Introduction
Meat is an important source of nutrition for human health due to high amount of protein, minerals, and various bioactive compounds (Kim et al., 2018; Kim et al., 2019). In particular, pork is one of the most popular and widely-consumed forms of meat in Korea. According to the Organization for Economic Cooperation and Development (OECD), in Korea, the annual pork consumption (31.6 kg per capita) was higher than that of beef (11.9 kg per capita) and poultry (18.8 kg per capita) (OECD, 2021). Pork belly has been indicated as a strong preference among the various cuts of pork available in Korea (Choe et al., 2015; Hoa et al., 2021). During distribution and storage of pork belly meat, quality can be decreased and various metabolites can be generated (Triki et al., 2018). As one of the metabolites generated during storage, biogenic amines (BAs) is considered as an indicator of freshness of meat (Jairath et al., 2015).
BAs are basic nitrogenous compounds which can be produced by the action of microbial decarboxylases on free amino acids (Fan et al., 2015; Jairath et al., 2015). BAs are mainly produced in foods with high protein content, such as meat, fish, and dairy products (Linares et al., 2012; Prester, 2011; Vinci and Antonelli, 2002). Generally, the most common BAs in meat and meat products are putrescine (PUT), cadaverine (CAD), histamine (HIM) and tyramine (TYM), and spermidine (SPD) has also been reported as amine present at significant levels in raw meat (Stadnik and Dolatowski, 2010). BAs are formed by spoilage bacteria (Enterobacteriaceae and Pseudomonas spp.), gram positive bacteria, and lactic acid bacteria (LAB) (Bover-Cid et al., 2003; Katikou et al., 2006; Ruiz-Capillas and Jiménez-Colmenero, 2005). As high levels of BA intake can result in toxicity, its consumption can be important for human health. Research has indicated that PUT and CAD can potentially be converted to carcinogenic N-nitrosamine as precursor substances (Lee and Kim, 2012), and SPD can also be harmful as a precursor of carcinogenic N-nitrosamines through heating of meat products (Drabik-Markiewicz et al., 2011). In addition, high intake of TYM and HIM can cause migraine headaches and food poisoning, respectively (Balamatsia et al., 2006).
To inhibit the BAs formation in meat and meat products, various natural substances have been applied in meat. The use of natural substances to meat inhibit not also the formation of potential carcinogens such as BAs (Naila et al., 2010), but also enhance meat flavor and quality (Yusop et al., 2010). For example, green tea, cloves, cumin, and spearmint were reported to be strong inhibitor of BAs production in meat (Abu-Salem et al., 2011; Cai et al., 2015; Naila et al., 2010). According to these research, these natural substances had high antioxidant activity, antibacterial activity due to high phenolic compounds (Abu-Salem et al., 2011; Cai et al., 2015). These natural substances may inhibit the BAs formation by inhibiting the growth of the bacteria producing BAs or inhibiting the biosynthesis of BAs (Mah et al., 2009).
Black currant can be a good candidate as natural substance to inhibit BAs formation in meat, because of its high content of polyphenol (e.g., ferulic acid) and antibacterial activity (Borges et al., 2013; Widén et al., 2015). Black currant (juice or extract) demonstrated effective antibacterial activity against pathogenic and spoilage bacteria such as Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes, Escherichia coli and Pseudomonas aeruginosa (Denev et al., 2014; Krisch, 2008; Widén et al., 2015).
Although black currant extracts has antibacterial activity, its effect on the reduction of BAs contents in meat during storage is limited. Therefore, the aim of this study was to evaluate the effect of black currant marination on the formation of BAs in pork belly during cold storage.
Materials and Methods
The BCJ was commercial BCJ used in food industry, and it was purchased from local market. The process of marination and storage of the pork belly are shown in Fig. 1. The pork belly was cut into rectangular slabs of 10 cm×5 cm×0.4 cm (length×width×thickness). Pork belly marination was carried out with 95.15% of pork belly, 1.43% of BCJ, 0.57% of salt, and 2.85% of water, selected marinating formula according to previous sensory evaluation (data not shown). The pork belly marinated with BCJ (PBB) was placed into a vacuum pack to ripen for 24 hours at 5°C. The ripened PBB and raw pork belly (RPB) were stored at 9±2°C for 10 days and analyzed at days 0, 5, and 10 of storage. Each experimental day, pork belly (n=10) was finely ground using a food mixer (HMF-3800SS, Hanil Electric, Seoul, Korea) and used for analysis.
The pH values of BCJ, PBB, and RPB were measured by pH meter (Orion 230A, Thermo Fisher Scientific, Waltham, MA, USA). Ten grams of each sample were homogenized with 90 mL distilled water (DW) using a homogenizer (PolyTron® PT-2500E, Kinematica, Lucerne, Switzerland).
The CIE L* (lightness), a* (redness), and b* (yellowness) values of the BCJ was measured with a Minolta chromameter (Model CR-400, Minolta, Tokyo, Japan), with the device calibrated using a white calibration plate (L*=93.69, a*=–0.22, b*=4.15).
ABTS radical-scavenging activity was analyzed by the method of Kim et al. (2019). The stock solution of the ABTS+ radical was made by mixing equal volumes of 14 mM ABTS+ solution and 4.9 mM potassium persulfate solution, and left to react for 12 h at 23±1°C in the dark. The stock solution was diluted with DW (absorbance of 0.700±0.02 at 735 nm) and assessed using a spectrophotometer (SpectraMax M2, Molecular Devices, San Jose, CA, USA) at 30°C. The sample (50 μL) was reacted with ABTS+ radical solution (950 μL) at 30°C for 30 min in the dark. The standard curve was established using Trolox (Sigma-Aldrich, St. Louis, MO, USA), and the ABTS values were expressed as mmol Trolox equivalent (TE)/g.
The FRAP assay was conducted by the method of Kim et al. (2019) with slight modifications. The FRAP working solution was made with acetate buffer (300 mM) of pH 3.6, 2,4,6-tripyridyl-S-triazine in 40 mM HCl (10 mM), and FeCl3·6H2O solution (20 mM) at a ratio of 10:1:1 (v/v/v), respectively. The 25 μL of the sample was reacted with FRAP working solution of 175 μL at 37°C for 30 min in the dark. The reacted solution absorbance was determined at 590 nm using a spectrophotometer (Molecular Devices). FRAP activity were expressed as mmol TE/g.
DPPH radical scavenging activity was conducted by the method of Kim and Jang (2021) with slight modifications. The 100 μL of sample was added to wells of a 96-well microplate with 100 μL methanolic solution containing DPPH radicals (0.2 mM). The plate was shaken for 10 s and allowed to react for 30 min at 25°C in the dark. The absorbance was measured at 517 nm using a spectrophotometer (Molecular Devices). A standard curve was established using Trolox, and DPPH values were expressed as mmol TE/g.
The ORAC assay was carried out using the method of Kim et al. (2019). The modified ORAC assay used potassium phosphate buffer (75 mM) of pH 7.4 at 37°C. The 25 μL of the sample was mixed with 150 μL of fluorescein (80 nM) and incubated for 15 min at 37°C. The 25 μL of 2,2’-azobis (2-amidinopropane) hydrochloride (150 mM) was mixed with generate peroxyl radicals. The change in the absorbance of the reacted sample was measured every minute at emission wavelength of 520 nm and excitation wavelength of 480 nm at 37°C by spectrophotometer (Molecular Devices). A standard curve was established using Trolox, and ORAC values were expressed as mmol TE /g.
TPC was measured using the Folin-Ciocalteu colorimetric method as described by Kim et al. (2021). BCJ was diluted using methanol. 0.5 mL of diluted sample was mixed with 5 mL DW and Folin-Ciocalteu phenol reagent (Sigma-Aldrich), and left for 3 min. After that, the mixture was added to 1 N Na2CO3 and reacted for 90 min at 25°C in the dark. The absorbance of the reacted sample at 760 nm was measured using a spectrophotometer (Molecular Devices). A standard curve was established using gallic acid, and TPC was expressed as mg gallic acid equivalent (GAE)/g.
The BAs content was analyzed according to the method of Eerola et al. (1993). PUT, CAD, TYM, HIM and SPD stock solutions were diluted from 0.078 to 10 μg/mL using 0.4 M perchloric acid (PCA). Two grams of samples (BCJ, RPB, and PBB) were homogenized with 10 mL of 0.4 M PCA and centrifuged (1,763×g, 4°C, 10 min). After centrifugation, the homogenate was filtered using Whatman No. 1 filter paper and the remaining pellet was re-extracted using 10 mL of 0.4 M PCA. The filtrated solution was collected, and made to 25 mL with 0.4 M PCA. The extracted solution (0.2 mL) was then mixed with 40 μL of 2 N NaOH, 60 μL of saturated NaHCO3 and 0.4 mL dansyl chloride (10 mg/mL in acetone), and incubated at 45°C for 40 min. Following the incubation, the solution was mixed with 20 μL of ammonium hydroxide to remove the dansyl chloride from the solution, and incubated in the dark for 30 min. The solution was thereafter mixed with 280 μL of acetonitrile, centrifuged for 10 min at 589×g, and filtered using 0.22 μm membrane filter (Rephile Bioscience and Technology, Shanghai, China) for using HPLC analysis.
Quantification of BAs was performed using Agilent 1260 HPLC (Agilent Technologies, Santa Clara, CA, USA) with Poroshell 120 EC-C18 (4 μm, 4.6×150 mm) column (Agilent Technologies). The HPLC analysis used a gradient elution program with solvent A (0.1 M ammonium acetate) and solvent B (acetonitrile). The flow rate was 1.0 mL/min. The gradient started with a 50% of solvent B and then, it proceeded linearly for 19 min at 90% of solvent B. This ratio was changed linearly over 5 min to 50% of solvent B and it was kept for 5 min (the total run time was 29 min). The column temperature was 40°C. Samples of 20 μL volume each were injected, and BA amounts were quantified by UV-absorption measured at 254 nm.
To evaluate its antibacterial activity, BCJ was lyophilized and stored at –20°C until analysis. The antibacterial activity of BCJ was assessed against Escherichia coli (E. coli, KCCM 11234) and Pseudomonas aeruginosa (P. aeruginosa, ATCC 27853). The bacteria strains (E. coli and P. aeruginosa) were streaked on Mueller Hinton agar (MHA, MB Cell, Seoul, Korea) and incubated at 37°C for 24 h. A single colony of each test organism from the culture plates were inoculated into 10 mL sterile Mueller Hinton broth (MHB, MB Cell) and incubated at 37°C. Subsequently, the culture was sub-cultured 3 times and used for the paper disc diffusion assay, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) analyses.
The paper disc diffusion assay for evaluating antibacterial activity was conducted according to the method of Ramos et al. (2006) with slight modifications. The lyophilized BCJ powder was dissolved in DW at different concentrations: 30, 20, 10, 5, 2.5, and 1.25 mg/disc. The samples were filter sterilized using a 0.45 μm hydrophobic membrane filter (Rephile Bioscience and Technology). Test organisms were inoculated by transferring a loopful of culture into 10 mL of sterile MHB and incubated at 37°C for 48 h; following the incubation period, the culture was adjusted to 8 Log CFU/mL and incubated in MHA. Sterile 8 mm paper discs (Advantec, Toyo Roshi Kaisha, Tokyo, Japan) were aseptically placed on MHA surfaces, and each sample was immediately added to disc in volumes of 50 μL. The negative control was 50 μL of DW added to a sterile paper disc, and the positive control was 50 μL of streptomycin added to discs at concentrations of at 0.01 mg/disc for E. coli and 0.05 mg/disc for P. aeruginosa. The loaded plates were incubated for 24 h at 37°C. After incubation, the inhibition zone diameters (mm) were measured using a digital caliper (A&D Company, Tokyo, Japan).
The MIC, which was the least concentration of sample that inhibit microbial growth, was determined by microdilution of MHB in 96-well plates; 130 μL MHB, 20 μL microorganism suspension, and 50 μL sample were loaded to each well. The BCJ was diluted to concentrations of 600, 400, 200, 100, 50, 25, and 12.5 mg/mL, then inoculated with each test bacterial strain. The plates were incubated in a 37°C incubator for 24 h, and the absorbance of each sample concentration at 600 nm was then measured using a spectrophotometer (Molecular Devices) at 37°C. The MIC was defined as the lowest concentration of BCJ showing no detectable growth.
The MBC, which was the least concentration of sample required to kill microorganisms, was determined by the testing concentrations listed above and performing subcultures on MHA medium. The plates were incubated at 37°C for 48 h. The MBC was defined as the lowest concentration showing no bacterial colonies in media.
To evaluate the total aerobic bacteria (TAB), LAB, Enterobacteriaceae, and Pseudomonas spp. counts, each sample (10 g) was transferred into a sterile stomacher bag with 90 mL of sterile saline solution. Then, samples were homogenized for 40 s using a stomacher (Bag Mixer 400, Interscience, St. Nom, France). A serial dilution was performed using sterile saline solution, and 1 mL diluent was seeded onto petri dish in media. TAB counts were performed on plate count agar (PCA; MB cell) and incubated at 37°C for 48 h. The man rogosa sharpe (MRS) agar (MB cell) was used for counting LAB as selective media and incubated at 37°C for 48±2 h in the anaerobic jar. The Enterobacteriaceae and Pseudomonas spp. counts were carried out on violet red bile glucose (VRBG) agar (MB cell) and cetrimide (CN) agar (MB cell), respectively, as selective media and incubated at 30°C for 24±2 h. The numbers of colony-forming units (CFU) per gram of pork belly were calculated.
VBN content was analyzed by the micro diffusion method, as described by Kim et al. (2020). Ten gram of ground RPB and PBB was homogenized for 30 min in 50 mL DW on a magnetic stirrer, and the homogenate was filtered by filter paper (Whatman No. 1). The filtrate in Conway unit was incubated with H2SO4 (0.01 N) at 25°C for 1 h. Following incubation, 20 μL of Brunswik indicator added to the Conway unit inner chamber, and titrated against NaOH (0.01 N). The VBN value was calculated as follows and expressed as mg/100 g.
where a is the volume of NaOH (0.01 N) added to the sample (mL), b is the volume of NaOH (0.01 N) added to the blank (mL), F is the standard factor for NaOH (0.01 N), W is the weight of sample (g).
Data were analyzed using the SAS program (ver. 9.2; SAS Institute, Cary, NC, USA). T-test was performed to comparing means of treatment (marination) and a one-way analysis of variance (ANOVA) was performed for data of storage time. Significant differences was determined by Tukey’s test (p<0.05). Additionally, a two-way ANOVA was carried out to evaluate any interactions between treatment and storage.
Results and Discussion
The pH, color, antioxidant activity, TPC, BAs of BCJ were analyzed (Table 1). The pH of BCJ was 3.17 and the CIE L*, CIE a*, and CIE b* of BCJ were 16.81, 0.19, and 0.25, respectively. BCJ showed the antioxidant activity across four traits; ABTS radical scavenging activity: 112.64 mmol TE/g; FRAP activity: 126.46 mmol TE/g; DPPH radical scavenging activity: 104.85 mmol TE/g; ORAC activity: 231.57 mmol TE/g. TPC of BCJ showed 8.58 mg GAE/g, which had similar values compared to previously published research (5.80–7.42 mg GAE/g black currant extract) (Bakowska-Barczak and Kolodziejczyk, 2011). The BCJ contained four types of BAs: PUT: 4.83 μg/g; CAD: 14.41 μg/g; TYM: 0.50 μg/g; SPD: 1.19 μg/g. The PUT contents of BCJ (4.83 μg/g) was higher than that of other vegetable [Chinese cabbage (1.8 μg/g), Endive (2.8 μg/g), Radicehio (4.3 μg/g)], while the contents of TYM and SPD in BCJ (0.50 and 1.19 μg/g, respectively) was lower than that in Chinese cabbage (1.2 and 15.1 μg/g, respectively), Endive (11.3 and 1.5 μg/g, respectively), Iceberg lettuce (0.9 and 7.8 μg/g, respectively), and Radicehio (2.3 and 11.3 μg/g) (Simon-Sarkadi et al., 1993).
ABTS, 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) radical-scavenging activity; FRAP, ferric reducing antioxidant power; DPPH, 1,1-diphenyl-2-pricrylhydrazyl radical scavenging activity; ORAC, oxygen radical absorption capacity; BA, biogenic amine; PUT, putrescine; CAD, cadaverine; HIM, histamine; ND, not detected; TYM, tyramine; SPD, spermidine.
The antibacterial activity of BCJ against spoilage bacteria (E. coli and P. aeruginosa) was evident by the presence of inhibition zones, shown in Table 2 and Fig. 2. BCJ at a concentration from 10 to 30 mg/disc showed inhibition zones against E. coli and P. aeruginosa. BCJ had antibacterial activity against E. coli, with the range of inhibition zones between 9.71 and 14.87 mm, while an inhibition zone of P. aeruginosa of between 8.76 to 12.52 mm was clearly observed. The inhibition zones formed by BCJ against E. coli and P. aeruginosa indicated a concentration-dependent effect of BCJ from 10 to 30 mg/disc (p<0.05). However, there were no inhibition zones in the concentration range between 1.25 and 5 mg/disc.
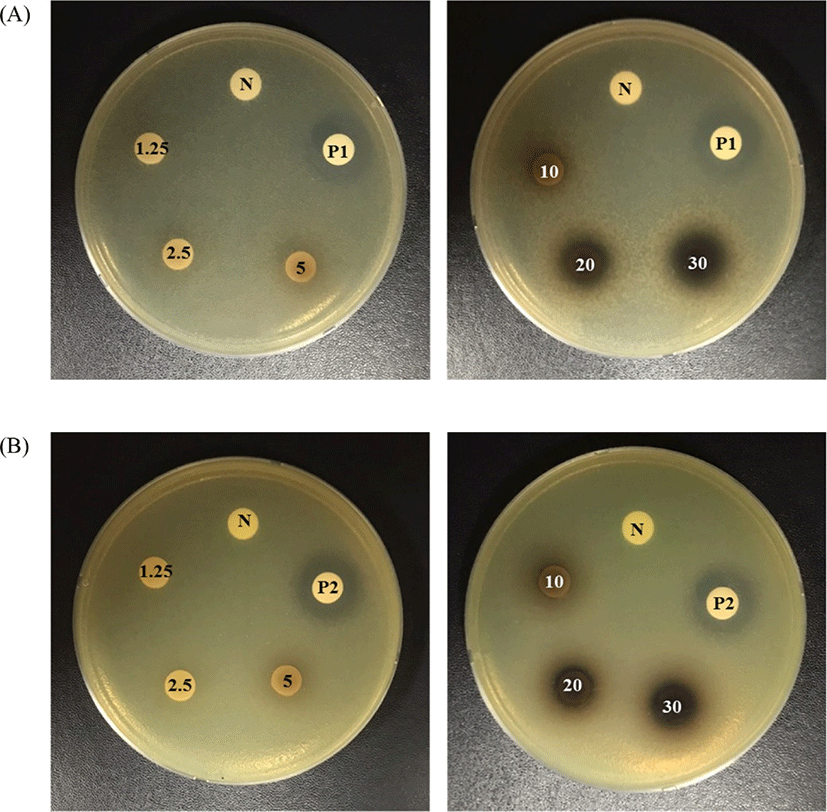
The antibacterial activity of BCJ against E. coli and P. aeruginosa was also evidenced by the MIC and MBC shown in Table 3. The MIC of BCJ was determined to be 100 mg/mL against both bacterial strains. For the MBC, the inhibitory effect of BCJ was higher in P. aeruginosa than in E. coli. In a previous study, BCJ, water and methanol extract inhibited the growth of E. coli by less than 25% (Krisch, 2008). Meanwhile, a study by Widén et al. (2015) showed that a higher level (10%) BCJ (pH 4.7) effectively inhibited P. aeruginosa growth due to the low pH of the black currant, in concordance with our results (Table 1). According to previous studies, the high antibacterial activity of black currant is resulting from its high levels of phenolic acids such as ferulic acid and chlorogenic acid (Borges et al., 2013; Widén et al., 2015). In particular, the abundance of ferulic acid (113.1 μg/mL) in black currant, and BCJ, is higher than that of other phenols (Widén et al., 2015). Ferulic acid is one of several phenols with antibacterial activity against pathogenic bacteria. Several reports found that ferulic acid had high antibacterial activity against E. coli and P. aeruginosa (MIC of E. coli and P. aeruginosa were 100 μg/mL) (Borges et al., 2013; Cho et al., 2017). The possible mechanism for antimicrobial effect of phenolic compounds are known as altering microbial cell permeability, which interfering with membrane function such as nutrient uptake, electron transport, enzyme activity, protein and nucleic acid synthesis (Abu-Salem et al., 2011).
| Antibacterial activities (mg/mL) | Microorganisms | |
|---|---|---|
| E. coli | P. aeruginosa | |
| MIC | 100 | 100 |
| MBC | 200 | 100 |
Changes in pH value of RPB and PBB during storage were shown in Table 4. The pH values of RPB and PBB significantly decreased on day 10 when compared to day 0 of storage. Of note, the pH values of PBB were low compared to RPB on all storage days (p<0.05). In pH, the treatment (marination) and storage had a high degree of interaction with each other (p<0.0001). Duffy et al. (2000) previously reported that pH values of vacuum-packed minced beef gradually declined during storage at 0°C and 10°C due to predominant growth of LAB in vacuum-packed minced beef. Additionally, Chung et al. (2018) reported that the pH of Hanwoo tteokgalbi treated with black currant powder (pH 5.31) was lower than that of control tteokgalbi (pH 5.40) at day 0 of storage, which agrees with our results. Moreover, an earlier study also reported that black currant contains high level of ascorbic acid (Iversen, 1999). Thus, the low pH value of PBB may be affected by the low pH of BCJ (Table 1), and the vacuum conditions may contribute to the predominance of acid-forming microbes during storage.
Bacterial contamination is an unavoidable consequence of meat processing and storage. As such, bacterial counts are an important factor in determining the freshness of meat and meat products. To evaluate the antibacterial activity of BCJ on the BCJ-marinated pork belly, microorganism counts in RPB and PBB are shown in Table 4. There were no significant differences in the microorganism abundance between RPB and PBB at day 0 of storage. As the storage period increased, the counts of TAB and LAB were higher in PBB than in RPB, and were evident at both day 5 and 10 of storage (p<0.05). On the other hand, amounts of spoilage bacteria (Enterobacteriaceae and Pseudomonas spp.) were lower in PBB than in RPB by days 5 and 10 of storage (p<0.05), evidencing the antibacterial activity of BCJ. Indeed, in the present study, BCJ demonstrated effective antibacterial activity against E. coli (the type species of the type genus of Enterobacteriaceae) and P. aeruginosa (the type species of Pseudomonas) (Table 3). In addition, the vacuum condition used here can inhibit the growth of Pseudomonas spp. and contribute predominance of LAB (Castellano et al., 2008). The low pH of BCJ (pH 3.17) and vacuum packaging can also contribute to providing an optimal environment for LAB growth, while low pH can concurrently inhibit the growth of spoilage bacteria during storage. The treatment (marination) and storage was highly interacted with each other for TAB, Enterobacteriaceae and Pseudomonas spp. (p<0.01–p<0.0001). Therefore, our study demonstrated that PBB can effectively inhibit growth of spoilage bacteria (Enterobacteriaceae and Pseudomonas spp.) due to the antibacterial activity of black currant, and the vacuum conditions.
As well as the number of bacteria, VBN value is also important indicators for determining the freshness of meat. The VBN value of RPB and PBB are shown in Table 4. The addition of BCJ was not associated with a significant difference between the VBN value of RPB and PBB (6.60 and 6.04 mg/100 g, respectively) at day 0 of storage. However, the VBN value of RPB and PBB increased as the storage period increased, and significant differences were observed from day 5 of storage (p<0.05). On days 5 and 10 of storage, the VBN values of PBB (8.35 and 15.10 mg/100 g, respectively) was lower than that of RPB (15.77 and 32.92 mg/100 g, respectively) (p<0.05). Moreover, the treatment (marination) and storage had a strong interaction for VBN value (p<0.0001). In Korea, the upper limit of the VBN value is 20 mg/100 g for fresh meat (Kim et al., 2020). During storage for 10 days at 9°C, it was observed that the VBN content for PBB did not exceed 20 mg/100 g, in contrast to the RPB. VBN is mainly produced by enzymatic decarboxylation of specific amino acids, which is associated with growth of Enterobacteriaceae and Pseudomonas spp. (Li et al., 2019). In the present study, the addition of BCJ significantly reduced the VBN values of PBB from day 5 of storage, which is consistent with the results of microbial analysis (Table 4), indicating that addition of BCJ effectively inhibited the growth of spoilage bacteria in PBB from day 5 of storage.
BAs are formed by the action of bacterial decarboxylases in meat. Factors involved in the formation of BAs include factors pertaining to the specific raw meat material (such as meat composition, free amino acids, fat content, pH, etc.), microbial growth, processing conditions, and storage conditions (Ruiz-Capillas and Jiménez-Colmenero, 2005). In particular, microorganisms can be an important factor in the formation of BAs; thus, controlling microbial levels using natural antibacterial agents can be a direct strategy for controlling BA content.
The effect of BCJ on the formation of BAs in the pork belly during refrigerated storage are shown in Table 5. The PUT, CAD, HIM, and TYM contents of RPB and PBB were gradually increased from day 0 to 10 of storage (p<0.05). On the other hand, the SPD content of RPB tended to decrease as the storage period increased (p<0.05), while the SPD content of PBB showed irregular fluctuation throughout storage. Although there is no criterion for judgment about BAs in fresh meat, according to previous study, total BAs content in fresh pork meat reported in range of 3.8–8.0 μg/g (Favaro et al., 2007; Kalač, 2006; Min et al., 2007; Ngapo et al., 2017; Triki et al., 2018) and even up to 32.8 μg/g (Halász et al., 1994). The addition of BCJ effectively inhibited the formation of BAs in pork belly during the storage period. In the PBB, an inhibitory effect on PUT content was observed from day 0 to 10 of storage (p<0.05). Further, a significant inhibitory effect on the formation of CAD and TYM was found on day 5 and 10 of storage in PBB. However, HIM was detected only at day 10 of storage, and there was no significant differences between PBB (1.12 μg/g) and RPB (1.45 μg/g). Additionally, no clear reduction of SPD in pork belly was observed in association with addition of BCJ during storage. All BAs contents were strongly affected by BCJ treatment (p<0.0001), and treatment and storage had high degree of interaction with each other for PUT, CAD, TYM, Total BAs (p<0.001) and HIM (p<0.05).
PUT, CAD, and TYM can be formed by spoilage bacteria in fresh pork, and can therefore be used as spoilage indicators in pork (Li et al., 2014). The formation of PUT and CAD is mainly associated with Pseudomonas spp. and Enterobacteriaceae, respectively (Bover-Cid et al., 2003; Geornaras et al., 1995; Katikou et al., 2006; Ruiz-Capillas and Jiménez-Colmenero, 2005), while the formation of TYM usually has responsibility with the LAB strains (Bover-Cid et al., 2001). Prior research has indicated that Pseudomonas spp. is also strongly correlated with TYM formation in chilled pork (Li et al., 2014). In the present study, PBB showed low levels of spoilage bacteria (Enterobacteriaceae and Pseudomonas spp.) and a low VBN value when compared to RPB. According to Min et al. (2007), VBN values are highly correlated with levels of BAs (PUT, CAD, and TYM) in beef, pork, and chicken during storage. Here, the content of PUT, CAD, and TYM were significantly low in PBB compared to RPB; these results may therefore be related to low levels of spoilage bacteria and the low VBN value in PBB during storage. Taken together, our results indicate that addition of BCJ to pork belly can inhibit the growth of spoilage bacteria in pork belly, and can reduce the formation of PUT, CAD, and TYM by spoilage bacteria during chilled storage. The lack of difference in HIM content between RPB and PBB could be attributed to the low inhibitory efficiency of black currant on gram-positive bacteria; HIM is formed by decarboxylase of histidine, which is widely distributed in the gram-positive genera (Geornaras et al., 1995). In a previous study, black currant was shown to include high of ferulic acid, and the level of intracellular content (K+) release of gram-positive bacteria was lower upon exposure to ferulic acid than that of gram-negative bacteria (Borges et al., 2013; Widén et al., 2015). Thus, BCJ marination would have had a lesser effect on HIM in PBB than its effect on other BAs (PUT, CAD, and TYM) more susceptible to its mechanisms. Additionally, in the present study, the average level of SPD in RPB and PBB was 3.27 and 3.31 μg/g during storage, respectively. A previous study found that the average level of SPD in meat is usually around 3.0 mg/kg (Hernández-Jover et al., 1997), which agrees with our results. During storage, there was an irregular reduction of SPD content associated with BCJ marination. This could be due to different mechanisms of SPD formation. Generally, PUT, CAD, HIM, and TYM are formed on the surface of meat by the activity of bacteria, while SPD is formed naturally in fresh pork meat because it exists as a natural constituent of living cells (Cai et al., 2015; Hernández-Jover et al., 1997; Paulsen et al., 2006). Therefore, it is reasonable that marination with BCJ would have a lesser effect on SPD. In other studies, a small decline in SPD content has been reported due to the conversion of SPD to PUT (Larqué et al., 2007) or deamination by microbial polyamine oxidase enzymes (Razin et al., 1959) during the storage. Similarly, in our study, the SPD content of RPB and PBB tended to decrease at day 10 of storage than that of day 5 of storage. The total BA content in RPB and PBB gradually increased up to 242.73 and 151.96 μg/g during day 10 of storage time, respectively. At day 0 of storage, there was no significant difference in total BA content between RPB and PBB. However, the inhibitory effect of total BAs in PBB was observable at day 5 and day 10 of storage (p<0.05). In particular, the PBB had a higher rate of total BA inhibition (73.16%) at day 5 of storage.
Conclusion
In our study, the addition of BCJ was found to increase shelf life via inhibiting the growth of spoilage bacteria and reduce BAs contents formed during storage in pork belly. Overall, this study suggests that black currant can be used as a natural additive on meat industry and can provide consumer health benefits via reducing BAs in meat products. However, in order to commercially apply black currant to meat, additional studies for the optimal addition concentration of black currant on various meat are needed in consideration of economic efficiency and organoleptic characteristics.













