Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease that has multifaceted characteristics, such as pruritus, edema, xerosis, erythema, and lichenification (Yang et al., 2020). The age of disease occurrence is not fixed but the disease commonly develops by 5 years (Eichenfield et al., 2014). The prevalence of AD has been on the rise since the 1970s and especially in the advanced countries, AD has occurred 2–3 times more (Hadi et al., 2021). Although the studies on AD have been increasing, etiology of AD has not been fully understood (Kim et al., 2019). Though, genetic factors, environmental factors, immune system dysregulation (system failure to function properly) and epidermal barrier disruption are considered convincing causes. Among them, skin barrier dysfunction, allergen and immune dysfunction are essential factors of AD (Kim and Leung, 2018). Progression of AD is commonly divided into three phases: acute, subacute, and chronic AD (Berke et al., 2012). Chronic AD is deeply related to lichenification because patients suffering from pruritus repeat scratching their skin. Repeatedly scratching exacerbates the condition of the skin barrier. Accordingly, the skin gets thickened and leathery, which is known as ‘lichenification’, a severe symptom of AD (Nam et al., 2021).
AD is mainly treated with corticosteroids, topical immunosuppressants and antibiotics (Bieber, 2022). Specifically, corticosteroids are primarily used to treat AD, however, its severe side effects including skin atrophy, hypopigmentation, telangiectasia, steroid acne, adrenal suppression, growth retardation, Cushing’s syndrome and cataracts have been reported in prior studies (Gómez-Escobar et al., 2020). Hence, developing more safe and effective treatment has great significance and crude drug preparations for AD have been actively researched currently.
In terms of Korean traditional medicine, skin affliction is caused by various factors, such as heat of blood, dryness of blood, stasis of blood, or the exhaustion of kidney and liver (Jeon and Lee, 2016). Several studies have been conducted to find complementary and alternative medicines with better efficacies and less adverse effects (Liu et al., 2015; Tan et al., 2013). Most Korean traditional AD treatments are more focused on using plant-origin medicines than using animal-origin medicines. Though, animal-origin medicine has been reported to control the expression of cytokines, which means that animal-origin medicine can also be used as an effective treatment to remedy AD (Prokopov et al., 2019).
Cicadidae Periostracum (CP) is the skin of Cryptotympana pustulata or Cryptotympana dubia that is sloughed when they become an adult (Song et al., 2016). The Korean traditional medicine drug, CP, is known for treating skin affliction including AD as a traditional medicine in East Asian (Lim et al., 2019). In terms of Korean traditional medicine, CP can dissipate the heat from the lungs and lower the fever of liver through ‘hae-pyo-to-jin (解表透疹)’ efficacy and ‘ge-gyeng-toei-ye (止痙退翳)’ efficacy (Kim and Chae, 2015). Moreover, the fact that surgical skin diseases such as tetanus and tumors can be ameliorated with anti-oxidative and anti-inflammatory effects of CP is pharmacologically demonstrated (Xu et al., 2006).
According to the previous research, various alternative therapies which use traditional medicine for AD have been studied to reduce the side effects of steroid therapy, which is widely used to alleviate AD in recent years (Park et al., 2021). CP has been found out to have the effect of lowering the expression level of helper T (Th)1/Th2 cytokines by regulating nucleotide-binding domain, leucine-rich–containing family, pyrin domain containing 3 inflammasome. However, we suggest that more research is needed to fully understand the efficacy and potential possibility of CP on AD. Therefore, in this study, we present the alternative therapy for AD that can be induced by the 2, 4-dinitrochlorobenzene (DNCB) reaction, identifying and using treatment mechanisms of the CP, one of the animal-origin medicines.
Materials and Methods
The dried Cicada slough derived from C. pustulata (Cicadidae), also known as CP, was purchased from Dong-Yang Herb (Seoul, Korea). Ten grams of CP was washed with distilled water and extracted with 300 mL of distilled water for 2 h by using reflux extractor. The extract was filtered under a 10 μm filter paper and concentrated by a rotary vacuum evaporator (Eyela, Tokyo, Japan). The residue was lyophilized using a freezing dryer (Ilshin Bio, Dongducheon, Korea) to 16.4% yield for 72 h. Sample was named CP and stored at −20°C until use.
Female BALB/c mice aged 6 weeks old were procured from DBL (Eumseong, Korea). All experiments were conducted according to the guidelines of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by Committee on Care and Use of Laboratory Animals of the Kyung Hee University (KHSASP-20-053). The mice were maintained under a 12-h light/dark cycle at a controlled 20°C–25°C temperature and 50±5% humidity. All mice were freely fed with diet and autoclaved water. The adaptation period was 1 week. AD mice were established by sensitization of DNCB (Choi et al., 2018). Briefly, mice were separated into 4 groups (n=6); NOR, normal control; DNCB, negative control, DNCB-sensitized AD mice with vehicle treatment; dexamethasone (DEX), positive control, DNCB-sensitized AD mice with DEX treatment; CP, DNCB-sensitized AD mice with CP treatment. For the sensitization, 200 μL of 1% DNCB in acetone/olive oil (4:1, v/v) was topically administered to the skin of mice in dorsum once daily for 3 days. After then, mice were resting for 4 days. For the challenge, 0.5% DNCB was topically applied to the same region with the 4% sodium dodecyl sulfate (SDS) to make samples to get through the skin barrier. AD-like mice with DNCB were topically treated with 200 μL of 10 μM DEX and 100 μg/mL CP daily for 2 weeks.
The severity of the AD-like skin lesions was estimated based on the standard of dermatitis score (Nam et al., 2021). Dermatitis score was summed with erythema/hemorrhage, dryness/scarring, edema and erosion/excoriation from 0 to 3 score, respectively. All measurements were conducted as blind test by 3 different experts. Dermatitis score was scored on Day 4, 7, 14, and 21, respectively. At the end of experiment, the mice were assigned into a separated cage right after 0.5% DNCB application to the dorsal skin. The scratching behavior was recorded for 20 mins using a video camera. The number of scratching was counted as 1 when mice turned into the back and scratched. While, scratching of the face by the hind paw was excluded. All test was conducted as blind test by 3 different experts. The count was averaged per mice.
After scratching behavior recording, all mice were sacrificed under anesthesia. The skin tissues of dorsum were collected and fixed in a 10% neutralized formalin. After 24 h, the specimens were washed and dehydrated with ethanol and xylene. Paraffin-embedded skin tissues were sectioned in a 4 μm thickness and stained with a Hematoxylin and Eosin (H&E) staining solution and toluidine blue staining solution. The thickness of epidermis and dermis were analyzed using an Image J (National Institutes of Health, Bethesda, MD, USA). The number of mast cells was counted every slide per mice and averaged.
The blood was centrifugated and serum was collected to measure serum immunoglobulin E (IgE) levels. According to the instruction, serum IgE level was detected using a mouse IgE ELISA kit (BD Biosciences, Franklin Lakes, NJ, USA). All experiments were performed in triplicate and repeated three times.
Human keratinocyte HaCaT cells were grown in Dulbecco’s modified Eagle’s medium, with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. 1×105 cells were incubated in each well of a 6-well culture plate. The three concentrations of CP at 1, 10, and 100 μg/mL were used for treatment in the presence of 20 ng/mL of tumor necrosis factor (TNF)-α and 20 ng/mL of interferon (IFN)-γ. DEX was added at the 1 μM concentration. The cells were incubated with the samples for 24 h and harvested to extract RNA and protein.
The skin tissues and cell lysates were soaked with Trizol reagent to isolate total RNA according to the manufacturer’s instruction. One microgram of RNA was synthesized into complementary DNA (cDNA) using a Maxime RT Premix (iNtRON Biotechnology, Sungnam, Korea). After normalization of gene expression by confirming housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the cDNA was synthesized with Maxime polymerase chain reaction (PCR) premix (iNtRON Biotechnology) and specific primers, respectively. GAPDH, interleukin (IL)-4, -13 and -22, kallikrein related peptidase (KLK)-5 and -7, macrophage-derived chemokine (MDC), regulated on activation, normal T cell expressed and secreted (RANTES), Serine Peptidase Inhibitor Kazal Type (SPINK) 5 and thymus and activation-regulated chemokine (TARC) were amplified (Table 1). The amplification program started with a pre-denaturation of 94°C for 5 min; followed by 35 cycles that consisted of denaturation at 94°C for 30 secs, annealing at 55°C–65°C for 1 min and extension at 72°C for 2 min and ended with heating at a temperature of 72°C for 7 min and cooling at 4°C. Each PCR product was separated by 1.5% agarose gel. The mRNA expressions were visualized by a unified gel documentation system (DAIHAN, Daegu, Korea). The bands were normalized to GAPDH. The expression values were quantified using an Image J (National Institutes of Health).
PCR, polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; KLK, kallikrein related peptidase; MDC, macrophage-derived chemokine; RANTES, regulated on activation, normal T cell expressed and secreted; SPINK, serine peptidase inhibitor Kazal type; TARC, thymus and activation-regulated chemokine.
The skin tissues and cell lysates were soaked with radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail tablet (Roche, Penzberg, Germany) to isolate total protein. The total proteins were separated onto 10% SDS polyacrylamide gel electrophoresis gels and then transferred into polyvinylidene fluoride membranes. The membranes were incubated overnight with primary antibodies in Tris-buffered saline with Tween 20. After washing, secondary antibodies conjugated to horseradish peroxidase was applied to membranes for 1 h at room temperature. Bands were detected with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Uppsala, Sweden). The expression values were quantified using an Image J (National Institutes of Health).
Results
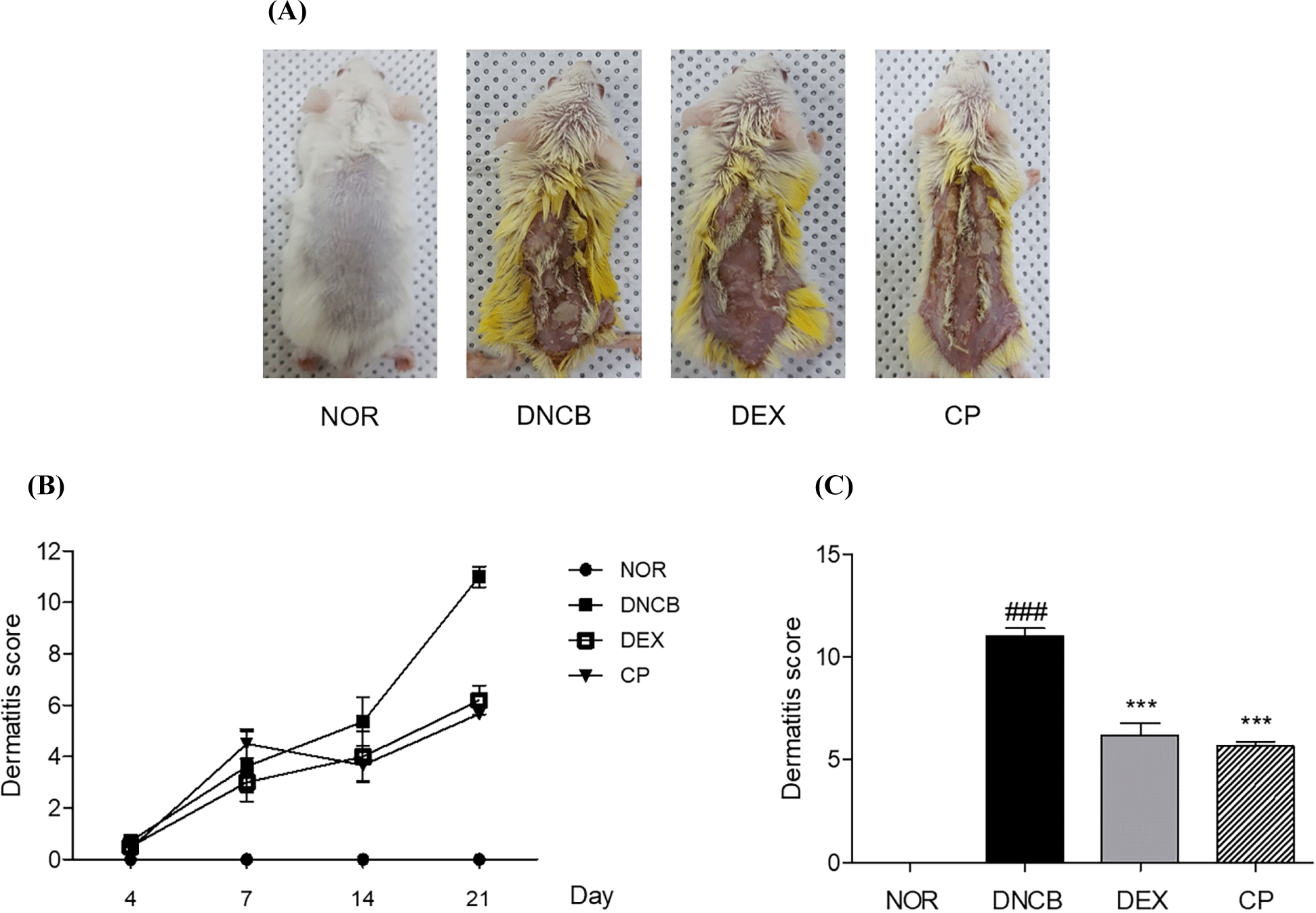
We used DNCB to cause AD reaction on the skin of mice, and as a control, DEX was used as a positive control to confirm the effectiveness of CP on AD. The morphological improvements of erythema and hemorrhage, the commonly observed symptoms of AD, can be observed on the mice induced with CP (Fig. 1A). The dermatitis score including erythema and hemorrhage levels, were checked on day 7, day 14, day 21, and it tends to increase over time in response of DNCB (Fig. 1B). Assuming that the dermatitis score of mice induced with DNCB was 11.00, the dermatitis score of mice treated with CP in AD-like skin legion was 5.67, which shows that the score decreases 48.5% than DNCB group (Fig. 1C).
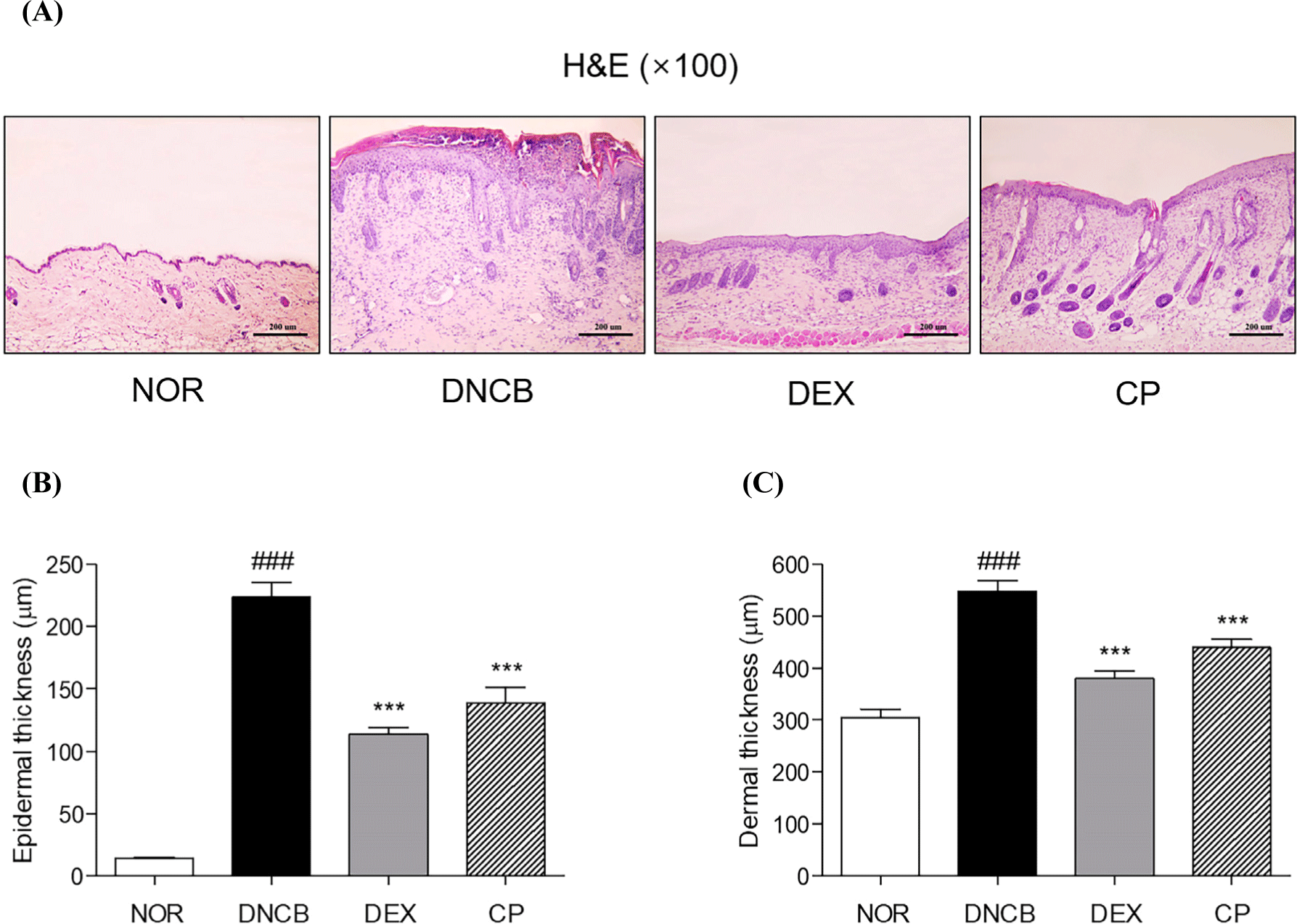
From H&E staining, which can show the histopathological features of dorsal skin tissue, the epidermal thickness, epidermal hyperplasia and hyperkeratosis were showed in DNCB-induced AD-like legion, leading to skin lichenification. The epidermal thickness was 37.95% lower in the mice treated with the CP, compared to the DNCB group (Figs. 2A and B). Also, the inhibition rate of dermal thickness in CP-treated group was 19.70% compared to DNCB group (Figs. 2A and C).
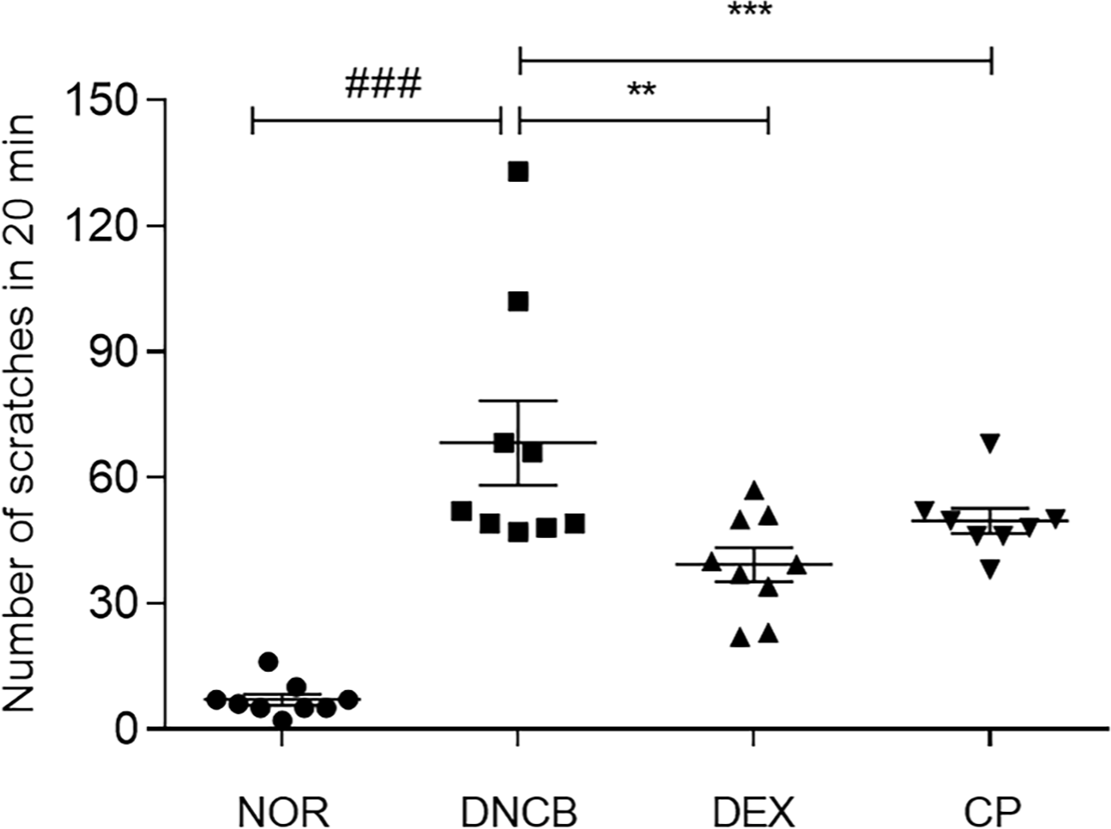
Scratching behavior is appeared in both human and mice in AD. In other words, the degree of AD can be evaluated by checking the number of scratching behavior. We measured the number of scratching behaviors of mice for 20 mins. DNCB induced 66.8 times increase of scratching behavior in mice. The mice treating with CP showed a 27.2% decline of scratching behavior compared to the DNCB group (Fig. 3).
The number of mast cells can be counted by toluidine blue staining. It can be visually confirmed that the number of mast cells was significantly increased by DNCB induction 12.14-fold. The number of mast cells in dermal skin tissues was 27.94% lower in the mice treated with the CP, compared to the DNCB group (Figs. 4A and B).
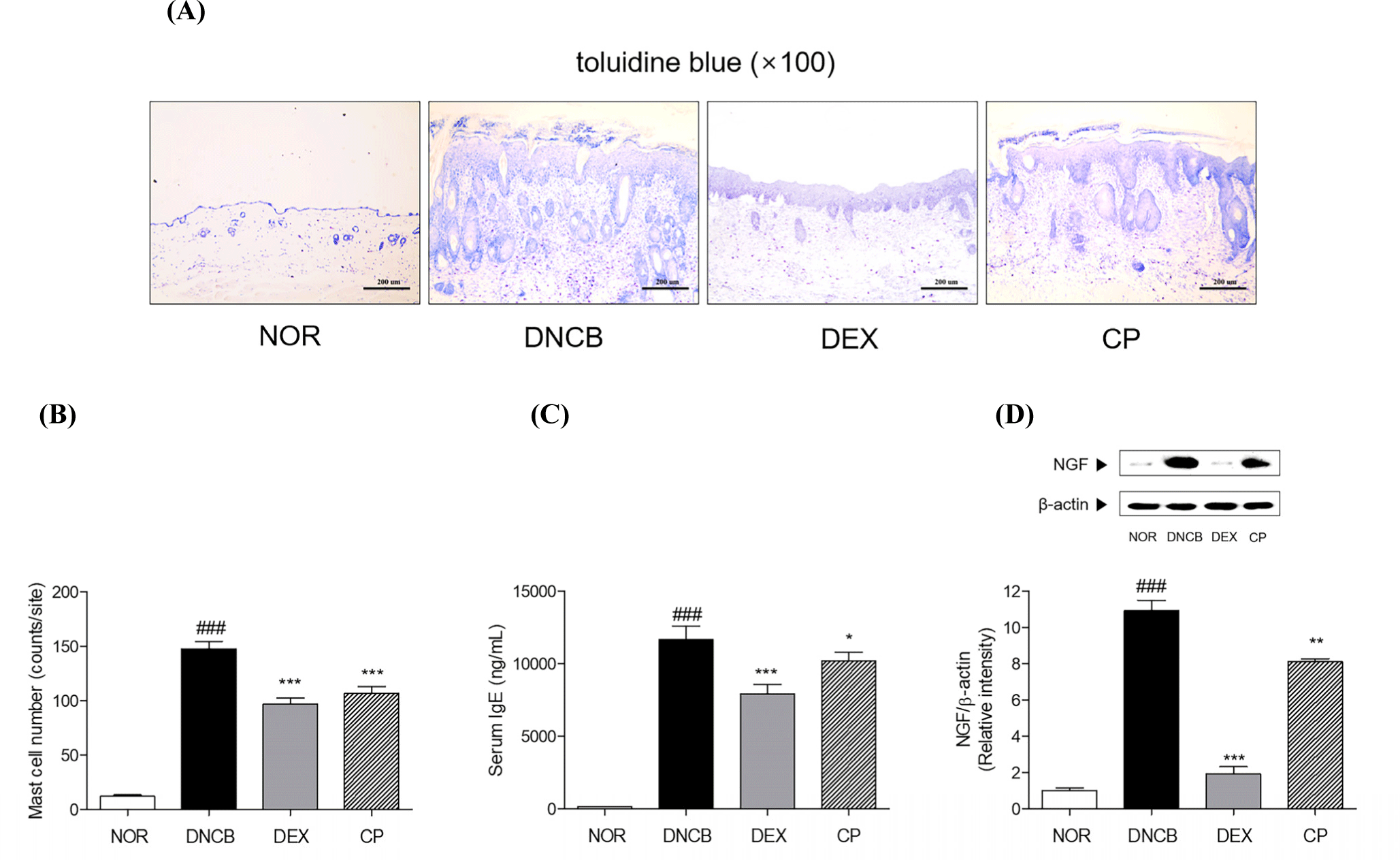
IgE is one of the antibodies that is involved in immune responses, especially in AD. In order to evaluate the degree of atopic dermatitis, IgE involved in this was measured and it was 87.79 times increased by DNCB induction. The mice treating CP topically showed 12.74% decline of serum IgE level compared to the DNCB-induced AD mice (Fig. 4C).
There was a significant elevation of NGF protein expression by 10.9-fold in AD-like skin legion. CP tends to decrease 25.72% of NGF in skin tissues of DNCB-induced AD mice (Fig. 4D).
Marked decreases of skin barrier-related genes, filaggrin and claudin-1, were showed by DNCB sensitization in skin tissues. CP treatment significantly increased the protein expressions of filaggrin and claudin-1 by 109.87% and 102.65% compared to DNCB group (Fig. 5A). In addition, the expressions of KLK5 and KLK7, factors that can disrupt the skin barrier, were decreased, while that of SPINK5, a factor that regulate the expression of KLK5 and KLK7, was increased by DNCB sensitization. CP has been shown to effectively regulated those factors. CP has been proven that it can increase the expression of SPINK5 by 125.72% in AD-like skin legion. CP reduced the 8.35-fold and 6.06-fold elevated expressions of KLK5 and KLK7 by DNCB sensitization in the skin tissues by 78.62%, and 70.75%, respectively (Fig. 5B).
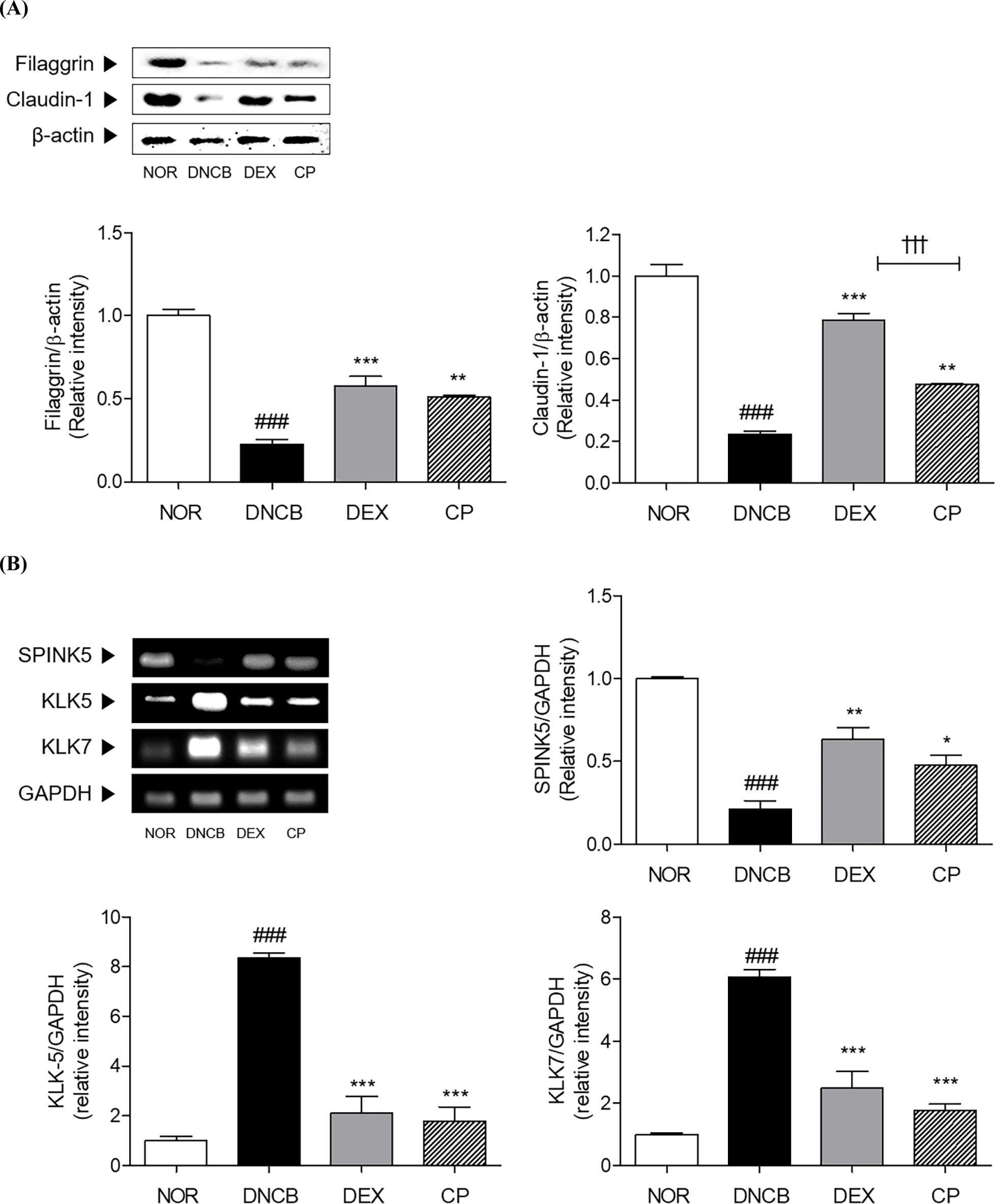
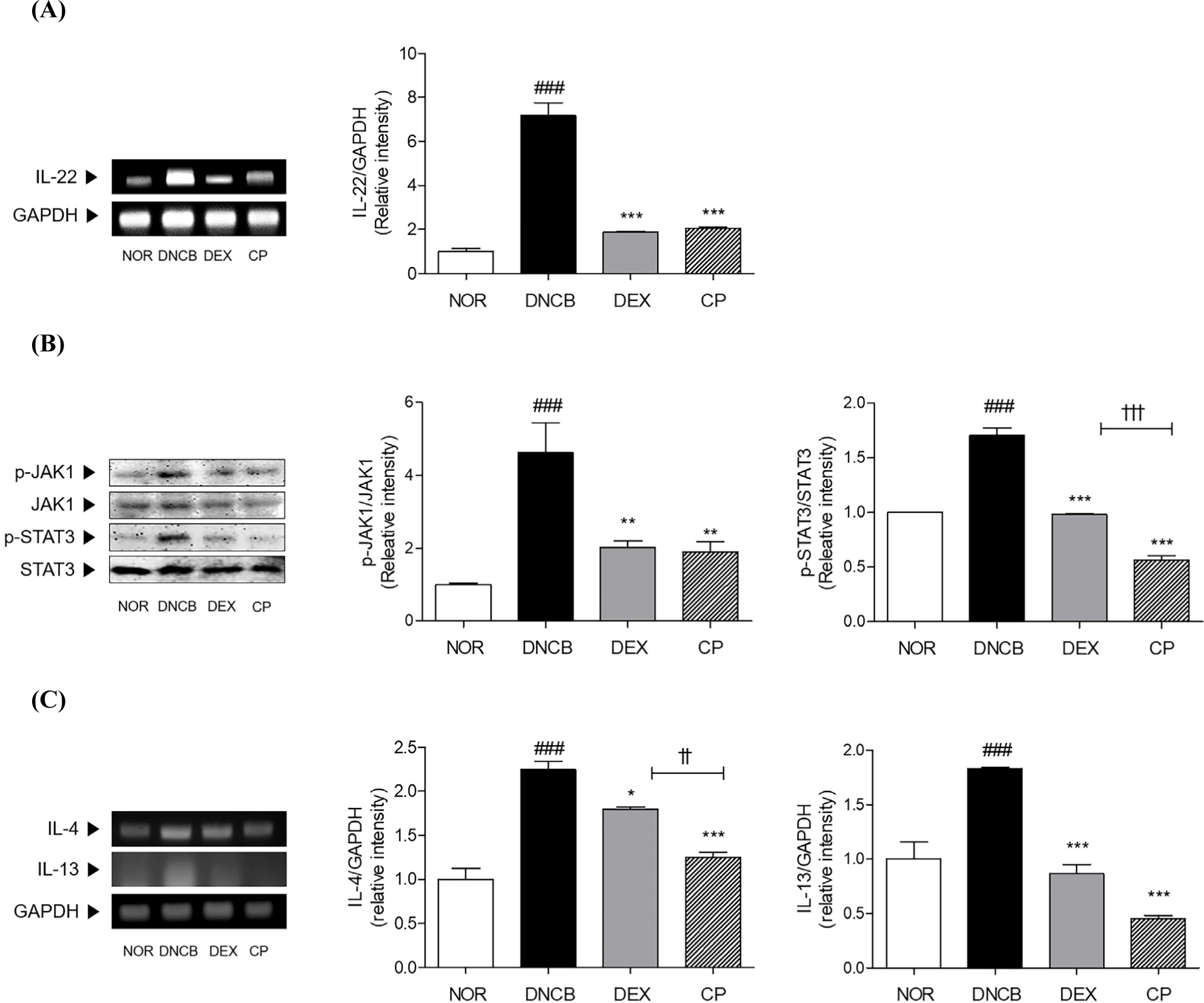
The mRNA level of IL-22 was apparently 7.16-fold increased in the DNCB-induced AD group compared with that in the normal group. After topical treatment with CP, there was significant decrease by 71.33% in the level of IL-22 in comparison with that upon DNCB treatment (Fig. 6A). Additionally, the JAK1 and STAT3 were 4.62 times and 1.71 times phosphorylated in the skin tissues by DNCB sensitization. The protein expressions of phosphorylated JAK1 and STAT3 were significantly decreased by 58.85% and 66.75% by CP treatment in AD mice (Fig. 6B). Moreover, the expressions of IL-4 and -13 in DNCB group was 2.24-fold and 1.83-fold higher than NOR group. Treatment of CP significantly reduced those expressions about 44.21% and 75.60%, respectively, compared with DNCB group (Fig. 6C).
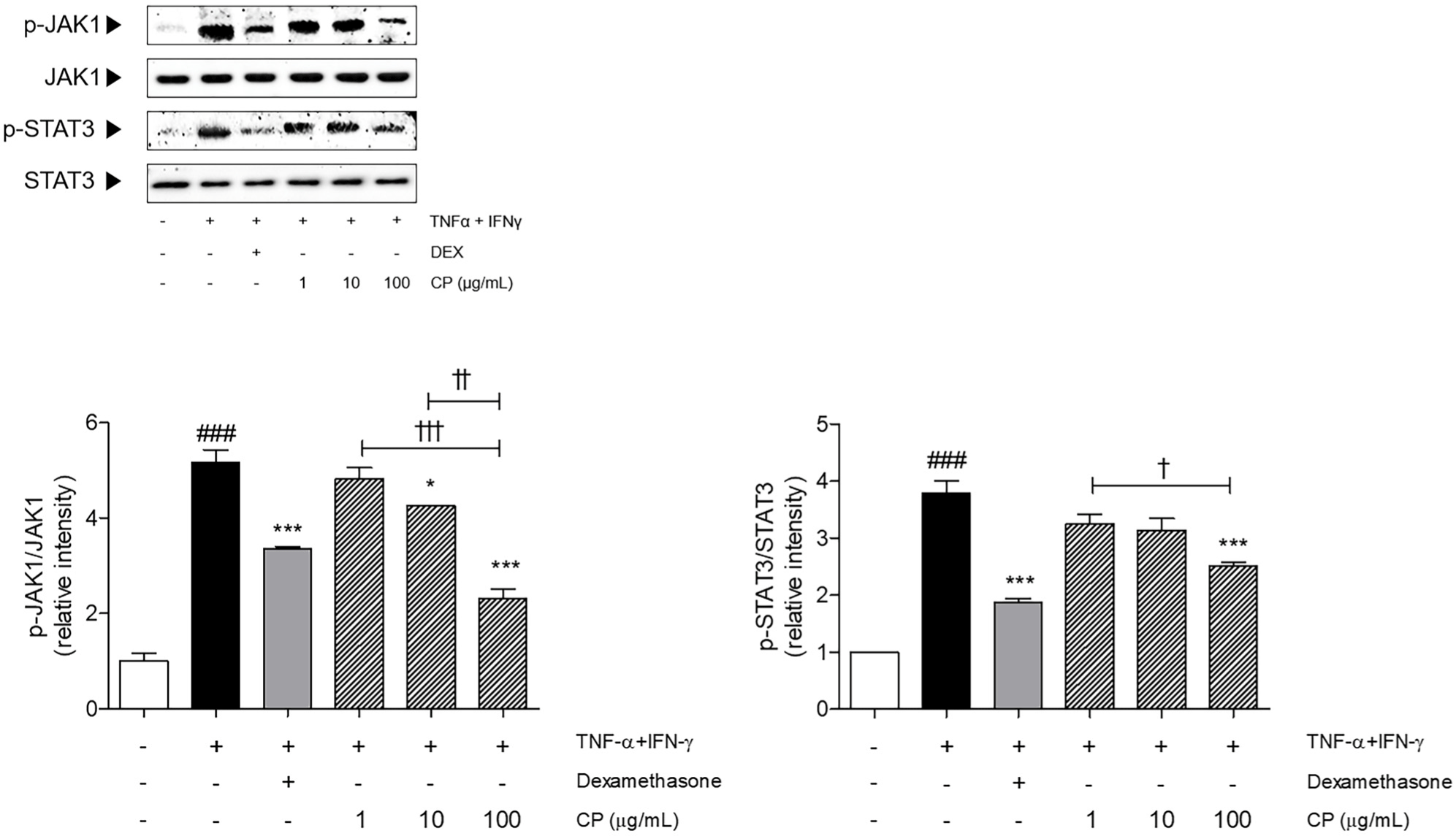
Compared with the non-treated cells, the expressions of phosphorylated JAK1 and STAT3 were 5.51-fold and 3.79-fold elevated in the TNF-α and IFN-γ-induced keratinocytes. The CP treatment at the concentration of 100 μg/mL significantly reduced the phosphorylated JAK1 and STAT3 expression levels compared to only TNF-α and IFN-γ-sensitized cells by 53.34% and 33.86%, respectively (Fig. 7).
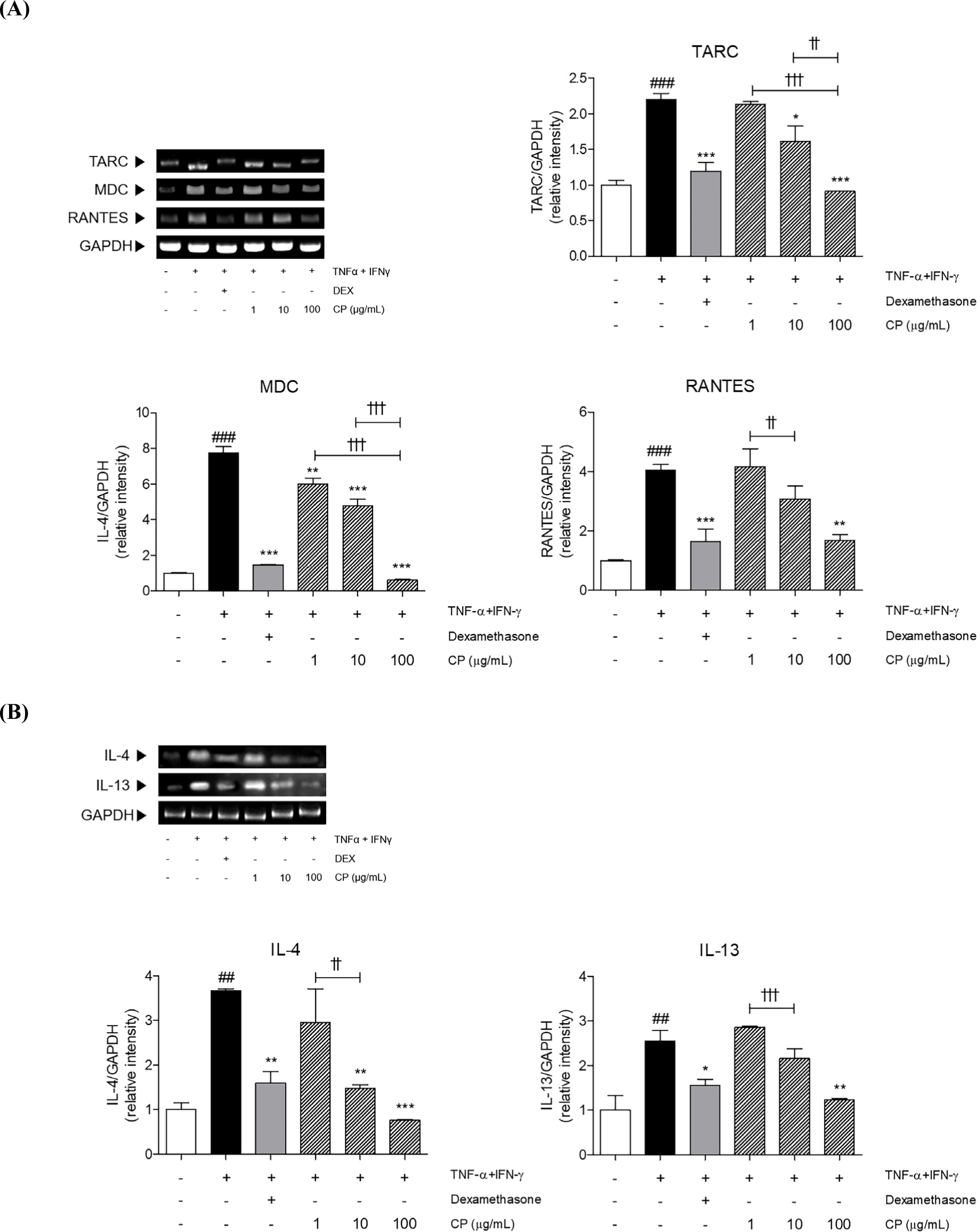
There are several mediators to induce allergic inflammation in AD. We measured the expressions of IL-22/JAK1/STAT3 pathway-mediated chemokines and Th2-specific cytokines in TNF-α and IFN-γ-induced keratinocytes. The mRNA levels of TARC, MDC and RANTES were significantly increased 2.20 times, 7.74 times, and 4.04 times by TNF-α and IFN-γ sensitization in HaCaT cells. One hundred micrograms per milliliter of CP treatment decreased those chemokines by 58.69%, 92.08%, and 58.64%, respectively compared to only TNF-α and IFN-γ-sensitized cells (Fig. 8A). Additionally, the 3.67-fold and 2.55-fold increased mRNA expressions of IL-4 and IL-13 were effectively down-regulated by CP treatment at the concentration of 100 μg/mL by 79.22% and 51.51%, respectively, compared to only TNF-α and IFN-γ-sensitized cells (Fig. 8B).
Discussion
Crude drugs have been explored for decades, as safer alternatives to synthetic pharmaceutics (Cheng et al., 2009). They have been used to treat a variety of diseases in Asian countries for thousands of years. AD is one of the diseases that can be effectively treated with crude drug administration. Two routes of administration are mainly used: topical and oral application. Commonly, topical administration is regarded as a preferred route for the treatment of AD with no side effects and faster effects, compared to oral administration (Nygaard et al., 2018). In previous studies, the topical administration of AD with CP has been supported as an effective therapy (Park et al., 2021). 3% CP ointment has therapeutic effects on AD development without any side effects. In addition, herbal product including 2.5 mg of CP decreased the total lesion score with regard to erythema, surface damage, pruritus and sleep scores in refractory AD patients compared to placebo group with no significant adverse effects (Cheng et al., 2010). Max 514 μg/kg of CP would have no toxicity by conversion from human to mice dosage through human equivalent dose formula, although that study could be applied to the oral administration. In this study, topical treatment of 100 μg/mL of CP showed any toxicity and adverse effects. CP as a medicine derived from animal source can be used as effective AD treatment in that it has anti-inflammatory and antiallergic actions. The decrease in the total lesion score in the treatment group at 8 weeks was significantly greater than that of the placebo group (79.7±5.8% vs. 13.5±7.64%; p<0.001). There was also a statistically significant difference between the treatment and placebo groups.
AD is a complicated inflammatory skin disease that is characterized by atopic pleats, cheilitis, hyperlinear palms, ichthyosis, keratosis pilaris, lichenification, papules, urticaria and more (Correale et al., 1999). In this study, we used various biomarkers to identify symptoms of AD. To objectively evaluate the process, the dermatitis score is used to check overall atopic dermatitis mechanism by using dermatitis score. There are erythema and hemorrhage on the skin in DNCB-induced AD. It has been proven that CP has visual improvement of AD such as erythema and hemorrhage, indicated by dermatitis score.
One of the most serious problems of AD is pruritus (Hong et al., 2011). It is important to reduce pruritus so that it prevents recurrence or deterioration of AD (Kahremany et al., 2021). The previous study has reported that inflammatory reactions due to atopic dermatitis can precipitate pruritus (Frazier and Bhardwaj, 2020). The results of this study have shown that CP can reduce pruritus by regulating inflammatory reactions. The pruritus can cause repeated scratching behavior that leads to epidermal hyperplasia, that makes skin barrier weaken and induces the hyper keratinized epithelium, lichenification (Yosipovitch et al., 2019). In addition, mast cells are known for causing inflammatory reactions on skin, the variation of the number of mast cells can affect inflammatory reaction and pruritus (Thangam et al., 2018). The results of this study also have pointed out that CP can diminish the number of mast cells in keratinized epithelium. As a result, it has been proven that CP reduced scratching behavior in AD. Previous researchers have showed that CP declined NGF with claudin, increasing filaggrin that can alleviate skin barrier disorder (Yang et al., 2018). Epidermal and dermal thickness of skin treated with CP, the results of present study, correspond well with those found that CP has effect on skin disorder.
Th2-mediated AD is mainly focused on in this study. In Th2-mediated allergic inflammation response, Th22 cells secrete the cytokines, IL-22, due to the disrupted outer skin by foreign antigen (Jiang et al., 2021). These IL-22 cells decline Fillaggrin by activating JAK1-STAT3 pathway in keratinocyte, and it can increase the skin barrier decomposition enzymes, KLK5 and KLK7, that provoke damaging skin barrier by lipid barrier disruption (Kasparek et al., 2017). The results of present study have shown that CP can decline IL-22 secreted by Th22 cells, which means it has anti-inflammatory effects and prevents chronic AD. The effects of CP in this mechanism, preventing water loss and reducing inflammatory response, is similar to previous results. There are various immunological hypotheses about AD such as skin barrier dysfunction, genetic susceptibility, and dysregulation of the immune system. About induction of allergic inflammation, imbalance between Th1 and Th2 cells is considered the main factor (Zhang et al., 2014). Development of AD goes through two stages: ‘sensitization’ and ‘elicitation’. When allergen infiltrates the skin, Langerhans cells phagocytize and transfer the allergen to local lymph nodes (Matsui et al., 2020). Reacting to the antigen, memory T lymphocytes secrete diverse kinds of cytokines including IL-1, IL-2, IL-3, IL-6, IFN-γ, TNF-α, granulocyte-macrophage colony-stimulating factor that induce inflammatory response (Dong, 2021). The imbalance between Th1 and Th2 can occur from increasing thymic stromal lymphopoietin (TSLP) which induces Th2 mediated immune response (Meng et al., 2021). Increase of TSLP activates Th2 cells, causing Th1/Th2 imbalance. After topical administration of CP, the expressions of IL-4 and IL-13 at animal model were decreased along with the decrease of serum IgE level. Consequently, it is demonstrated that CP can correct the imbalance between Th1 and Th2.
Pathophysiologically, there are two main theories about the cause of disease: Inside-out hypotheses and Outside-in hypotheses (Silverberg and Silverberg, 2015). In these theories, the point is on the function of the skin barrier. For skin barrier to function regularly, the FLG genes of which mutations are pivotal risk factors causing AD are important elements with other factors such as lack of skin barrier proteins, increase of peptidase activity, defect of specific protease inhibitors, and lipid disorder (Scharschmidt et al., 2009). When foreign substances like allergens and microbes enter the body, Th1, -17 and -22 cells secrete cytokines like IL-1, 17, 22 (Furue, 2020). They inhibit FLG genes and it leads to lichenification, which exacerbates the disease. Therefore, we aim for developing new external preparation which protects filaggrin genes from mutation and finally getting anti-pruritus effects.
At the change from acute to chronic AD, the most influential factors are IL-1, IL-17, and IL-22 each from Th1, Th17, Th22 cells. Among these cytokines, IL-22 phosphorylates JAK1, activates STAT3 and consequently induces chronic phase of AD with abnormality of keratinocytes and damaged skin barrier (Lejeune et al., 2002). The role of CP treatment is highly critical for control of the JAK1/STAT3 pathway. Through regulating the JAK1/STAT3 pathway, CP administration can inhibit the activity of IL-22 and it can block Th2 production caused by increasing TSLP (Park et al., 2021). In conclusion, CP treatment can recover Th1/Th2 balance. Additionally, CP has inhibitory effects on KLK5, KLK7 which impair skin barrier function, protecting the structure of our skin barrier.
While IL-4 secreted from Th2 cell is related with acute atopic eczema development, IFN-γ, IL-17, IL-22 secreted from Th1 and Th17 cell is related with chronic atopic eczema development (Eyerich and Novak, 2013). It is demonstrated that CP has an inhibitory effect on both of two cases. In other words, it is suggested that CP has an ameliorating effect on atopic dermatitis caused by imbalance between Th1 and Th2 from cytokine overproduction. Taken together, CP effectively regulated the IL-22-mediated allergic inflammatory response, leading to the recovery of barrier disruption and amelioration of lichenification in AD (Fig. 9). IL-22-activated JAK1/STAT3 signaling pathway impair both of epidermal barrier homeostasis and Th1/Th2-mediated allergic inflammation, and CP reversed by modulating the IL-22 signaling pathway. Eventually, treatment of CP apparently inhibited the exacerbation of itching and attenuated the abnormal epidermal hyper-proliferation and skin barrier dysfunction via IL-22-mediated allergic inflammation in the development of AD.
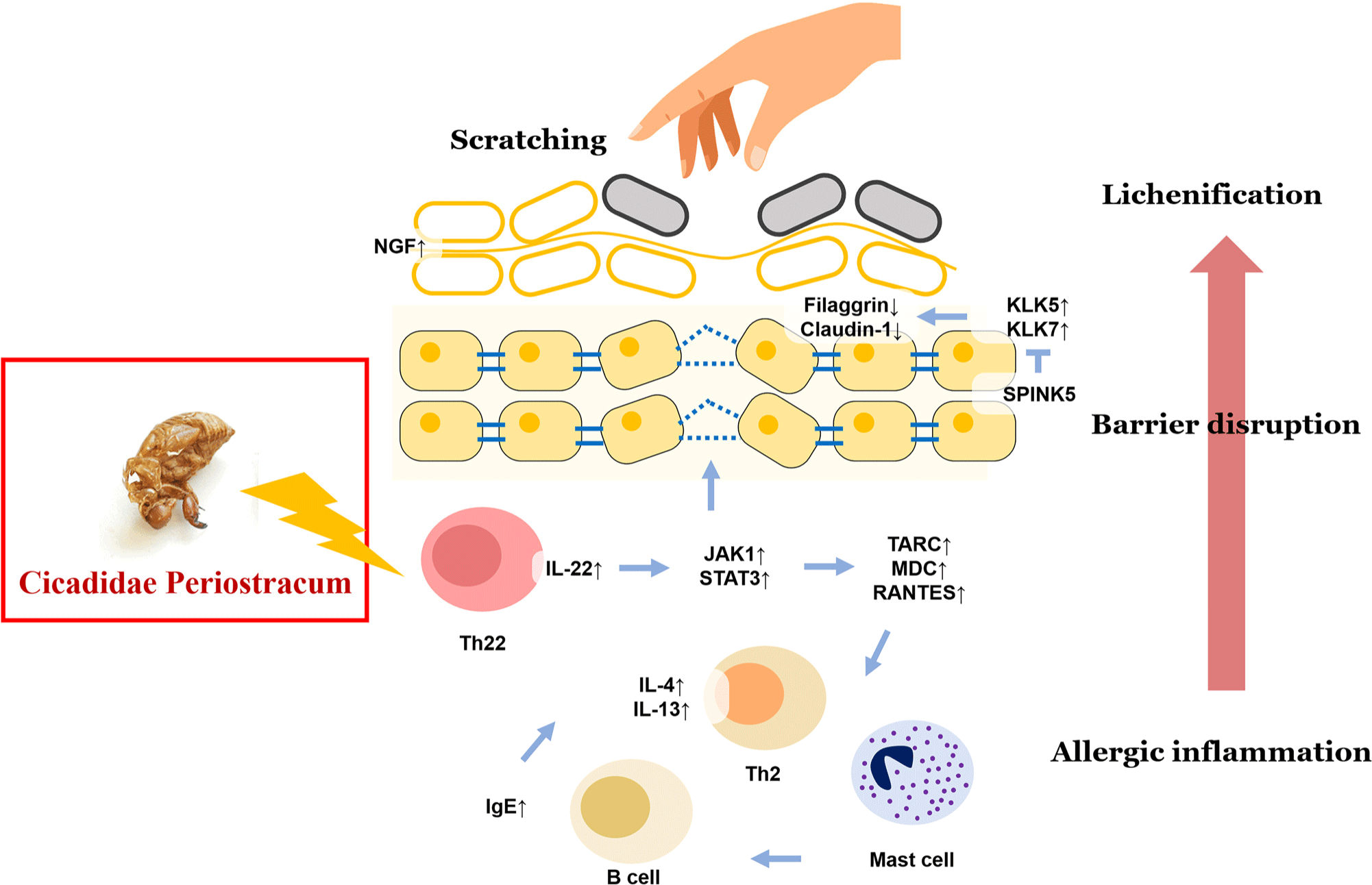
In conclusion, CP has considerable importance in ameliorating and treating the development of AD disease. By using CP as an external preparation, we can achieve not only a more direct effect on symptoms of AD such as pruritus, lichenification, edema but also more safe treatment of AD disease.
Above all, it has great significance that CP treatment can systematically ameliorate symptoms and fundamental problems of AD.













