nitrogen plasma and lemongrass oil | 500 W, 120 s and lemongrass oil 5 mg/mL, 30 min |
L. monocytogenes | 2.8 | Cui et al. (2017) |
| Fresh and frozen pork | Corona discharge plasma jet | 58 kHz, 20 kV, 90–120 s |
E. coli
L. monocytogenes |
1.5 1.0 |
Choi et al. (2016). |
| Pork butt | Flexible thin-layer DBD plasma | N2/O2, 100 W, 10 min | L. monocytogenes E. coli S. Typhimurium |
2.04 2.54 2.68 |
Jayasena et al. (2015) |
| Pork | Pulsed plasma | He, Ar, 0.8 MPa, 20–100 kHz, 1.2 kVA, 10 min | Psychrotropes Yeast and Mold |
2.70 (He)/1.20 (Ar) 2.13 (He)/2.57 (Ar) |
Ulbin-Figlewicz et al. (2015a) |
| Pork | Low pressure plasma | He, Ar, 20 kPa, 10 min | Psychrotropes Yeast and Mold |
1.60 (He)/1.20 (Ar) 1.90 (He)/0.41 (Ar) |
Ulbin-Figlewicz et al. (2015b) |
| Pork loins | DBD plasma | He/He+O2, 3 kV, 30 kHz, 10 min, 3 mm distance between sample and DBD actuator | L. monocytogenes E. coli | 0.43 (He)/0.59 (He+O2) 0.34 (He)/0.55 (He+O2) |
Kim et al. (2013) |
| Beef jerky | Clove oil and encapsulated atmospheric pressure plasma | Air, 8.4 kV, 2.2 kHz, 4 min, 0.05% clove oil concentration | E. coli O157:H7 | >7.5 | Yoo et al. (2021) |
| Beef jerky | Plasma beam system | N2 or air, 20 kHz, 300 W, brine (sodium nitrite) solution | L. innocua | 0.85 | Inguglia et al. (2020) |
| Beef jerky | Encapsulated atmospheric pressure plasma and nisin (100 ppm) | Air, 2.2 kHz, 8.4 kV, 5 min (beef jerky) and 9 min (sliced ham) | E. coli O157:H7 | 0.80 | Lee et al. (2023) |
| Beef jerky | Flexible thin-layer DBD plasma | Air, 15 kHz, 10 min | L. monocytogenes E. coli S. Typhimurium Aspergillus flavus |
2.36 2.65 3.03 3.18 |
Yong et al. (2017a) |
| Beef jerky | Radio-frequency atmospheric pressure plasma | Ar, 20,000 sccm, 200 W, 3 min | S. aureus | 3–4 | Kim et al. (2014b) |
| Pork jerky | DBD plasma | Air, 4 kHz, 3.8 kV, 40 min |
S. aureus
Bacillus cereus |
7.00 6.00 |
Yong et al. (2019) |
| Bacon | Atmospheric pressure plasma | He/He+O2; 125 W; 14 MHz, 90 s | L. monocytogenes E. coli S. Typhimurium |
2.06 (He)/2.60 (He+O2) 1.57 (He)/3.00 (He+O2) 1.32 (He)/1.73 (He+O2) |
Kim et al. (2011) |
| Chicken ham | Atmospheric pressure plasma jet | He, N2, O2, 2 kV, 50 kHz, 2 min | L. monocytogenes | 1.94–6.52 | Lee et al. (2011) |
| Sliced ham | Encapsulated atmospheric pressure plasma and nisin (100 ppm) | Air, 2.2 kHz, 8.4 kV, 5 min (beef jerky) and 9 min (sliced ham) | E. coli O157:H7 | 1.96 | Lee et al. (2023) |
| Chicken patties | DBD plasma | 65% O2+30% CO2, 70 kV, 1% rosemary, 180 s | Total plate count | 0.55 (plasma 0.80 (plasma+rosemary) |
Gao et al. (2019) |
| Ready-to-eat meat product (bresaola) | Cold atmospheric pressure plasma | 70% Ar+30% O2, 27.8 kHz, 27 kV, 15.5, 31, and 62 W, 2–60 s | L. innocua | 0.8–1.6 | Rød et al. (2012) |
Cold plasma-based hurdle technologies have emerged as innovative strategies for microbial decontamination in the food industry. These technologies combine cold plasma with other hurdles such as mild heat, chemical antimicrobials (organic acids, essential oils), ultrasound technique, biocontrol agents, and nanomaterials have recently been utilized as novel microbial decontamination strategies (Xinyu et al., 2020). A recent study by Lee et al. (2023) investigated the synergistic bactericidal effect of nisin and cold plasma on beef jerky and sliced ham. The hurdle treatment combining nisin and plasma demonstrated a 100% reduction rate in both E. coli and L. monocytogenes surpassing the effectiveness of individual treatment. Similarly, when atmospheric DBD plasma technology was coupled with acetic acid (i.e. plasma-activated acetic acid), it caused a reduction in S. Typhimurium counts more effectively than did acetic acid alone and improved the chicken meat quality (Kang et al., 2022). The hurdle treatment of cold plasma and peracetic acid applied to inactivate S. Typhimurium in raw poultry showed a greater log reduction (3.8 to 5.3 Log CFU/cm2) compared to individual treatment with peracetic acid (0.6 to 1.3 Log CFU/cm2; Chaplot et al., 2019). Moreover, cold plasma treatment was shown to increase the inactivation of L. monocytogenes in pork loin when coupled with lemongrass oil (2.80 Log CFU/g) compared to application of individual cold plasma (0.96 Log CFU/g) or lemongrass oil treatment (0.59 Log CFU/g; Cui et al., 2017). It is important to note that the efficacy of these hurdle treatments may vary due to differences in plasma treatment conditions (such as power, time, and gas composition), which generate different reactive species. Additionally, other factors illustrated in Fig. 2 can also influence the effectiveness of cold plasma in microbial inactivation.
Numerous studies have conducted to elucidate the effects of cold plasma technology on the physicochemical attributes of meat and meat products, but the findings have been contradictory. The color values has not been changed in chicken breast or chicken thigh skin surface as well as in pork when treated with cold plasma (Cui et al., 2017; Dirks et al., 2012; Moon et al., 2009). However, the application of plasma technology has led to a reduction in CIE a* of ready to eat bresaola, beef, pork, and poultry (Chaplot et al., 2019; Jayasena et al., 2015; Rød et al., 2012). According to Stoffels et al. (2008), the impact of cold plasma treatment of meat on pH value is negligible. In contrast, Kim et al. (2013) reported a significant reduction in the pH of pork following the plasma treatment.
The findings of the very few studies conducted on sensory data on plasma-treated meat and meat products have shown that cold plasma has certain negative effects on some sensory parameters of meat. The application of cold plasma technology has a negative impact on sensory properties of meat such as appearance, color, odor, and acceptability (Kim et al., 2013). However, the sensory analysis on cooked pork butt and beef loin samples treated with the flexible thin-layer DBD plasma revealed no differences in the pork and beef samples with respect to appearance, color, off-flavor, general acceptability, and texture parameters such as hardness, gumminess, springiness, cohesiveness, and chewiness. The DBD plasma treatment, however, had a negative impact on consumers' preferences for the flavor of both meat samples (Jayasena et al., 2015).
Formation of radicals and ROS during plasma treatments could induce the lipid oxidation and production of related by products such as malondialdehyde (MDA) and hexanal (Kim et al., 2016a). This might contribute to the variations in sensory attributes of meat and meat products upon plasma treatments, particularly in high-fat meat sources such as pork (Jayasena et al., 2015). The cold plasma treatments increased the level of lipid oxidation in beef, pork, poultry, and their products such as bresaola and beef patty (Cui et al., 2017; Gavahian et al., 2018; Huang et al., 2019; Jayasena et al., 2015; Kim et al., 2013; Rød et al., 2012; Wang et al., 2021; Yong et al., 2017a). Nevertheless, several other authors found that cold plasma treatment of meat and meat products had no impact on lipid oxidation (Jung et al., 2017b; Kim et al., 2011; Lee et al., 2016a; Lee et al., 2018; Moutiq et al., 2020). Accordingly, it is clear that the level of lipid oxidation occurred in meat and meat products is generally influenced by plasma power, treatment time, meat type, and storage (Akhtar et al., 2022; Rød et al., 2012). In addition, scientists have proposed several strategies to limit lipid oxidation by cold plasma treatment, such as eliminating O2, applying a lower voltage, using shorter treatment time, reducing fat and unsaturated fatty acid concentration in meat or meat products to be treated by plasma, and adding antioxidants (Gavahian et al., 2018). Table 3 provides an overview of recent studies examining the impact of cold plasma on the physicochemical properties of meat and meat products.
| Meat/meat product | Plasma source | Processing parameters | Key findings | Reference |
|---|---|---|---|---|
| Beef | Atmospheric pressure plasma jet | Air, 600 W, 1 min, plasma activated water | Comparable lipid oxidation levels in samples thawed by plasma activated water and traditional thawing methods. No detrimental effect on physicochemical and sensory quality traits by PAW thawing compared to traditional thawing methods. |
Xinyu et al. (2020) |
| Chicken | Plasma-activated acetic acid (PAAA) | 2.2 kHz, 8.4 kVpp, 30 min, and 0.8% (v/v) acetic acid | pH, TBARS, and CIE b* decreased and CIE L* increased in PAAA-treated samples. | Kang et al. (2022) |
| Chicken breast | In package DBD-ACP | Air, 233 W, 100 kV, 60 Hz, 5 min | MDA content was comparable between untreated and treated samples. | Moutiq et al. (2020) |
| Chicken breast | In package DBD-ACP | Air, 70 kV, 5 min, 5-d storage | Similar CIE a* and CIE b* in control and plasma treated samples, however, plasma treatments increased the CIE L*. | Zhuang et al. (2019) |
| Chicken breasts | Flexible thin-layer DBD plasma | Air, 100 W, 15 kHz, 10 min | Lipid oxidation was not affected by plasma treatment. However, it increased the CIE L* and CIE b* and decreased the CIE a*. | Lee et al. (2016a) |
| Pork loin | DBD plasma | CO2+N2+O2, 85 kV, 60 s | Oxidation of lipids and the production of carbonyls in the oxidation of proteins were increased. | Huang et al. (2019) |
| Pork loin | Cold plasma and lemongrass oil | N2, 500 W, 120 s, and lemongrass oil 5 mg/mL, 30 min |
TBARS values were increased upon cold plasma treatment. | Cui et al. (2017) |
| Fresh and frozen pork | Corona discharge plasma jet | Air, 58 MHz, 20 kV, 90–120 s | Plasma treatment improved the peroxide value of frozen pork. However, the lipid content of unfrozen meat was not influenced. TBARS values were not changed due to plasma treatment. | Choi et al. (2016) |
| Pork butt and beef loin | Flexible thin-layer DBD plasma | N2/O2, 100 W, 15 kHz 10 min | Lipid oxidation value was increased and CIE a* was significantly lowered. CIE L* not significantly affected. | Jayasena et al. (2015) |
| Pork | Pulsed plasma | N2, He, Ar, 0.8 MPa, 20–100 kHz, 1.2 kVA, | Comparable colour parameters and pH values after cold plasma treatment. | Ulbin-Figlewicz et al. (2015a) |
| Pork loins | DBD plasma | He/He+O2, 3 kV, 30 kHz, 10 min, 3 mm distance between sample and DBD actuator | Plasma treatment increased the TBARS values. The pH and CIE L* decreased, but CIE a* and CIE b* showed no changes. |
Kim et al. (2013) |
| Fresh pork | Atmospheric pressure plasma | 2.45 GHz, 1.2 kW; process gas air | pH decreased, CIE a* increased and CIE b* decreased upon plasma treatment. | Fröhling et al. (2012) |
| Pork | DBD plasma | 0.30 W/cm2 in ambient air, with a gap of 5.0 mm | Increase in CIE L*. Decrease in surface moisture. |
Moon et al. (2009) |
| Beef jerky | Plasma beam system | N2 or air, 20 kHz, 300 W, brine (sodium nitrite) solution | Comparable texture and lipid oxidation values in samples cured in plasma-activated brine as opposed to standard curing. Significantly higher CIE a* in samples cured in plasma-activated brine. | Inguglia et al. (2020) |
| Beef jerky | Flexible thin-layer plasma system | Linear electron-beam RF accelerator (2.5 MeV, beam power 40 kW) | Plasma treatment decreased the CIE L* and increased the CIE a* and ΔE values. | Yong et al. (2017a) |
| Pork jerky | DBD plasma | Air, 4 kHz, 3.8 kV, 40 min | Jerky made with plasma treatment for 40 min had similar color values, nitrosoheme pigment, lipid oxidation, and texture properties as opposed to jerky made with sodium nitrite (100 ppm). | Yong et al. (2019) |
| Bacon | Atmospheric pressure plasma | He/He+O2; 125 W; 14 MHz, 90 s | Plasma treatment increased the TBARS values in bacon after a 7-d storage CIE L* of the bacon surface was increased. | Kim et al. (2011) |
| Chicken patties | DBD plasma | 65% O2+30% CO2, 70 kV, 1% rosemary, 180 s | Plasma treatment increased lipid oxidation. However, MDA level decreased upon the addition of rosemary extract to the product. | Gao et al. (2019) |
| Canned ground ham | DBD plasma | Air, 600 W, 25 kHz, 30 min | Plasma treatment had no effect on lipid oxidation. | Lee et al. (2018) |
| Ground ham | Atmospheric non-thermal plasma (ANP) | Air, 1.5 kW, 60 kHz, 30 min | Temperature and residual nitrite levels increased when cured by remote infusion of ANP (RANP) compared to sodium nitrite. The color and MDA content of ground hams did not differ between RANP and sodium nitrite during storage. | Jo et al. (2020) |
| Ready to eat ham | DBD plasma | 3,500 Hz, 300 W, 0–28 kV | Plasma treatment had significantly induced the MDA levels, but with no changes in CIE L* and CIE b* compared to untreated samples. However, a significant increase in CIE a* was detected. | Yadav et al. (2019) |
| Pork based batter | DBD plasma | Air, 550 W, 25 kHz, 60 s | Plasma treatment did not induce the lipid oxidation in meat batter. The CIE a* of cooked meat batter gradually increased. | Jung et al. (2017b) |
| Ready-to-eat meat product (bresaola) | Cold atmospheric pressure plasma | 70% Ar+30% O2, 27.8 kHz, 27 kV, 15.5, 31, and 62 W, 2–60 s | Higher plasma power with longer treatment duration and storage period increased the TBARS values. Significant reductions in CIE a*. | Rød et al. (2012) |
Nitrite the most commonly used curing agent in the meat industry contributes to the development of cured colour and flavor in meat products (Parthasarathy and Bryan, 2012; Sebranek et al., 2012). Additionally, it plays a role in inhibiting lipid oxidation and contamination by pathogenic microbes including Clostridium botulinum in cured meat products (Jung et al., 2017b; Sebranek et al., 2012). However, due to the increasing consumers’ negative perception towards synthetic food additives, the scientists have shifted their focus on natural alternatives.
It has now been well documented that cold plasma treatment of liquids can generate nitrite (Ercan et al., 2016; Kojtari et al., 2013; Oehmigen et al., 2010). Plasma-activated water contains nitrate and nitrites, and the detailed reactions involved in the formation of nitrite and nitrate in plasma-activated water are explained in the review published by Lee et al. (2017). Since cold plasma technology contains RNS and nitrogen oxides, including NO2, NO3, N2O, N3O, and N2O5, which could form nitric and nitrous acids by reacting with water molecules and subsequently decompose into nitrate and nitrite, it could be a potential nitrite source for curing of processed meat (Jung et al., 2015a; Jung et al., 2015b; Lee et al., 2017). It is noteworthy that the nitrite formed by plasma under alkaline conditions can persist (Jung et al., 2015b; Lukes et al., 2014). For example, cold plasma-treated distilled water containing sodium pyrophosphate can contain up to 782 mg/L of nitrite (Jung et al., 2015b). Therefore, cold plasma has been identified as a potential novel curing agent for meat products because it can provide similar characteristics to synthetic nitrites (Jung et al., 2015b).
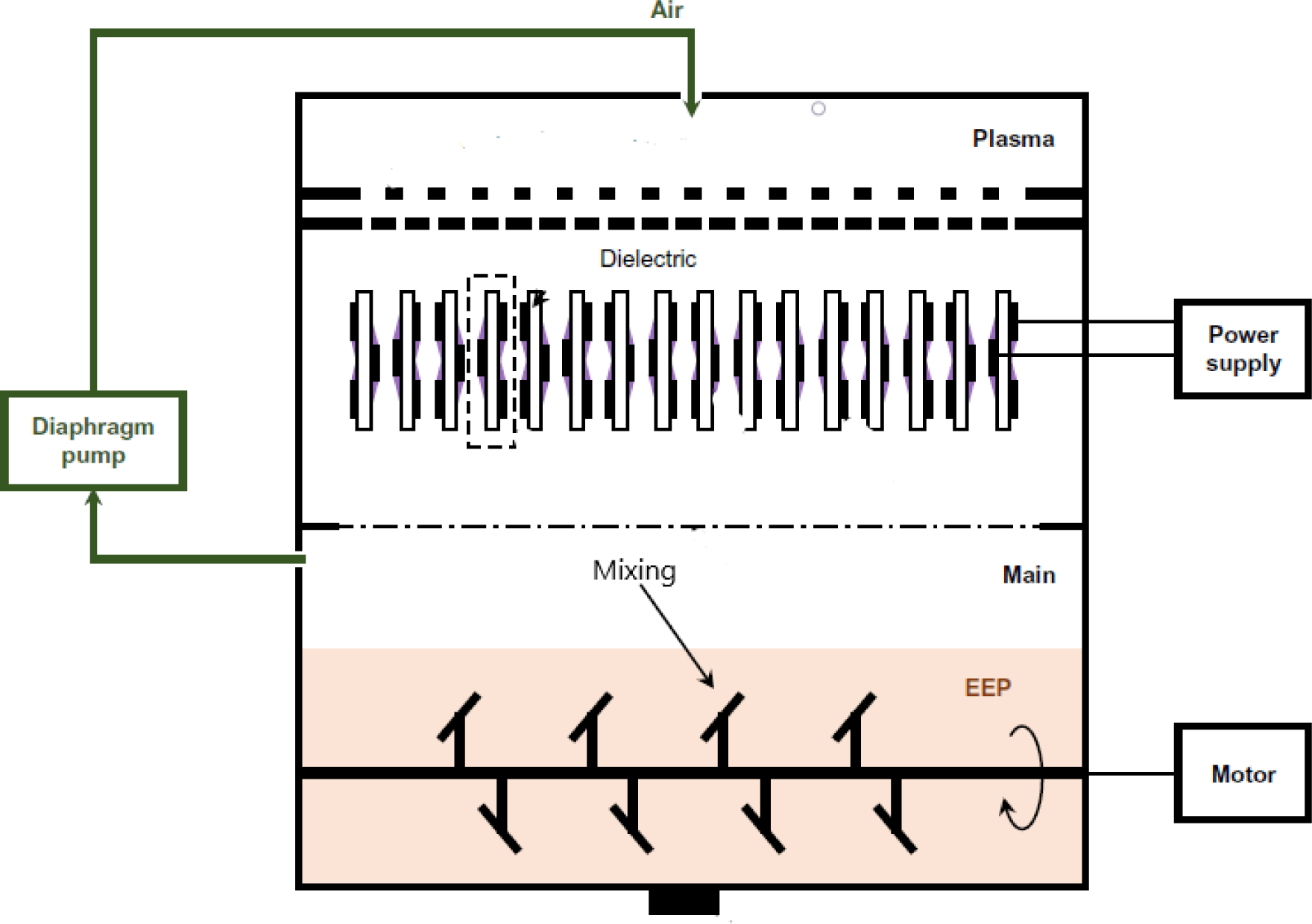
Comparable meat quality traits such as color, lipid oxidation, and sensory characteristics were reported in emulsion sausages and pork loin hams when they were cured using plasma-treated water and sodium nitrite. Importantly, the residual nitrite contents of the two products cured with plasma-treated water was lower than those cured with sodium nitrite (Jung et al., 2015b; Yong et al., 2017b). Moreover, Jung et al. (2017b) and Lee et al. (2018) have explored the potential use of cold plasma treatment to generate nitrite in meat batter with levels ranging from 42 to 65.96 mg/kg. Fig. 4 shows the cold plasma curing system used to treat meat batter and ethanolic extract of Perilla frutescens by Jung et al. (2017a). In addition, canned ground ham prepared from meat batter treated with cold plasma exhibited similar properties in terms of color, residual nitrite content, texture, and sensory attributes compared to those cured at similar nitrite levels using sodium nitrite or celery powder (Lee et al., 2018).
Yong et al. (2018) studied the mechanism of green discoloration of myoglobin induced by cold plasma and proposed that nitroso-myoglobin, which is a major compound for desirable pink color, can be produced in the reduced meat after plasma treatment. Furthermore, Kim et al. (2021) reported an effective way of enriching nitrite level in onion powder using plasma treatment to be used as natural materials with additional meat curing ability. Interestingly, natural nitrite has been derived from Perilla frutescens a plant with no original nitrate content following cold plasma treatment. In addition, the resultant lyophilized powder following plasma treatment has shown increased antimicrobial activity against C. perfringens and S. Typhimurium as opposed to that without plasma treatment (Jung et al., 2017a).
Limitations and Future Directives
Many authors have studied the optimal balance between plasma treatment conditions to maximize the bactericidal effects. However, the quality attributes of plasma treated meat is still less researched (Misra and Jo, 2017). The lipid oxidation might be induced in meat and meat products with high fat contents upon plasma treatment. The development of some off-flavors in meat and meat products has been reported due to rancidity development during subsequent storage (Lee et al., 2016a). In addition, meat discoloration and texture deterioration in plasma-treated meat have been detected (Jayasena et al., 2015; Kim et al., 2013; Lee et al., 2016a). Hence, there is a need for research to focus on retarding lipid oxidation in plasma-treated meat and meat products.
Data on the chemical residual effects and potential toxicity of plasma-treated meat and meat products are limited. Several studies reported no mutagenicity in meat and meat products treated with cold plasma (Kim et al., 2016b; Lee et al., 2016a) or cured with plasma-treated water (Yong et al., 2017b). However, further studies are required to fully confirm the safety of cold plasma-treated meat and meat products which would be vital in guiding decision and regulation.
The end reaction products from the reaction of plasma reactive species and other chemical agents such as essential oils are still not fully understood (Xinyu et al., 2020). Moreover, there is a need to investigate the precise mechanisms of chemical interactions with food ingredients and their impact on quality attributes of meat products. This will lead to the development of safe and high-quality meat products using cold plasma technology. Plasma-treated meat and its products could become microbiologically unsafe unless handled carefully post-treatment. Therefore, correct packaging methods and materials need to be applied to minimize post-treatment contamination. Therefore, further research is required for establishing cold plasma technology for meat and meat products and understanding the quality attributes of meat and its products to optimize the technology for specific applications in the meat processing industry.
Conclusion
In the context of growing concern over foodborne pathogens, ensuring safety and quality of meat and meat products to consumers poses significant challenges for the meat industry. Recently, non-thermal food processing technologies have attracted the focus in various sectors of the food industry, including meat, and poultry processing. Cold plasma is an emerging cost-effective non-thermal technology with high microbicidal efficacy without the need for temperature abuse, making it a promising alternative to traditional meat preservation methods. The reactive oxygen and nitrogen species generated by plasma not only effectively inactivate microorganisms but also enable researchers to safely apply this technology to biological materials, including food. In addition, plasma-treated liquids have been shown to generate nitrite, which can act as a curing agent in cured meat products.













