Introduction
Over the last decades, there has been a worldwide demand continuously rising for ready-to-eat poultry products. However, consumers now highly associate these kind of products as an unhealthy diet source in terms of high levels of fat, cholesterol and saturated fatty acids, sodium and synthetic food additive content (Decker and Park, 2010; Felisberto et al., 2015; Hygreeva et al., 2014; Olmedilla-Alonso et al., 2013). In this line, incorporation of clean-label ingredients is a growing trend representing one of the new strategies for development of healthier and additive-free meat product formulations.
Phosphates, the sodium or potassium salts of phosphoric acid, are one of the widely used additives in meat processing industry (Long et al., 2011). Phosphates have a common use to improve quality of various meat products by shifting the pH away from isoelectric point, increasing protein solubility, water-holding capacity, emulsion stability, protein binding, tenderness, juiciness and palatability, thereby enhancing sensory and textural quality attributes and product yield (Hayes et al., 2006; Hurtado et al., 2012; Lampila, 2013; Long et al., 2011; Petracci et al., 2013; Sebranek, 2008). Besides, they act as antioxidants (Lampila, 2013; Petracci et al., 2013; Sebranek, 2008; Trout and Dale, 1990) and indirect antimicrobials (Akhtar et al., 2008; Lee et al., 1994).
Despite those mentioned advantages, due to the emerging and newly identified health risks associated with phosphates, there has been a tendency to decrease the amount of these additives in meat product formulations (Dimitrakopoulou et al., 2005; O’Flynn et al., 2014; Petracci et al., 2013). Recent medical studies have reported that excessive amounts of dietary inorganic phosphates may considerably disrupt the hormonal regulation of phosphate, calcium and vitamin D, which contributes to disordered mineral metabolism, vascular calcification, impaired kidney function and bone loss (Calvo and Uribarri, 2013; Takeda et al., 2014; Watanabe et al., 2016). Therefore, today incorporation of natural ingredients as inorganic phosphate replacers in meat product formulations has come into prominence as a novel research topic. Recently, some healthier alternatives, such as porcine blood plasma (Hurtado et al., 2012), dried plum products (Jarvis et al., 2012), blends of citrus flour and sodium carbonate (Casco et al., 2013), potato starch and sodium carbonate (Prabhu and Husak, 2014), rice starch and fructo-oligosaccharides (Resconi et al., 2016a), potato and rice starch (Resconi et al., 2016b) have been suggested as phosphate replacers in formulation of different types of meat products, which presented favorable results in improving product quality.
Jerusalem artichoke (Helianthus tuberosus L.) is a natural raw material which highly contains fiber, minerals and vitamins, and its tubers can be cultivated in all over the world, including Europe, Asia and North America (Khuenpet et al., 2017; Radovanovic et al., 2015; Takeuchi and Nagashima, 2011; Yang et al., 2015). The tubers are known to be a health-promoting food source which contain inulin instead of starch as a carbohydrate reserve (Bach et al., 2012; Khuenpet et al., 2017; Takeuchi and Nagashima, 2011). Inulin is known as a valuable prebiotic dietary fiber enhancing textural, sensory and technological properties of meat products (Öztürk and Serdaroğlu, 2017), as well as providing a number of health benefits such as managing increased risk of chronic diseases, improving digestive health, reducing cholesterol and lipids and enhancing mineral absorption from colon (Barclay et al., 2010; Radovanovic et al., 2015). Thus, as a main source of inulin, JA is a promising ingredient which could be used as an economic source of functional components in meat product formulations.
Sodium carbonate is a cutter process aid that is stated to be used in natural products to increase the meat pH (Bodner and Sieg, 2009; Prabhu and Husak, 2014). Yet no study has addressed the utilization of the combination of Jerusalem artichoke powder (JAP) and sodium carbonate (SC) as inorganic phosphate replacers so far. In line with this target, in the present study we investigated the effects of JAP and SC incorporation on emulsion stability, physical, chemical and technological characteristics of phosphate-free emulsified chicken meatballs.
Material and Methods
Fresh Jerusalem artichoke tubers were obtained from a local producer in Izmir, Turkey which were harvested in winter season. The tubers were packaged and stored at 4°C. Fresh boneless post-rigor chicken breast muscle (Musculus pectoralis) and chicken skin as fat source were supplied from the production line of Lezita Integrated Meat Processing Plant (Abalıoğlu Co., Izmir). Breast muscles were trimmed of visible fat and connective tissues and stored at 4°C with chicken skin until used. Food-grade sodium tripolyphosphate (STPP), sodium carbonate (SC), rosemary extract and yeast extract were supplied from Tunçkaya Chemicals Co. (Turkey), Ömeroğlu Agricultural Products Co. (Turkey), A&D Chemicals Co. (Turkey) and Frutarom-Etol Co. (Turkey), respectively. Other spices were purchased from local market. All chemicals used were of analytical grade.
Jerusalem artichoke powder (JAP) was produced from fresh and non-damaged tubers. The tubers were washed with tap water, peeled and immediately immersed in citric acid (1%) solution to avoid enzymatic color changes. The tubers were then sliced into 0.2 mm thickness with a slicing machine (Berkel Slicers, Italy). The slices were then air-dried in an industrial drying oven (Defne Spices Co., Turkey) at 60 ± 5°C for 8 hrs (the slices had 5.5% moisture after drying operation). The dried slices were ground through a hammer mill (Brook Crompton, UK) and sieved through 0.5 mm. The powdered samples were stored in glass jars prior to meatball production.
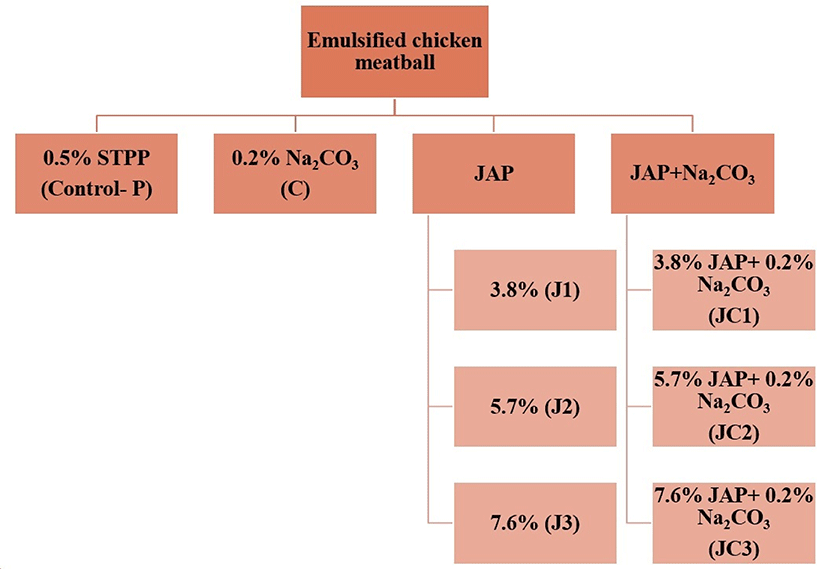
Chicken meatballs were formulated either with STPP (control) or JAP and/or Na2CO3 (SC) as STPP replacers. Treatment combinations consisted of eight different formulations as follows: Meatballs prepared with 0.5% STPP (Control-P), meatballs prepared with 0.2% Na2CO3 (C), meatballs prepared with 3.8% JAP (J1), 5.7% JAP (J2) or 7.6% JAP (J3), and finally meatballs prepared with 3.8% JAP+0.2% Na2CO3 (JC1), 5.7% JAP+0.2% Na2CO3 (JC2), 7.6% JAP+0.2% Na2CO3 (JC3). The experimental design of the study is presented in Fig. 1.
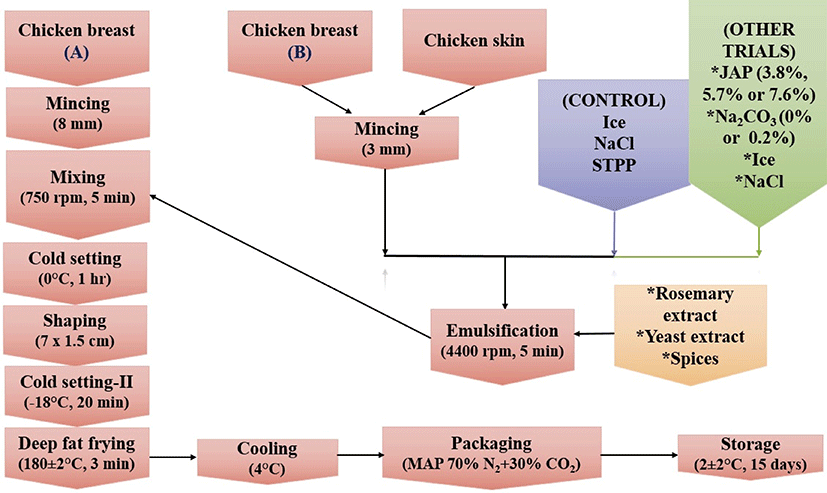
The production flow chart of meatballs could be seen in Fig. 2. Breast muscle-A (70%) was minced through 8 mm plate (K+G Wetter, Germany). Breast muscle-B (30%) and chicken skin (10%) were minced through 3 mm plate. The amount of all other ingredients were calculated based on total meat and skin weight. Breast muscle-B and chicken skin were emulsified with NaCl (1%), STPP (0.5%), rosemary extract (150 ppm) and ice (to have approximately 72% moisture) at 4400 rpm for 5 min (control) in a bowl cutter (K+G Wetter, Germany). Other treatments were prepared using 3.8%, 5.7% or 7.6% JAP, with or without 0.2% SC as STPP replacers. After the emulsification procedure, the emulsions were mixed with breast muscle-A, yeast extract (0.1%) and other spices (0.65%) at 750 rpm for 5 min. After cold-setting of the meatball batters at 0°C for 1 h, the batters were portioned with molds (7.0 × 1.5 cm) and a second cold-setting was performed at −18°C for 20 min. Meatballs were then deep-fat fried with canola oil in an electric fryer (Inoksan, Turkey) at 180 ± 2°C for 3 min. The samples were cooled at 4°C and packaged under modified atmosphere consisted of 70% N2 and 30% CO2 (Multivac 240, Germany) and stored for 15 days at 2 ± 2°C.
Methods
Total moisture, protein, lipid and ash analysis were performed according to AOAC (2012) and total sugar was determined by Luff-Schoorl Method as described by Lees (1975) in powdered samples to evaluate chemical composition.
Water-holding capacity was measured according to Rodríguez-Furlán et al. (2014). One g of JAP (Wo) was weighed and mixed with 10 mL distilled water for 5 min at 5°C for 30 min. The sediment was weighed (W2), dried in a stove for 30 min and re-weighed (W1). WHC was calculated according to the equation below:
WHC= (W2-W1) / (W0)
Oil binding capacity (OBC) of JAP samples was determined using the method of Chakraborty (1986). 1 g of sample (Wo) was weighed and mixed with 10 mL of vegetable oil (V1). After 30 min, the mixture was centrifuged (3-30 KS, Sigma, Germany) at 20.000 rpm for 5 min at 5°C for 30 min. The supernatant volume was then recorded (V2). OBC was calculated according to the equation below:
OBC= (V1-V2) / W0
Emulsifying capacity (EC) of the powders were determined according to Rodríguez-Furlán et al. (2010). 1 g of sample was mixed with 200 mL of distilled water for 2 min before addition of 500 mL vegetable oil under continous homogenization (Ultra-Turrax® T25, IKA, Germany). Blending was stopped every 2 min to check for emulsion breakage. When a clear emulsion breakage was observed, the total volume of oil added was recorded and EC was calculated as volume (mL) of oil emulsified by per g of JAP.
Volumetric gel index (VGI) was analyzed to express the degree of gel formation of JAP, according to Kim et al. (2001). Different concentrations (5%, 10% and 25%, w/v) of aqueous JAP solutions were prepared by mixing JAP with distilled water at 250 rpm with a magnetic stirrer (MS-20D, Wisd, South Korea) and VGI was expressed as the volume of gel (VG) over the total volume (VT) multiplied by 100.
pH value of raw meatball emulsions was measured from three different points by using a pH-meter (pH 330i/SET, WTW, Germany). Jelly and fat separation (JFS) of emulsion batters was measured in triplicate according to Bloukas and Honikel (1992). 200 g of raw emulsion sample was placed in glass jars, sieved and heated in boiling water bath (Wisd, South Korea) for 35 min (with core temperature about 90°C). After that, the jars were cooled to room temperature (25 ± 2°C) and stored at 4°C for 24 h. Jars were then re-heated at 45°C for 1 h. The fluid jelly and fat were drained in a volumetric cylinder and measured (mL). JFS was calculated as a percentage of the original weight of batter.
Water-holding capacity (WHC) of the emulsions was determined in triplicate according to Hughes et al. (1997) with slight modifications. 10 g batter was weighed (W1), placed into glass jars and heated in 90°C water bath for 10 min. After cooling to room temperature, the samples were wrapped in cotton cheesecloth and centrifuged at 1400 rpm (NF400, Nüve, Turkey) for 15 min and weighed again (W2). Water-holding capacity (WHC) was calculated from the equation below:
% WHC= 1− T/M * 100 = 1− (W1−W2)/M * 100
(T: Water loss after heating and centrifugation, M: Total moisture content of the sample)
Emulsion stability (ES) was measured in triplicate according to Hughes et al. (1997). 25 g of the raw emulsion was centrifuged for 1 min at 4000 rpm (NF400, Nüve, Turkey) . The samples were heated in a water bath for 30 min at 70°C and centrifuged again for 3 min at 4000 rpm. The pelleted samples were removed and weighed and the supernatants were poured into pre-weighed crucibles and dried overnight at 100°C. The volumes of total expressible fluid (TEF) and the expressible fat (EFAT) were calculated from the equations as follows:
TEF = (Weight of centrifuge tube + Weight of sample) − (Weight of centrifuge tube + Weight of pellet)
% TEF = TEF/ Weight of sample * 100
% EFAT = [(Weight of crucible + Weight of dried supernatant) − (Weight of empty crucible)] / TEF *100
Chemical composition of chicken meatballs was determined in triplicate by analyzing total moisture (AOAC, 2012), protein (AOCS, 2004), lipid and ash content (AOAC, 2012). Energy value was calculated from the chemical composition as total calorie estimates (kcal) on the basis of 100 g sample using Atwater values for fat (9 kcal/g) and protein (4.02 kcal/g) (Mansour and Khalil, 1997). pH value was measured from three different points by using a pH-meter (330i/SET, WTW, Germany) equipped with a penetration probe. The surface color of the samples was measured in triplicate using a portable colorimeter (CR-200, Konica Minolta, Japan) with D65 illuminant setting and 10⁰ standard observer and expressed as CIE L* (lightness), a* (redness) and b* (yellowness). Cook yield was determined according to Murphy et al. (1975) from three different meatballs by measuring the difference in the weight before and after cooking and calculated according to the equation below:
Cook yield (%) = (Weight of cooked meatballs) / (Weight of uncooked meatballs) * 100
Sensory evaluation of the samples was performed by an untrained panelist group consisted of 10 volunteers from Ege University Food Engineering Department. A representative portion of meatballs were served warmed to the panelists with randomly coded numbers. A 9-point hedonic scale was used to score appearance, color, texture, juiciness, saltiness, sweetness and overall acceptability, where 1 represented dislike extremely and 9 represented like extremely.
Analysis of 2-thiobarbituric acid reactive substances (TBARS) was performed according to Witte et al. (1970). 20 g of sample was homogenized with 50 mL cold solution containing 20% trichloroacetic acid (TCA) in 2 M phosphoric acid for 2 min. 50 mL distilled water was then added and homogenized again for 1 min. After that, the slurry was filtered through Whatman No.1 filter paper into a 100 mL flask. The volume was completed to 100 mL by 1:1 TCA:distilled water. 5 mL of the filtrate was then pipetted into a test tube while another 5 mL of fresh chilled TBA (0.02 M in distilled water) was added. The tubes were incubated at 80°C for 35 min and cooled to room temperature. The absorbance of the solution was measured with a spectrophotometer (T-60, PG Instruments, UK) at 532 nm against blind solution prepared with 1:1 TCA:distilled water. The results were expressed as TBARS values (mg malonaldehyde/kg sample), which was calculated by multiplying the absorbance by 5.2. Each sample was analyzed in triplicate at each storage time.
The data was statistically evaluated by One-way Analysis of Variance (ANOVA) using the IBM SPSS Statistics 21.0 for Windows. The data was analyzed using General Linear Model (GLM) procedure, least squares differences (LSD) were utilized for comparison of mean values among formulations and Duncan’s multiple range test was performed to identify significant differences between formulations and storage time, at a confidence interval of 95%. Partial Pearson Correlation was also applied to measure the strengths of association between emulsion parameters at a significance of 0.05 level.
Results and Discussion
Most of the plant products are harvested seasonally in large amounts and due to their high moisture content, the products need further treatments as drying, which is the most common method to inhibit microbial growth and enzyme activity as well as to decrease the weight and simplify transport and storage (Ozdikicierler et al., 2014; Prosapio and Norton, 2017). JAP samples had 5.31% moisture, 21.87% sugar, 11.96% protein, 5.81% ash and 2.09% lipid. The rest of the composition is estimated to be inulin (approximately 53%), which was similarly reported by Khuenpet et al. (2017) as 54.86 g/100 g dry mass of JA tuber powders. Our raw materials had an average moisture content of 82.9%, so approximately 93% of the water in the material could be removed by drying process, that resulted in increased total solid material content. Earlier studies reported that the fresh JA tubers are composed of about 80% moisture, and the remaining 20% of solids consist of inulin for about 85%, ash for about 5%, and protein, cellulosic material and sugars for the rest (Fleming and GrootWassink, 1979). Protein and ash content of our samples were also in accordance with findings of Afoakwah et al. (2015). Khuenpet et al. (2017) stated that the composition could change depending on species, harvesting maturity, storage time, growing conditions and processing technique.
Evaluating the technological characteristics of JAP samples, the results were WHC: 3.03 ± 0.60 mL water/g product; OBC: 3.63 ± 0.45 mL oil/g product and EC: 150.0 ± 7.0 mL oil/g product. Afoakwah et al. (2015) reported similar WHC (4.30 ± 0.10 g water/g) in oven-dried JAP. They reported an OBC value of 2.06 ± 0.03 g oil/g and stated that OBC of JAP are higher than many other fibers. In their study, freeze-dried JAP samples had higher OBC (3.02 ± 0.14 g oil/g) compared to oven-dried samples, but the values were not as high as our results, probably due to the different origin of the raw materials. Rodríguez-Furlán et al. (2014) reported that chicory inulin had 100 ± 5 mL oil/g product EC. This result showed that JAP had a better emulsification ability compared to industrial inulin.
The gel formation ability of JAP is important as the amorphous region of inulin from JAP can hydrate and swell to form a gel (Afoakwah et al., 2015). Especially in emulsified meat products, gelation of the fiber has critical impact on water-holding and textural properties of the product. VGI of JAP samples was found to be 10.1 ± 1.2%, 53.3 ± 4.8% and 100.0 ± 0.0% in concentrations of 5%, 10% and 25%, respectively. Thus, it was shown that a total gel formation was obtained in 25% JAP solution. Afoakwah et al. (2015) found that oven-dried JAP formed a gel at a concentration of 12%, but subsequent increases from 12-20% led to a progressive increase in the least gelation concentration. Kim et al. (2001) reported that 30 and 35% inulin suspensions made 100% gel structure. Thus, JAP samples could form a gel structure in lower concentrations compared to inulin, in other words gel formation ability was higher in JAP samples than in inulin samples.
Meatball emulsion batters had pH levels between 6.12 ± 0.02 and 6.67 ± 0.11 (data not shown), significant differences were obtained among formulations (p<0.05). C and JC1 samples had higher pH compared to P samples (p<0.05), which could be a result of the higher effectiveness of SC in altering the pH value. JC2 and JC3 samples had similar pH to P samples. The formulations including JAP alone (J1, J2, J3) were recorded to have the lowest pH values among treatments (p<0.05), probably due to the natural characteristics of the raw material.
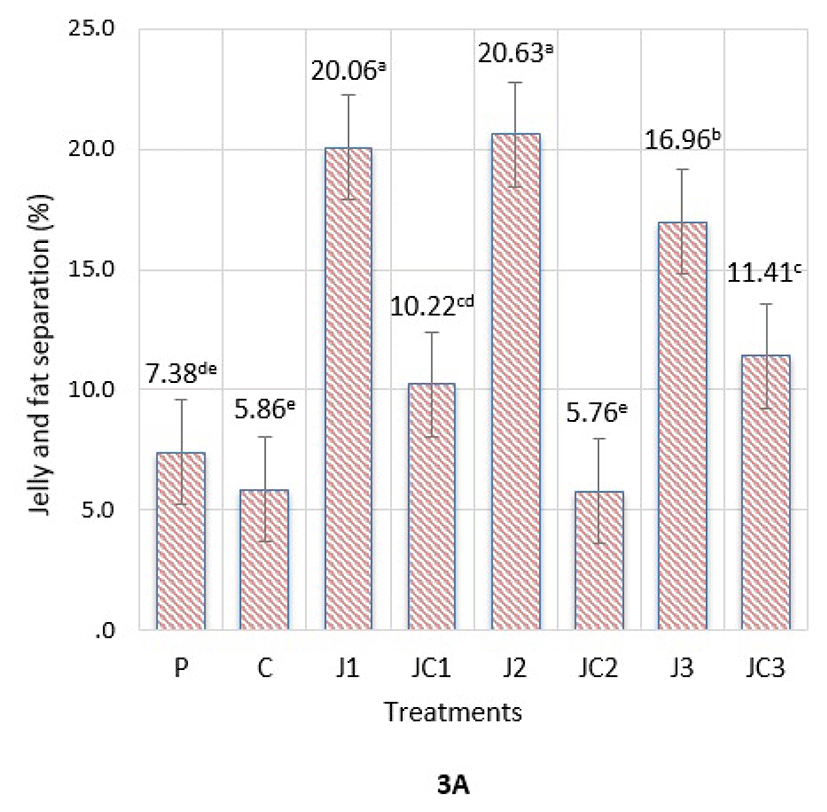
Jelly and fat separation (JFS) refers to the amount of total liquid released from an emulsion at a certain period and temperature. JFS of meatball emulsions is presented in Fig. 3A. The values were between 5.76-20.63%, where significant differences were detected among formulations (p<0.05). Among phosphate-free formulations, C and JC2 samples had the lowest JFS compared to others (p<0.05). P samples had similar JFS to C, JC1 and JC2 samples. The highest JFS was measured in J1 and J2 samples compared to other treatments (p<0.05). The second highest values were measured in J3 samples, compared to J1 and J2 samples (p<0.05). Thus, utilization of JAP alone in formulations had an undesired impact in preventing liquid leakage from the structure with the effect of heat treatment and storage. We hereby found that application of the average level of JAP in combination with SC showed the most favorable effect in lowering the separation of liquid phase.
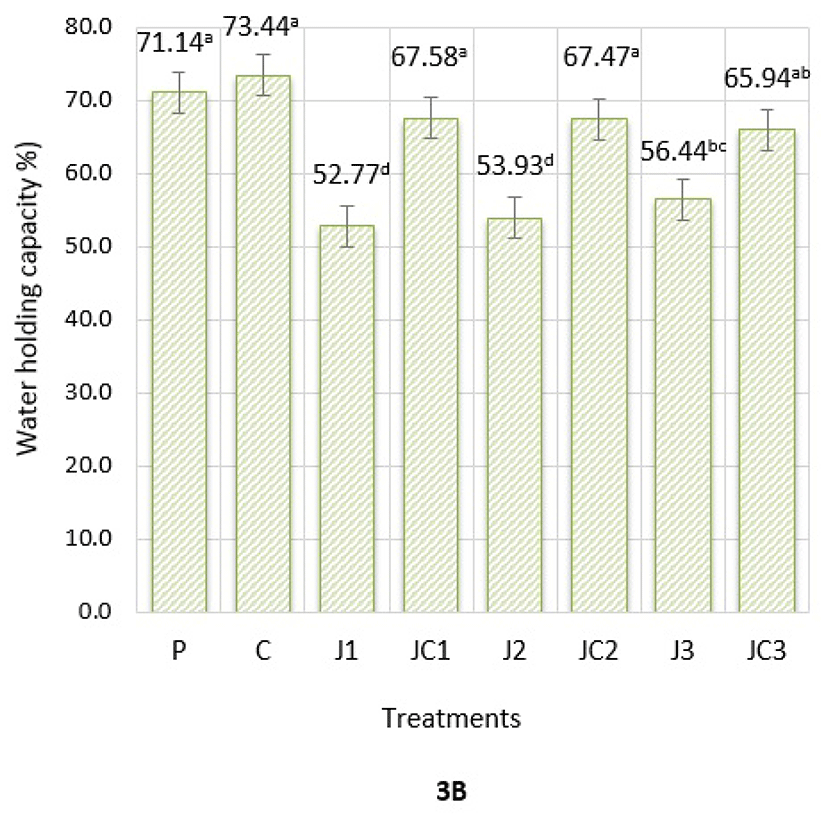
Water-holding capacity (WHC) is defined as the ability of meat to retain moisture. The results of WHC of emulsion batters are presented in Fig. 3B. The values were recorded between 52.77-73.44%, and the formulation had a significant effect on WHC of emulsions (p<0.05). C, JC1, JC2 and JC3 samples had similar WHC compared to P samples. Therefore, usage of JAP mixed with SC had an enhancing effect in WHC, regardless of JAP level. This result is a probable sign of the potential of JAP and SC combination to increase WHC and provide desired technological quality attributes in phosphate-free formulations. On the other hand, J1 and J2 samples had lowest WHC among treatments (p<0.05). Hence, when used solely, JAP samples lead a decrement in WHC, exceptionally J3 group had similar WHC compared to JC3 group. In case of increment in the amounts of JAP alone, WHC might tend to rise, however in this case final product quality such as visual parameters and sensory attributes should also be considered.
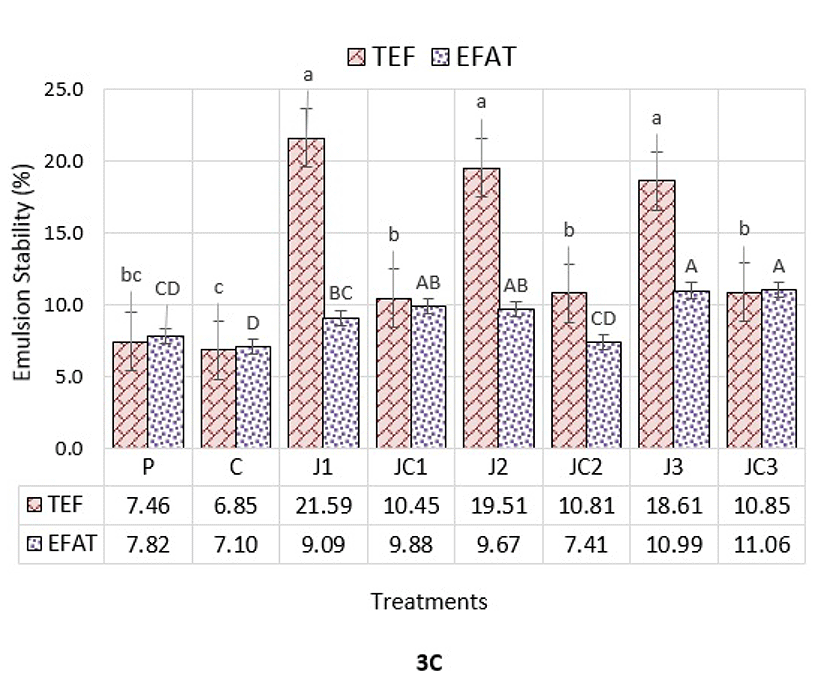
The term emulsion stability (ES) refers to the ability of an emulsion to resist changes in its characteristics over time. ES of meatball batters in terms of total expressible fluid (TEF%) and expressible fat (EFAT%) is presented in Fig. 3C. The values were recorded between 6.85-21.59% and 7.11-11.06% for TEF and EFAT, respectively. The formulation of meatballs was found to be significantly effective on ES (p<0.05). J1, J2 and J3 samples had the highest TEF compared to other samples (p<0.05), so that these samples were reported as the most unstable groups against fluid release from the structure. All the samples prepared with JAP and SC mixture (JC1, JC2, JC3) had similar TEF to samples prepared with STPP. This result showed that a rising trend was identified in liquid stability of the batters by utilization of JAP and SC together. Regarding EFAT values, it was found that the most effective phosphate-free treatment in maintaining fat retention was JC2, which had similar EFAT values to both of the P and C groups, and that had altogether lower EFAT compared to other treatments (p<0.05). From the results, it could be concluded that the most useful approach to obtain similar traits to phosphate-free emulsions is to include an average amount of JAP in combination with SC for maintaining overall emulsion stability. This supports the idea of emulsified products prepared with these ratios could have stronger storage characteristics and better technological quality. Afoakwah et al. (2015) stated that since JAP contains both inulin and protein, it may be a good emulsifying agent.
The correlations which were analyzed between emulsion characteristics are presented in Table 1. The results showed that significant correlations were obtained between pH value and JFS, WHC and TEF (p<0.05). Among these parameters, the highest correlation coefficient belonged to pH-JFS (r=0.801), which might indicate that the strongest relationship was between pH and JFS compared to other parameters. Significant negative correlations were also recorded between JFS-WHC, WHC-TEF and WHC-EFAT (p<0.05), meaning that there was a significant increase in JFS, TEF and EFAT with the decrease in WHC. These results showed that the parameters used to express functional behaviors of emulsions mostly have a strong relationship and could be used together for a comprehensive evaluation. The results confirmed that functional properties regarding emulsion stability mainly depends on pH value and thus the lack of stability in the samples in which JAP was solely used could be attributed to the low pH of these emulsions. Most of the industrial phosphates and their blends are alkaline phosphates and their addition to meat product formulations leads to a rise in pH. When a movement further away from the isoelectric point takes place, it enhances the WHC of proteins because greater electrostatics repulsive forces create larger gaps between actin and myosin to bind larger amounts of water. In addition, phosphates could increase the WHC via the chelating ability and contributing to separate actin and myosin and via changing the ionic charges and increasing degree of swelling of the muscle (Long et al., 2011; Petracci et al., 2013; Puolanne et al., 2001). These mentioned features imply that phosphates are multi-functional compounds to enhance the quality and yield of the product. However, while phosphates assist in the development of the protein structure network, fibers could absorb water that is expelled from the network when the product is cooked, as well as they have nutritive functions (Bodner and Sieg, 2009; Resconi et al., 2016a). Bodner and Sieg (2009) pointed out that unlike phosphates, fibers are not able to work at the molecular level on the actin-myosin complex, but their high ability to bind water and improve texture can provide phosphate replacement in an appropriate pH medium in different types of processed meats.
*Correlation is significant at the 0.05 level (2-tailed).
JFS, Jelly&fat separation; WHC,Water-holding capacity; TEF, Total expressible fluid; EFAT, total expressible fat
Chemical composition, in terms of moisture, protein, lipid and ash contents, total energy and pH of emulsified chicken meatballs are shown in Table 2. Moisture, protein, lipid and ash content of the samples were between 64.37-69.62%, 16.29-21.36%, 7.77-11.62% and 1.98-2.12%, respectively. The highest moisture content was evaluated in JC1, JC2 and JC3 samples (p<0.05), namely incorporation of JAP-SC mix lead to an increase in total moisture content. The most probable reason for the differences in moisture content is the aid of JAP-SC mixture in water-holding capacity of the samples. During cooking, the treatments with higher WHC might decrease the fluid exuding from the protein network. A similar study reported that added JAP increased total moisture of the products due to high water absorption capacity of the fiber structure (Afoakwah et al., 2015). Protein content of the samples were pretty much similar to each other, except J1 samples that had higher protein content compared to JC1 samples (p<0.05). Although JAP samples had a pretty high protein content (11.96%), no observable increments in protein content of the samples were evaluated with JAP incorporation. A significant decrease in total lipid content was recorded in samples formulated with JAP-SC mixture, compared to phosphate treatment (p<0.05), which could be related to increased moisture content in these groups, leading a relative decrease in final lipid amount. Resconi et al. (2016b) reported that in reduced-phosphate restructured hams, fat and protein percentage were lowered by added rice and potato starches, since they provide carbohydrates, but they noted that their functionality in retaining water might also have influenced the results. In connection with lipid content, JC1, JC2 and JC3 samples formulated with JAP-SC mixture had lower energy value compared to P samples formulated with STPP (p<0.05). As energy value was calculated based on fat and protein content, energy values were decreased in relation to lower fat content of these samples, J2 and J3 samples had also lower calorie compared to P samples (p<0.05). No differences were observed in P, C and J1 groups. This result indicated that a level of at least 5.7% JAP incorporation is required to decrease total caloric value of the product. No differences were recorded among ash content of the samples, probably due to similar contribution of the additives to inorganic matter content.
1)The meatballs were formulated with P, 0.5% STPP; C, 0.2% Na2CO3; J1, 3.8% JAP; JC1, 3.8% JAP+0.2% Na2CO3; J2, 5.7% JAP; JC2, 5.7% JAP+0.2% Na2CO3; J3, 7.6% JAP; JC3, 7.6% JAP+0.2% Na2CO3. Data are presented as the mean values of replications ± standard deviation of the mean.
abcdMeans with the different letter in the same column are significantly different (p<0.05).
pH of the samples was recorded between 6.21-6.61. It was obvious that SC had a drastic impact on pH, and C samples had the highest value among formulations (p<0.05). Similar to raw emulsion results, utilization of JAP-SC mixture was effective to maintain the high pH values. Thus, similar pH values were recorded in JC1 and JC3 samples to P samples and even higher values were recorded in JC2 samples than P samples (p<0.05). J1 and J3 treatments had lower pH than P treatment (p<0.05). A strong correlation (r=0.927) was obtained between pH values of emulsion batters and final product (data not shown) (p<0.05). Thus, a similar trend was observed in both of the raw and cooked samples and so that cooking operation did not affect this trend between groups although the values individually changed. Jarvis et al. (2012) and Casco et al. (2013) evaluated no differences in pH of chicken marinades formulated with phosphate replacers as phosphate substitutes. In a study with inulin, Huang et al. (2011) reported that pH values of dried sausages formulated with inulin did not differ from the control samples without inulin. The contrasts in our study should be due to the natural characteristics of the raw material. Yang et al. (2015) stated that the suggested value of soil pH for growth of JA tubers ranges from 4.5-8.2. Therefore, the raw material that we used might have an initial low pH, and the citric acid used for the prevention of browning might also be a reason of decreasing the pH value. In this line, the best option is to utilize JAP-SC combination to maintain the pH values at desired level.
Color parameters (CIE L*, a*, b*) of the meatballs are given in Table 3. CIE L*, a* and b* values were recorded between 44.99-55.61, 5.15-6.02 and 20.91-29.71, respectively. The results showed that significant differences were obtained in color features depending on formulations (p<0.05). The most visible change was seen in CIE L* values, where JAP treatments had lower L* values compared to phosphate (P) and carbonate (C) treatments (p<0.05), regardless of JAP amount and SC added. The reason of lower values in JAP treatments is probably due to the natural behavior of JAP in giving a darker color when mixed with water. Similarly, Afoakwah et al. (2015) found that L* value of the sausages incorporated JAP decreased while a higher L* value was recorded in the control and corn starch sausages. The meatballs formulated with JAP-SC mixture (JC1, JC2 and JC3) had similar CIE a* values to P and C groups. a* values were similar to control groups in the other phosphate-free treatments, except J1 samples that had higher a* values than P (p<0.05), but a* values decreased as the addition level increased from J1 to J3, probably due to the natural characteristics of JAP and diluting effect in total myoglobin content when used in higher levels. Conversely, added JAP was reported to increase a* values of sausages, in which the reduction of a* values in other groups were attributed to lipid and hemo-pigments oxidation (Afoakwah et al., 2015). The highest CIE b* values were obtained in P and C samples in treatments (p<0.05). Addition of JAP to formulations decreased b* values regardless of SC (p<0.05). However, increment in JAP level was effective to change the values, this is why J1 and JC1 samples had higher b* values compared to other JAP treatments (p<0.05), but the values remained similar over 5.7% added JAP. Afoakwah et al. (2015) reported that b* values of sausages formulated with JAP increased with the increasing powder concentration. They also emphasized that the color of JAP, due to the presence of polyphenolic compounds, ascorbic acid and carotene may be carried over to the final product and thus can affect the color parameters of the product. Resconi et al. (2016a) proposed that a difference in objective measurements of color is not necessarily translated into a difference in color acceptability. Hence, sensory characteristics of the final product should be evaluated with further studies to consider visual satisfaction.
1)The meatballs were formulated with P, 0.5% STPP; C, 0.2% Na2CO3; J1, 3.8% JAP; JC1, 3.8% JAP+0.2% Na2CO3; J2, 5.7% JAP; JC2, 5.7% JAP+0.2% Na2CO3; J3, 7.6% JAP; JC3, 7.6% JAP+0.2% Na2CO3. Data are presented as the mean values of replications ± standard deviation of the mean.
abcMeans with the different letter in the same column are significantly different (p<0.05).
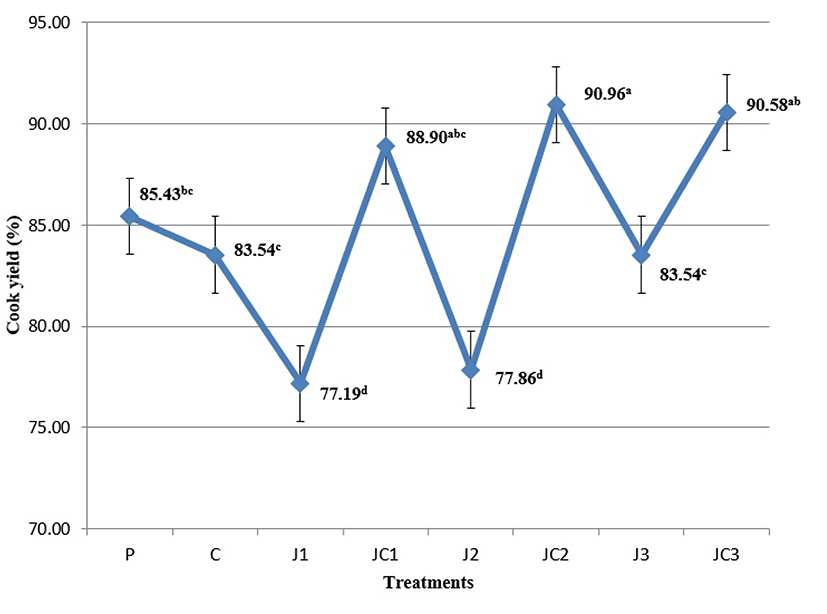
Cook yield (CY) of the samples could be seen in Fig. 4. CY of the samples were recorded between 77.19-90.96% and the changes in the formulation caused significant differences among treatments (p<0.05). A similar trend to emulsion stability parameters was observed in CY of the samples, where JC1, JC2 and JC3 groups showed equivalent CY to P group. Also C and J3 samples had similar yield to P, but these samples showed lower CY than JC2 group (p<0.05). Thus, JC2 samples showed a better performance to reduce fluid losses upon cooking. J1 and J2 samples had the lowest CY among the treatments (p<0.05). Consequently, in single-handed utilization of JAP in phosphate-free formulations, a level of more than 5.7% is required to supply similar cooking characteristics to that of formulations with phosphate. But, once again, the most effective way to maintain product yield seemed to be the combined application of JAP-SC. Fiber-sourced ingredients have a potential impact to meet the behaviors of phosphates by their water-holding ability. Álvarez and Barbut (2013) stated that the polysaccharides form a gel with heat treatment that favors the formation of a heat-induced protein matrix, diminishing the link with water. Similar to our results, Prabhu and Husak (2014) reported that native potato starch in combination with sodium carbonate increased CY of pork loins, compared to the effects of the ingredients used alone, but the CY was not different from the control samples with STPP. In a study investigating the effects of phosphate substitutes, it was found that cooked hams with rice starch retained the free water due to the starch gels that were formed (Resconi et al. 2016a). Likewise, Resconi et al. (2016b) found that with the addition of potato and rice starches to formulation of restructured hams, a 50% reduction in the use of STPP could be achieved, while maintaining a similar cook loss as obtained when adding the traditional levels. In another study, similar cooking losses were reported in case of substitution of sodium caseinate and polyphosphate by plasma, which was connected to the high hydrophilic character of plasma proteins (Hurtado et al. 2012).
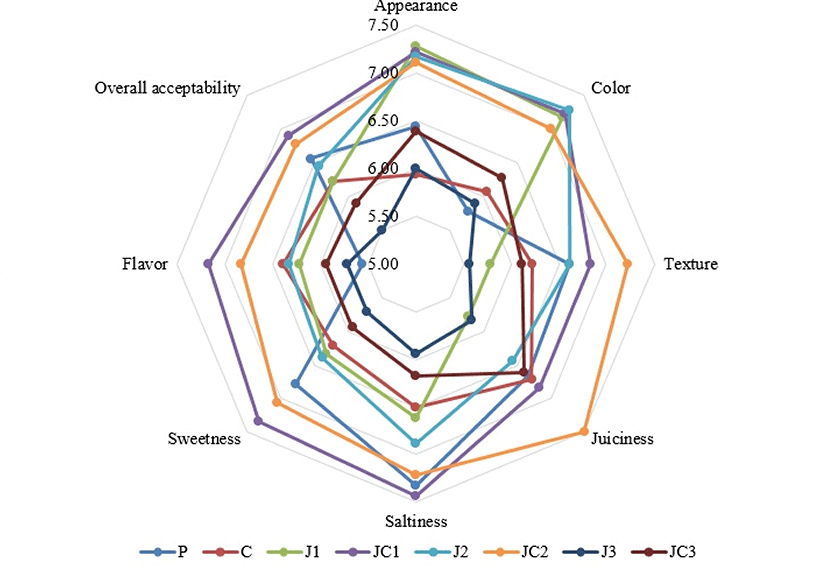
Phosphate reduction and addition of fiber ingredients are mentioned as two approaches that have potential to improve the perception and health profile of meat products (Resconi et al., 2016a). Besides the improvement of health profile, reduction and/or replacement of phosphates could negatively affect the sensory characteristics like leading color changes, off-flavors and drying of the final product. Thus it is an important point to minimize sensory changes and protect traditional appearance, texture and taste of phosphate-free meat products. The sensory scores of the meatballs are illustrated in Fig. 5. Different formulations were found to be significantly important on sensory characteristics (p<0.05), where appearance, color, texture, juiciness, saltiness, sweetness, flavor and overall acceptability were scored between 5.94-7.28, 5.78-7.28, 5.56-7.22, 5.78-7.50, 5.94-7.44, 5.72-7.33, 5.72-7.17, and 5.50-6.89, respectively. J1, JC1, J2 and JC2 samples had higher appearance scores compared to C and Y3 samples (p<0.05). Color scores were also higher in these groups compared to K and Y3 (p<0.05). Thus, visual attributes were better with low and medium level JAP samples, either alone or with SC. Statistically similar texture and juiciness scores were obtained in samples containing JAP-SC mixture compared to samples containing phosphate. This result indicated that an equivalent textural acceptability to phosphates could be provided in phosphate-free samples, probably due to desired water-holding ability leading good palatability and mouthfeel. Among samples, J3 samples had mostly lower scores in saltiness, sweetness and flavor compared to other JAP and SC mixture containing samples (p<0.05). Also, general acceptability was lower in J3 samples compared to JC1 and JC2 (p<0.05). This result indicated that increased concentrations of JAP could provoke negative changes in sensory parameters especially related with taste. Thus, medium levels of JAP and SC combination had enhancement effects on sensory parameters and thereby could meet phosphate’s sensory functions. The absence of phosphates in meat product formulations could be a challenge due to the change in sensory attributes, thus appropriate alternatives are necessary to meet consumer demands and customary habits. Jarvis et al. (2012) reported that the combination of plum powder and plum fiber marinade was found to have similar sensory characteristics when compared to STPP in chicken breast fillets for a majority of the attributes measured. However, despite of using in marinade solutions, utilization of the ingredient directly in the product formulations could influence the sensory scores. Hurtado et al. (2012) stated that the addition of plasma proteins and caseinate as phosphate replacers modified the flavor of frankfurters and panelists detected the presence of animal taste and odour in plasma containing sausages, nevertheless the overall acceptance was scored as 6.3 out of a 10-maximum scale. Resconi et al. (2016a) found that replacement of STPP with rice starch is associated with restructured hams being less juicy, salty and springy, but more adhesive and could negatively affect appearance. In comparison to this study, JAP and SC mixture used in our study seem to be a suitable alternative to phosphates as the sensory scores were in an acceptable range when this mixture was used in average levels in meatball formulations. Resconi et al. (2016b) emphasized that the used of labels “low in phosphates” and particularly “no added phosphates” in cooked hams positively influenced consumer choices. The addition of healthy ingredients in meat products and generally in foods could be appealing to consumers, especially for health conscious consumers. Therefore, the consumption of dietary fiber-enriched, phosphate-free and clean-label meat products at commercial level could be favored by this approach in the future.
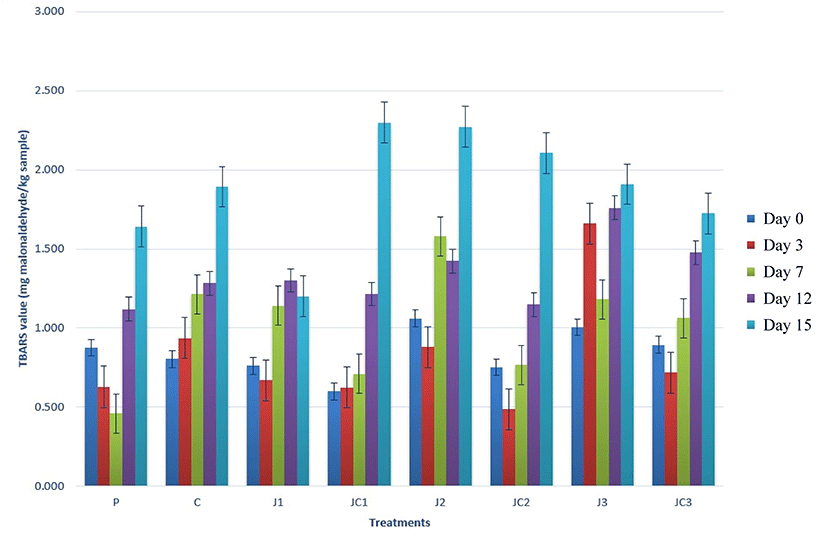
Lipid oxidation phenomena leads to negative effects on the quality of meat and meat products in terms of sensory attributes and nutritional value, as well as on health. TBARS value is an indicator of secondary products formed in fatty acid oxidation chain reactions. Phosphates have the ability of sequestrating the metal ions such as Ca2+, Mg2+, Fe2+, Fe3+, etc, which are presented in meat and thereby could reduce oxidative rancidity of the products (Long et al., 2011). TBARS values of meatball samples, presented in Fig. 6, were significantly affected by formulation and storage time (p<0.05). The values were between 0.600-1.061 mg malonaldehyde/kg at the beginning of storage. These values were not closer to zero, which could be an indicator of the oxidation process taken place during deep fat frying of the samples. Initial values were nearly similar to each other, except J2 and J3 samples having higher TBARS values than JC1 (p<0.05). But generally, it could be concluded that initial TBARS values did not change among formulations. During the storage period of 15 days, although some fluctuations were observed, in the end of the storage period, no significant differences were evaluated between TBARS values of the samples, which were between 1.201-2.299 mg malonaldehyde/kg. This result might be a good indicator that JAP had an advantage to delay lipid oxidation, due to the phenolic compounds, and it could present equivalent behavior to phosphates acting as an antioxidant agent. Yuan et al. (2012) reported that JA contains phenolic compounds such as polyacetylenic derivatives, sesquiterpenes and coumarins that can scavenge free radicals thus act as natural antioxidants. Afoakwah et al. (2015) formulated sausage with JAP and corn starch and found that freeze or oven-dried JAP sausages indicated antioxidant activity, in contrast starch showed inadequate lipid oxidation inhibition potential. They emphasized that JAP had the ability to prevent free radical-mediated oxidation as well as the ability to stabilize radicals created through intra-molecular hydrogen-bonding on further oxidation during the storage period. Contrary to this, Lowder et al. (2011) found that beef strip loins injected with dehydrated beef protein to replace phosphates had greater TBARS values than the loins injected with phosphate. It is worth noting that incorporation of JAP presents the opportunity to compensate for the oxidative changes in the phosphate-free products as well as enhancing natural and health benefits.
Conclusion
The results of our study indicated that utilization of JAP and SC in emulsified chicken meatballs have good potential to enhance physical, chemical, technological and sensory quality and offers a novel possibility for phosphate replacement in formulation of healthier poultry products. It might be suggested to utilize the medium ratios as 5.7% JAP in combination with SC in formulations to improve overall quality. This study presents earlier findings of usage of JAP, a healthy and economical dietary fiber source for phosphate replacement in emulsified poultry products. Further and comprehensive research is needed to assess issues related to different quality parameters of various phosphate-free meat products.