Introduction
Gingivitis and periodontitis are common chronic inflammatory diseases and these periodontal diseases occur in about 20%–50% of the world’s population. Moreover, periodontal diseases increase the risk of systemic diseases, such as cardiovascular disease (Nazir, 2017). The surgical intervention and antibiotic treatment may not be sufficient to control periodontitis because of the complex etiology involved in oral microbiota (Gatej et al., 2017).
Probiotics are defined as safe live microorganism exerting health benefits and disease prevention when consumed in adequate amounts (Pineiro and Stanton, 2007). The composition of oral microbiota was significantly different between healthy and periodontitis patients, and adequate oral lactobacilli, such as Lactobacillusparacasei and Lactobacillus plantarum, were able to reduce the occurrence of dental caries by inhibiting the growth and colonization of cariogenic bacteria (Kõll-Klais et al., 2005). Lactobacillus casei 393 and L. plantarum B719-fermented milk increased osteoblast activity and prevented bone loss in ovariectomized rats (Kim et al., 2009; Lee et al., 2020). In addition to probiotics, postbiotics refer to non-viable bacterial components or metabolites of probiotics, mitigate various inflammatory diseases, such as inflammatory bowel disease, rheumatoid arthritis, and obesity (Bungau et al., 2021; Cristofori et al., 2021).
As an ongoing effort to develop oral probiotics, Lactobacillus reuteri MG5346 (MG5346) has been selected from our preliminary screening study. L. reuteri is a Gram-positive bacterium that inhabits various locations in the human body, including the gastrointestinal tract, urinary tract and skin (Mu et al., 2018). L. reuteri showed an immune modulation effect by inducing anti-inflammatory regulatory T cells while reducing pro-inflammatory cytokines (He et al., 2017; Hsieh et al., 2016). L. reuteri lozenges helped to treat chronic periodontitis as an adjuvant treatment and delayed recolonization for up to 6 months in the follow-up study (Tekce et al., 2015). In our previous study, probiotic culture supernatant (CS) inhibited both Streptococcus mutans-induced biofilm formation and receptor activator of the nuclear factor B ligand (RANKL)-induced osteoclast formation (Jung et al., 2021). The inhibitory activity of CS on biofilm formation and osteoclastogenesis varied depending on the probiotic strain. These results suggested that the efficacy of oral probiotics, such as L. reuteri, are also strain-specific.
The objective of the present study was to examine the efficacy of MG5346 as an oral probiotic. To achieve this goal, the effects of the CS-MG5346 on RANKL-mediated osteoclastogenesis were analyzed. In addition, the effect of MG5346 administration on alveolar bone loss and tissue damage was evaluated using ligature-induced periodontitis rats.
Materials and Methods
Dulbecco’s modified Eagle’s medium (DMEM), -minimum essential Eagle’s medium (α-MEM), penicillin-streptomycin solution, and fetal bovine serum (FBS) were purchased from Welgene (Gyeongsan, Korea). TaqMan Gene Expression Master Mix, TaqMan probes (5’-fluorescein based reporter dye; 3’-TAMRA quencher), and High-Capacity RNA-to-cDNA Kit were purchased from Applied Biosystems (Foster City, CA, USA). RANKL was purchased from purchased ProSpec (Rehovot, Israel). All other reagents used in the experiment were purchased from Sigma-Aldrich (St. Louis, MO, USA).
MG5346 was originally isolated from fermented foods. CS-MG5346 was prepared by the method described previously (Jung et al., 2021) and kindly provided by Mediogen (Jecheon, Korea).
The gastrointestinal tolerance of MG5346 was determined by the method of Tokatlı et al. (2015) with slight modification. MG5346 was harvested (3,460×g, 10 min) after being cultured in MRS media at 37°C for 24 h. The MG5346 pellets were washed twice with sterile saline solution (0.85% NaCl, w/v) and resuspended to 107–108 CFU/mL in simulated gastric fluid (SGF; 3 g/L of pepsin in sterile saline solution, pH 2.5) or simulated intestinal fluid (SIF; 1 g/L of pancreatin, 0.3% bile salt in sterile saline solution, pH 8.0). The survival rate of MG5346 was determined after incubation at 37°C for 4 h in SGF and 6 h in SIF, respectively. The viable cells were counted on MRS agar and expressed by the following formula:
The antibiotic susceptibility of MG5346 was determined by the minimum inhibitory concentration (MIC) test strip method described previously (Jung et al., 2022).
The murine RAW 264.7 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10% FBS and penicillin-streptomycin (100 untis/mL) at 37°C in a 5% CO2 humidified atmosphere. Osteoclastogenesis was induced by replacing the α-MEM medium with the medium containing RANKL (100 ng/mL) and M-CSF (50 ng/mL). Cytotoxicity of CS-MG5346 was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Lee and Imm, 2017).
RAW 264.7 cells were seeded in 96-well plates at a density of 3×103 cells/well for 24 h and were cultured in the presence of RANKL (100 ng/mL), M-CSF (50 ng/mL), and CS-MG5346 for another 7 days. The cells were lysed using 0.05% Triton X-100/saline solution and were dispersed in 50 mM citrate buffer (pH 4.7) containing 10 mM sodium tartrate and 10 mM p-nitrophenylphosphate. TRAP activity was determined according to the method of Kim et al. (2019).
Animal experiments were conducted according to the guideline of the Institutional Animal Care and Use Committee (approval number: KNOTUS 21-KE-032). Male Sprague-Dawley rats (6 wks old; Orient Bio, Seongnam, Korea) were housed in an animal facility and maintained under the conditions of a 12-h light-dark cycle at 23±3°C, 55±15% humidity. After acclimation for 7 days, rats were randomly divided into three groups: 1) untreated control (n=10), 2) ligature+vehicle (n=10), 3) ligature+MG5346 (2×108 CFU/day; n=10). The ligature was placed around the right second molar of the mandible using a sterile 4-0 silk under anesthesia with zoletil 50 (Virbac, Carros, France) and xylazine (Rompun, Bayer AG, Leverkusen, Germany). Maintenance of the ligature was checked regularly during the entire experimental period (8 wk).
Mandibular jaws of all rats were scanned using a micro-CT (vivaCT 80, Scanco Medical AG, Brüttisellen, Switzerland) after 8 wk of ligation induction and MG5346 administration. The cement–enamel junction (CEJ)–alveolar bone crest (ABC) distance and degree of maxillary molar furcation involvement were used as an index of alveolar bone loss and periodontal tissue damage.
Total RNA was extracted using NucleoZOL reagent (Macherey-Nagel, Düren, Germany), and qRT-PCR analysis was performed using cell lysates [nuclear factor-activated T cells c1 (NFATc1), TRAP, c-Fos, TRAP, and cathepsin K] and rat gingival tissue [RANKL and osteoprotegerin (OPG)] as described previously (Jung et al., 2022). The relative expression of osteoclast-specific transcriptional factor and target genes were analyzed using the following probes: β-actin (Mm00607939_s1), TRAP (Mm00475698_m1), cathepsin K (Mm00484039_m1), NFATc1 (Mm00479445_m1), c-Fos (Mm00487425_m1), RANKL (Rn00589289_m1), and OPG (Rn00563499_m1). qRT-PCR was performed using the StepOne Plus Real-Time PCR System (Applied Biosystems), and the expression of target genes was normalized to the housekeeping gene, β-actin.
All analytical experiments were performed in triplicate, and SPSS Statistics (SPSS 26; SPSS, Chicago, IL, USA) software was used for statistical analysis. Data were expressed as mean±SD. Significant differences (p<0.05) were assessed using a one-way analysis of variance (ANOVA), followed by Duncan’s post-hoc test.
Results and Discussion
The acid resistance and bile salt tolerance of probiotics are the most important requirements to ensure health benefits to the host (Tokatlı et al., 2015). As shown in Table 1, MG5346 showed 91% and 92% survival rates in SGF and SIF, respectively. L. reuteri is one of the few resident Lactobacillus species found in various sites in the human body human body, including the gastrointestinal tract (Valeur et al., 2004). This high adaptability of L. reuteri might be related to its tolerance in the gastrointestinal environment. Chen et al. (2019) reported that L. reuteri WHH1689 contained various stress-resistant genes related to acid (FoF1-ATP synthase and the sodium proton antiporter) and bile (choloylglycine hydrolase and inorganic pyrophosphatae) tolerance.
| Strain | Initial count1) (Log CFU/mL) | Survival in SGF2) | Survival in SIF3) | ||
|---|---|---|---|---|---|
| Log CFU/mL | % | Log CFU/mL | % | ||
| L. reuteri MG5346 | 7.55±0.19 | 6.85±0.07 | 90.74 | 6.94±0.03 | 91.87 |
The antibiotic resistance of probiotics is a principal safety consideration because probiotics can be a source of transferable resistance genes to pathogens (Li et al., 2020). Thus, the MIC of eight antibiotics against MG5346 was determined. MG5346 showed much lower MIC values for eight antibiotics than corresponding cut-off MIC values. This result indicates that MG5346 does not have antibiotic resistance (Table 2). According to the report of Jose et al. (2015), probiotics generally show resistance to vancomycin, ciprofloxacin, gentamicin and streptomycin. Similar to the result of this study, 32 representative L. reuteri strains did not have any transferable or acquired antibiotic resistance. In addition, they did not show virulence potential in the gelatinase activity, and hemolysis test (Singh et al., 2012).
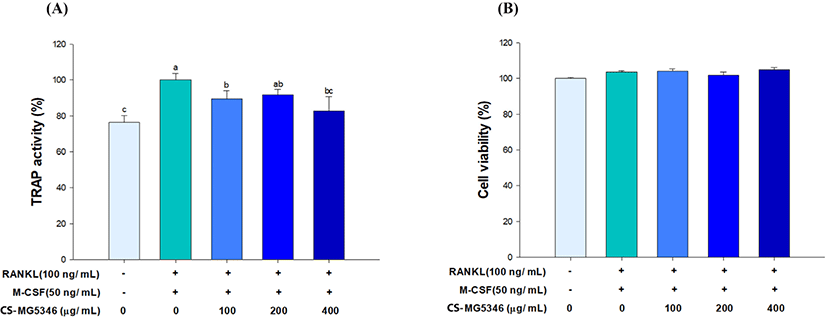
RANKL mediates the conversion of hematopoietic precursors, such as monocytes and macrophages into osteoclasts with the cooperation of M-CSF (Kong et al., 1999). TRAP is highly expressed in response to the conversion of macrophages to multinucleated osteoclasts, and increased TRAP activity is often used as a phenotype marker for osteoclasts (Tanaka et al., 2005). TRAP activity was significantly increased by RANKL stimulation, while the addition of CS-MG5346 decreased TRAP activity in a dose-dependent manner (Fig. 1A). This suggests that CS-MG5346 is able to inhibit osteoclast formation. Britton et al. (2014) reported that L. reuteri ATCC 6475 released compounds inhibiting RANKL-induced osteoclastogenesis. Although the exact nature of the inhibitory compounds for osteoclastogenesis was not clarified, histamine might be associated with the suppression of osteoclast differentiation. Thomas et al. (2012) demonstrated that histamine released from L. reuteri inhibited TNF- activity, which promotes osteoclastogenesis. In addition, CS-MG5346 did not show a cytotoxic effect in the MTT assay up to 400 g/mL. Thus, a further experiment proceeded within this non-cytotoxic concentration range (Fig. 1B).
Osteoclast differentiation requires transcription factors essential for the induction of target genes (Kim and Kim, 2014). The effects of CS-MG5346 on the gene expression of two key osteoclast-specific transcriptional factors (c-Fos and NFATc1) were analyzed. CS-MG5346 treatment significantly downregulated RANKL-mediated elevated c-Fos and NFATc1 gene expression in a dose-dependent manner (Figs. 2A and B). The binding of RANKL to RANK on the surface of osteoclast precursor cells recruits c-Fos at the early osteoclast differentiation stage, which in turn, activates NFATc1, a master regulator of osteoclastogenesis (Zhao et al., 2010). It has been reported that c-Fos-knock-out mice failed to undergo osteoclast differentiation (Wang et al., 1992). Thus, the downregulation of c-Fos and NFATc1 can be a major contributor to the inhibition of osteoclastogenesis.
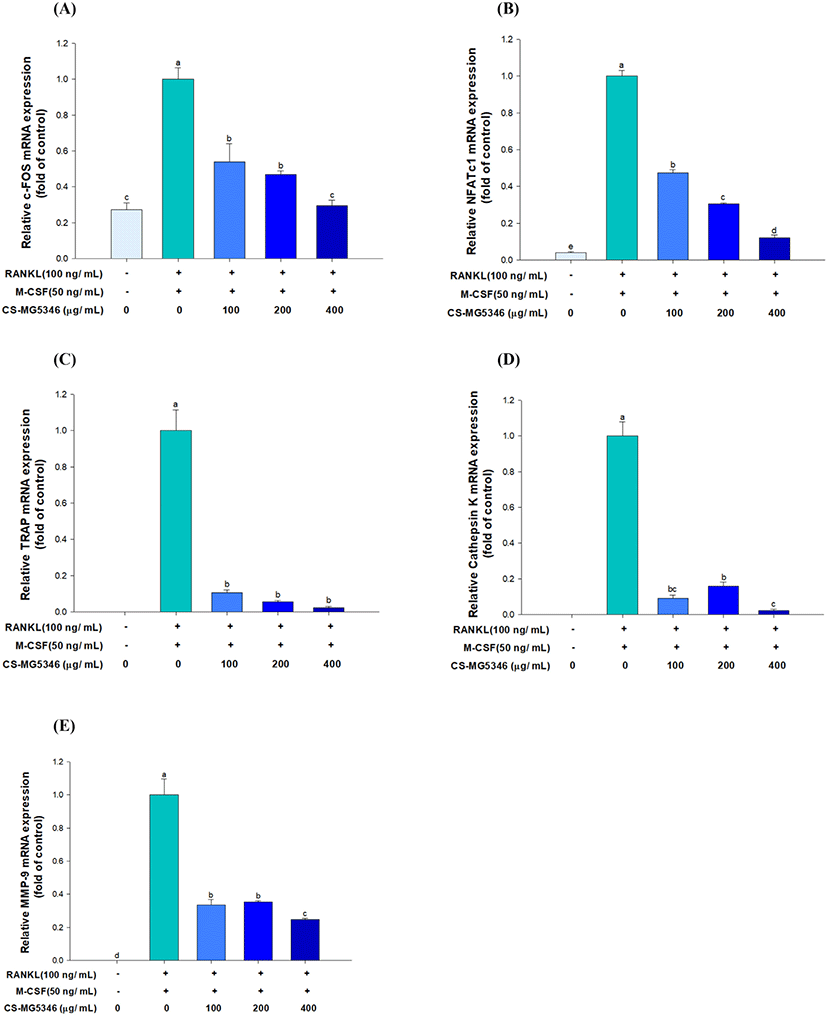
Stimulation of NFATc1 promotes the expression of osteoclast-specific genes, such as TRAP, cathepsin K, and metallo-proteinase-9 (MMP-9), that causing the degradation of bone extracellular matrix proteins (Asagiri and Takayanagi, 2007; Sundaram et al., 2007). Consistent with these reports, CS-MG5346 treatment significantly suppressed the expression of TRAP, cathepsin K, and MMP-9 (p<0.05; Figs. 2C, D, and E). The mRNA levels of TRAP, cathepsin K, and MMP-9 showed a high correlation with the level of bone resorption in patients with osteoarthritis and osteoporosis (Logar et al., 2007).
The mechanisms for the initiation and progression of periodontitis are still unclear and a large number of oral bacteria are involved in etiology of chronic periodontitis (Graves et al., 2008). The rat ligature model is one of the most frequently used non-primate animal periodontitis models. The ligatures around teeth cause plaque accumulation and induce periodontal inflammation and subsequent alveolar bone loss (Xu and Wei, 2006).
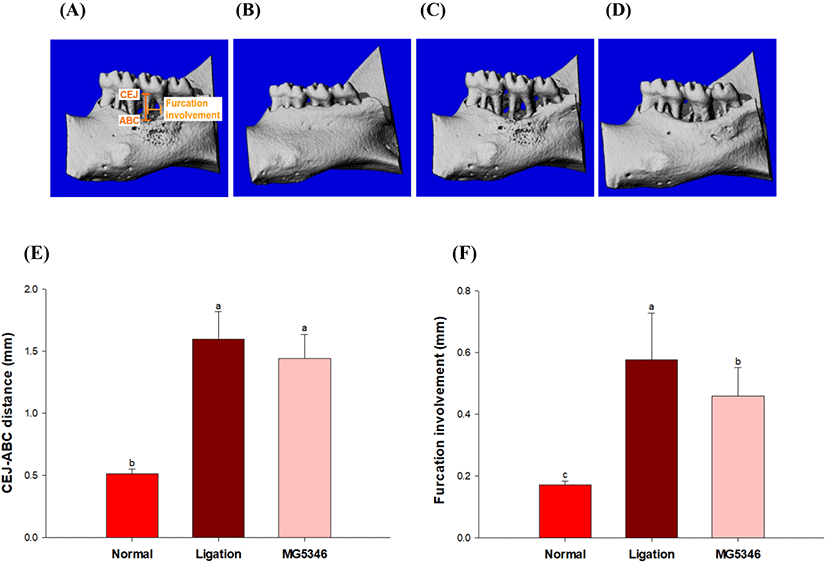
The effect of MG5346 administration for 8 wks on alveolar bone loss was determined in ligature-induced experimental periodontitis rats. The periodontal destruction was observed in both vertical (CEJ-ABC) and horizontal (furcation involvement) direction in multi-rooted teeth (Pilloni and Rojas, 2018). Micro-CT tomography indicated that ligation significantly increased both CEJ-ABC distance and furcation involvement. Although a decreasing tendency was observed in the CEJ-ABC distance by L. reuteri MG5346 administration, there was no significant difference between the ligation control and L. reuteri MG5346 group (1.60±0.22 vs.1.44±0.19). Conversely, furcation involvement was significantly decreased by administration of MG5346 (0.58±0.15 vs. 0.46±0.0.9; p<0.05; Fig. 3). The prolonged inflammation by periodontitis leads to bone resorption and furcation defect, and reduced furcation involvement significantly reduces the risk of bone loss (Parihar and Katoch, 2015). The adjuvant use of L. reuteri DSM 17938 (1×108 CFU/lozenge) for 21 days ameliorated chronic periodontitis by reducing gingival inflammation and deep periodontal pockets in smokers (Theodoro et al., 2019).
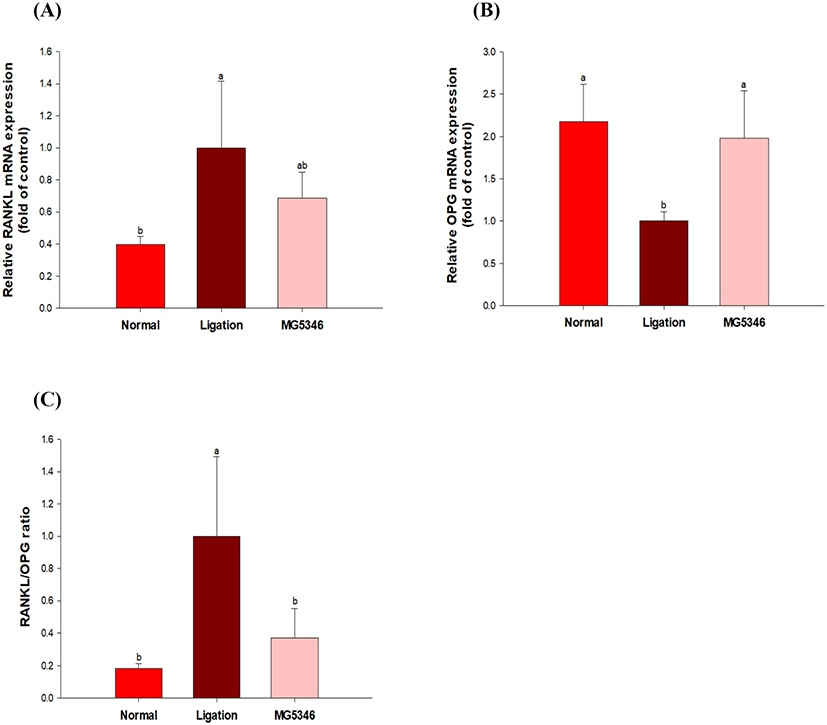
RANKL-RANK is a key pathway modulating the formation and differentiation of osteoclasts (Wada et al., 2006). OPG competitively binds to RANKL, subsequently interfering with the binding of RANKL with RANK. The imbalance in RANKL/OPG led to increased bone resorption (Boyce and Xing, 2008). Periodontal tissue was isolated from rats, and the expression of RANKL and OPG was analyzed using qRT-PCR. The expression of RANKL increased by ligation while it was decreased by MG5346 administration (Fig. 4A). The expression of OPG, which was decreased by periodontitis induction was significantly recovered by administration of MG5346 (p<0.05; Fig. 4B). The RANKL/OPG ratio in MG5346-fed groups was close to the non-ligation control group (Fig. 4C).
Although the modulation of bone metabolism by administration of probiotics has been reported (Britton et al., 2014; Jung et al., 2022; Yousf et al., 2015), the evidence is still inconclusive. Hu et al. (2021) reported that extracellular vesicles (EVs) released from L. reuteri might be involved in the mitigation of periodontitis. The administration of EVs from L. reuteri BBC3 exerted an anti-inflammatory effect in lipopolysaccharide-stimulated chicken macrophages and improved intestinal injury in chickens. Alternatively, the ability of probiotics to modulate C-X-C motif chemokine (CXCL8) was suggested as a potential mechanism of probiotic immune modulation (Mendi et al., 2016). CXCL8 is a chemokine released from various cell types, such as gum epithelial cells, and it recruits neutrophils to the site of infection (Yamamoto and Aizawa, 2021). Porphyromonas gingivalis-mediated CXCL8 inhibition reduced the host immune response and enhanced periodontal tissue damage (Sochalska and Potempa, 2017). Probiotics, such as L. rhamnosus ATCC 9595, L. casei 324 m, and L. reuteri upregulated CXCL8 gene expression and counteracted P. gingivalis-mediated CXCL8 suppression (Albuquerque-Souza et al., 2021; Allaker and Stephen, 2017; Mendi et al., 2016). Oral administration of L. reuteri tablet significantly reduced proinflammatory cytokine levels (TNF-α, IL-1β, and IL-17) in 18 out of 24 patients with chronic periodontitis. The clinical indices, such as bleeding index, periodontal probe depth, and clinical adhesion level, were also significantly improved (Szkaradkiewicz et al., 2014).
Conclusion
Probiotics are generally regarded as safe, except for specific health conditions, such as patients with immune-compromisation. The development of oral probiotics/postbiotics offers valuable options to prevent or alleviate periodontitis. Based on results in osteoclastogenesis and the ligature-induced periodontitis rat model, MG5346 can be a promising probiotic strain for oral health. Carefully designed clinical studies are required to warrant the efficacy of oral probiotics/postbiotics.













