Introduction
The use of synthetic antioxidants is limited nowadays due to their adverse health effects and toxic effects (Bougatef et al., 2010). Therefore, there is a tendency to use natural antioxidants, which have equal and possibly higher power than synthetic antioxidants, without entailing the associated health risks (Amissah, 2012). Compared to synthetic antioxidants, peptides can be readily absorbed without causing dangerous immune reactions because they have lower molecular weights and are more stable and have higher activity levels (Liu et al., 2010; Qian et al., 2008). Peptides are acknowledged to replace synthetic antioxidants in foods. On the other hand, ACE (Angiotensin Converting Enzyme) plays a central role in regulating blood pressure in humans. Peptides are able to inhibit ACE in vitro and then exhibit antihypertensive effect in the cell. Thus, these hold promise for the development of primary hypertension preventive therapeutics and functional foods (Hong et al., 2008). During ripening of fermented sausages, bioactive peptides are formed from sarcoplasmic and myofibrillar proteins as a result of proteolysis (Vaštag et al., 2010).
Although protein hydrolysates of sarcoplasmic and myofibrillar proteins, formed as a result of proteolysis, are known to be highly effective on specific organoleptic quality properties of fermented sausages, very little is known about their other functions and properties, such as bioactivity (Vaštag et al., 2010). Escudero et al. (2012) reported that there were effective antioxidant and antihypertensive peptides in the extracts of Spanish-type hams.
In recent years, these have acquired particular importance due to beneficial effects of certain bioactive components on health. New technologies are needed to develop new functional foods and to combine pharmaceutical components that support health without reducing their functionality and bioavailability. Encapsulation of bioactive components to protect them from harsh treatment and adverse storage conditions also ensures that the components can be moved to a target destination. It also provides controlled release of the components in a specific region. In addition, as some bioactive components often have very strong aroma, encapsulation can also be useful in terms of masking taste and odour (Peres, 2011).
In order to achieve long-term stability for the collagen hydrolysate, particles were formed, combining with polysaccharide matrix such as maltodextrin and gum arabic through spray drying method (in 5% and 25%). This new protein-carbohydrate system protects collagen hydrolysate from oxidation and they can be added to functional foods, especially drinks. This is because collagen hydrolysate-polysaccharide solution is colourless, odourless and tasteless (Peres, 2011).
The purpose of this study is to prevent direct effect of hydrolysates, obtained from fish skin collagen, on chemical, physicochemical properties of sucuk, and in case they have antioxidant activity, it is to determine the effects of the addition of encapsulated hydrolysates to ensure that they sustain such a property during processing and subsequent stages of storage.
Materials and Methods
Fresh boneless beef cuts and fat (sheep tail fat and beef back fat) used in the production of sucuk were obtained from local meat processors (butchers) in Afyonkarahisar, Turkey. The beef was trimmed of visible fat and connective tissue. The beef and fat were kept at temperatures of around 4℃. Natural casings and other sucuk ingredients such as salt, garlic and powder spices were provided by a company (Hilkan Food Industry and Trade Ltd. Co., Turkey) in Konya, Turkey.
Powdered maltodextrin Maldex 120 (dextrose equivalent [DE] 11 to 15) and Maldex 190 (dextrose equivalent [DE] 17 to 19.9) were obtained from Tereos Syral (Belgium). The Maldex was manufactured by spray drying liquid maltodextrin, derived from edible corn starch hydrolysis. The fish collagen hydrolysate (Peptan F 2000 HD) was obtained from Rousseoult Angouléme S.A.S. (Rue de Saint-Michel an Angouléme, France). The rest of the chemicals and standards were of analytical grade and obtained from Sigma or Merck (Germany) unless otherwise stated.
The collagen hydrolysate were encapsulated with maltodextrin 12 DE and maltodextrin 19 DE, using different coating/extract material in 90:10 and 80:20 ratio prepared as a 30% aqueous solution. The following analyses were conducted to characterize the encapsulated materials.
Six groups of sucuk samples were prepared, including control group, collagen hydrolysate and hydrolysates with 4 different encapsulations. The experiments were conducted in duplicate with repetitions.
Four aqueous dispersions were prepared in such a way as to contain 30% solid particles by weight. The solid particles with 90:10 and 80:20 coating material/extract material ratios and two different coating materials, maltodextrin 12 DE and maltodextrin 19 DE were used. After being mixed in a magnetic mixer, the products were homogenized at room temperature at 18,000 rpm for 2 minutes (Rocha et al., 2009).
The dispersions were dried using Buchi-B290, Flawil, Switzerland model, a laboratory type of spray dryer. Feeding the dispersion through a peristaltic pump, drying was conducted. The dispersions were prepared at room temperature (23-25℃), hot air inlet temperature was atomized as 140℃, and outlet temperature at 70-80℃. In this way, encapsulated peptide groups namely, MD1210 containing 90% MD12 + 10% collagen peptide, MD1220 containing 80% MD12 + 20% collagen peptide, MD1910 containing 90% MD19 + 10% collagen peptide and MD 1920 containing 80% MD19 + 20% collagen peptide were obtained. Microencapsulate material was stored dry at room temperature in glass bottles in such a way as not to be exposed to light (Rocha et al., 2009).
Meat and fat to be used in sucuk production were prepared in such a way as to represent the entire carcass of the cattle of 2.5, 3 year old Montofon race. The sucuk dough formulation was prepared by adding cumin (1%), black pepper (0.7%), red chillies (0.5%), red sweet pepper (0.5%), allspice (0.04%), salt (1.8%), garlic (1%) and NaNO2 (150 mg/kg) to 100 kg meat and fat (90% meat, 10% fat) mixture. The materials that were purchased from Afyonkarahisar market were brought to Afyon Kocatepe University Department of Nutrition and Dietetics Laboratory and divided into 12 equal pieces in duplicate as control, peptide, MD1210, MD1220, MD1910 and MD1920 added groups. The dough mixtures were prepared separately by adding 2% peptide and encapsulated peptides (MD1210, MD 1220, MD1910, MD1920) to the prepared sucuk spice mixture; about 100 g of this were stuffed into cattle small intestines as finger sucuks. Then they were taken into the ripening room at 20-22℃ and hung at 140-150 cm height from the floor, in such a way as not to block air circulation. The sucuks were allowed to undergo natural fermentation and ripening for 14 d and 14 d for storage in the ambient temperature varied in the range of 20-22℃, and the relative humidity varied in the range of 60-75%.
During the ripening period of the sucuk samples, moisture, water activity, pH and lactic acid tests were conducted on the 0th, 1st, 2nd, 3rd, 4th, 5th, 6th, 7th, 14th and 28th d; antioxidant activity, ACE inhibition activity, TBA tests were conducted on 0, 1st, 3rd, 7th, 14th, 28th d; protein, fat, ash contents were tested on 0th d and 14th d; and a texture profile analysis (TBA) was conducted on the 14th d.
Protein (as Kjeldahl nitrogen), moisture, fat (ether-extractable) ansd ash contents were determined according to (AOAC, 1990), and pH was measured in a homogenate prepared by blending 10 g of sucuk with 90 mL of distilled water for 30 s. Readings were taken with a Mettler-Toledo model pH meter. The number of replications was three for all the properties measured. Lactic acid content was determined as outlined (AOAC, 1990) and the results obtained were expressed as %w/w lactic acid. Water activity (aw) values were analysed in accordance with the method of Rödel et al., (1975). The water activity (aw) values of encapsulated products were determined using a water activity meter (LabTouch -aw, Novasina AG, Switzerland) at 251℃ after stabilization of the samples at the temperature for 1 h.
To determine lipid oxidation, a 10 g sample was homogenized with 97.5 mL distilled water at 50℃ and put into a Kjeldahl flask. The volume was completed to 100 mL by adding a 2.5 mL 4 N HCl solution (1:2 37% HCl : distilled H2O) to it. Soybean oil was used as the antifoaming agent. Fifty ml of distillate was collected with steam distillation in a precise manner. Five ml was taken from the distillate and 5 mL 0.02 M TBA reactive was added to it; it was kept in a boiling water bath for 35 min. The absorbance values of the cooled samples were read in the UV spectrophotometer at 538 nm wavelength. The amount of malondialdehyde (MDA) formed in the product was calculated by multiplying the absorbance values by the factor 7.8 (Tarladgis et al., 1960).
In measuring the antioxidant activities of the water-soluble proteins extracted from the sucuk samples, the method used by Morales and Jiménez-Pérez (2001) was modified.
Accordingly, 1 mL daily prepared DPPH solution (74 mg/L, in 96% ethyl alcohol) was added to a 200 μL aqueous sample. After being mixed for 30 min at room temperature, the mixture was centrifuged for 5 min at 5,000 rpm. Then, absorption values were read at 517 nm wave-length and antioxidant activities were calculated using the following formula
Antioxidant activity (%) = (1 − (A1 − A2) / A0) × 100
A1: 2 mL sample + 2 mL 0.1 mM DPPH solution absorbance
A2: 2 mL sample + 2 mL ethanol absorbance
A0: 2 mL DPPH solution + 2 mL distilled water absorbance
In determining ACE inhibitory activity, 10 mmol 80 μL hippuryl-His-Leu solution prepared in 0.2 mol/L potassium phosphate buffer (pH 8.3) and 10 μL 25 mU/mL ACE solution was added to 60 μL protein extract and it was incubated for 80 min at 37℃. The reaction was terminated by adding 110 μL 1 mol/L HCL solution. Hippuric acid formed by ACE from hippuryl-His-Leu was extracted with 1.5 mL ethyl acetate. One mL from the ethyl acetate layer was evaporated and hippuric acid was dissolved in 3 mL distilled water. Absorbance values were read at 228 nm (Yoshie-Stark et al., 2004).
In the analyses that conducted using a texture analysis device with 50 kg load cell (TA-HD Plus Texture Analyser, UK), compression tests were performed on the sucuk samples; thus, the texture analysis (TPA) profiles of the samples were determined.
Slices of same size were cut from the sucuk samples in each different group, readings were taken from the texture analysis device, and the results were evaluated. Texture profile analyses (TPA) were performed at 21℃ room temperature, using the software program of the texture analysis device. The sucuk samples were sliced in 1.5 cm thickness for texture measurement and analyses were performed in quadruplicate for each sample. Within the scope of this analysis, 50% compression was applied to the sucuk samples at room temperature; the analysis results were determined in terms of hardness (N), adhesiveness (Nxsn), springiness (mm), cohesiveness, gumminess (N), chewiness (N×mm) and resilience (Bozkurt and Bayram, 2006; Crehan et al., 2000; Herrero et al., 2007).
Each parameter was tested in triplicate samples with two replications. Conventional statistical methods were used to calculate means and standard deviations. The collected data was subjected to statistical analysis using SPSS 17. A multifactor analysis of variance (ANOVA) was used to evaluate the effect of ripening time (days) and treatment (peptide addition) on the parameters studied. When a significant main effect was found, values were further analysed, using Duncan’s Multiple Range Test.
Results
The beef used in the study had to contain approximately 67.46% moisture, 20.16% protein, 11.29% fat and ash 1.32% and pH 5.95.
The Duncan multiple comparison test results for the protein, fat and ash contents of the sucuk samples on the 0th and 14th d are given in Table 1.
a-dMean values followed by different superscripts within the same column indicate a statistically significant difference between the mean values (p<0.01). Values represent the mean ± standard error.
Peptide, Free collagen hydrolysate; MD1210, Collagen hydrolysate encapsulated with MD12 (%10 CH- %90 MD12); MD1220, Collagen hydrolysate encapsulated with MD12 (%20 CH- %80 MD12); MD1910, Collagen hydrolysate encapsulated with MD19 (%10 CH- %90 MD19); MD1920, Collagen hydrolysate encapsulated with MD19 (%20 CH- %80 MD19).
Moisture content of the sucuk samples on the 0th and 14th d were examined, the difference between the treatment groups on the 0th d was not statistically significant (p<0.05); on the 14th d, there was a statistically significant difference (p<0.01). Moisture content differences are due to the decrease of the pH values of encapsulated peptide added sucuk samples. pH decrease moves the meat proteins to isoelectric point, so the water holding capacity of meat proteins decreases too.
When the variance analyses of protein, fat and ash contents of the sucuk samples on the 0th and 14th d were examined, it was determined that there was no statistically significant difference between the groups on the both days (p<0.05).
It was determined that the effects of the ripening period (d), the treatment (peptide addition) and the ‘ripening period × treatment’ interaction on the pH values of the sucuk samples were statistically significant (p<0.01).
In the sucuk samples produced by adding encapsulated collagen peptides, the Duncan multiple comparison test results for average pH values depending on the ripening period (d) and the Duncan multiple comparison test results for pH values depending on the treatment (peptide addition) are given in Table 2.
a-gMean values followed by different superscripts within the same column indicate a statistically significant difference between the mean values (p<0.01). Values represent the mean ± standard error. 28 d of ripening.
Peptide, Free collagen hydrolysate; MD1210, Collagen hydrolysate encapsulated with MD12 (%10 CH- %90 MD12); MD1220, Collagen hydrolysate encapsulated with MD12 (%20 CH- %80 MD12); MD1910, Collagen hydrolysate encapsulated with MD19 (%10 CH- %90 MD19); MD1920, Collagen hydrolysate encapsulated with MD19 (%20 CH- %80 MD19).
The effect of the ‘ripening time × treatment’ interaction on the pH values of the sucuk samples is given in Fig. 1.
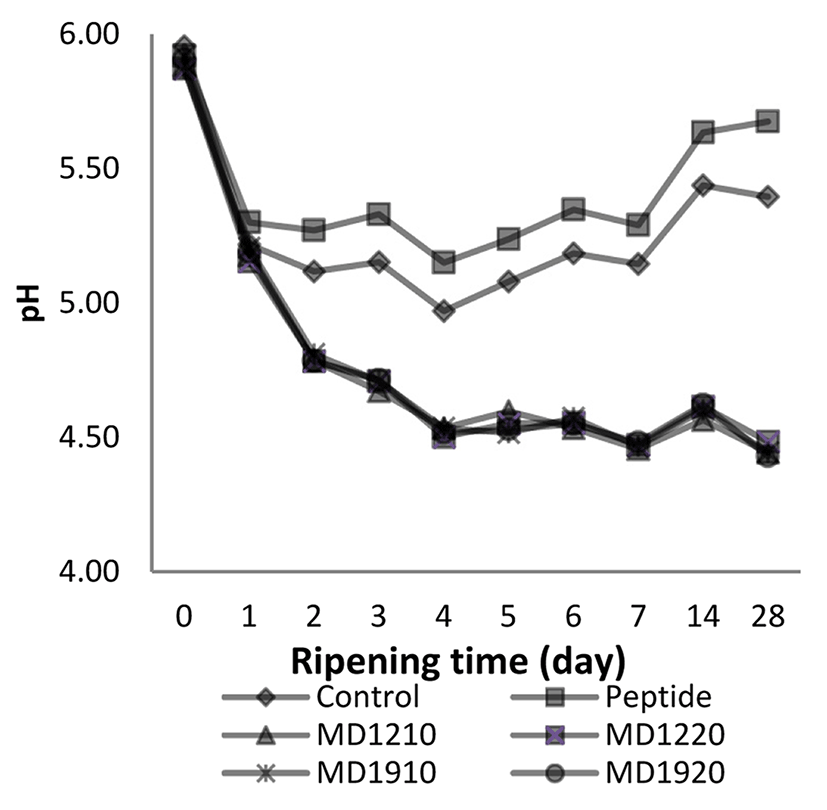
The pH values of the sucuk samples, which were taken before the ripening period, began to decline with the onset of ripening and reached the lowest value (4.7) on the 4th d. The difference between the average pH values, depending on the ripening period, was determined to be statistically significant (p<0.01). After the 4th d, the pH values began to rise.
Herranz et al. (2005) investigated the effects of free amino acid addition in the production of dry fermented sausage, they reported that the pH values of the sausage samples after ripening were around average 4.7.
Between the treatment groups, a statistically significant difference was determined in terms of average pH values (p<0.01), and the highest pH value (5.41) was measured in the samples to which peptide was added. The pH values of the sucuks, which were produced with the addition of encapsulated peptide, ranged between 4.76 and 4.77 and it was determined that they have lower pH values than the control group (5.26) in a statistically significant manner. The reason why the pH values of the peptide added group were higher than the other groups can be thought as occurring because the pH of the peptides ranged between 5.0-6.5 when they dissolved in the water and, this might cause an increase in the pH of the sucuk samples.
A drastic drop of the pH value of encapsulated peptide added sucuk samples is due to maltodextrin which used for encapsulation of peptides. Occurrence of lactic acid due to lactic acid bacteria and coagulase-negative staphylococci and the glycolysis etc. decreases the pH of the samples.
The effects of the ripening period (d), the treatment (peptide addition) and the ‘ripening period × treatment’ interaction on the lactic acid values of the sucuk samples were determined to be statistically significant (p<0.01).
The Duncan multiple comparison test results for the average lactic acid (%) contents depending on the ripening period (d) and the treatment in the sucuk samples produced with the addition of encapsulated collagen peptides, are given in Table 2.
The effect of the ‘ripening period × treatment’ interaction on the lactic acid content of the sucuk samples is given in Fig. 2.
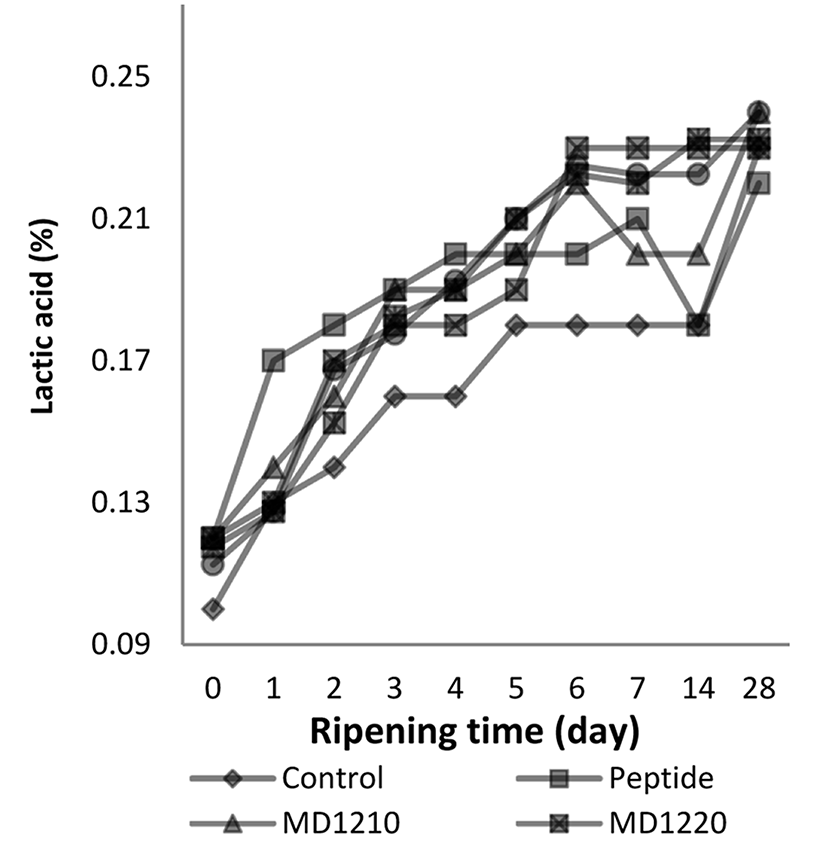
The lactic acid contents of the sucuks, produced by adding encapsulated collagen peptides, increased in a statistically significant way from the start of the ripening period (p<0.01). The lactic acid contents of the samples did not differ in a statistically significant way between the 6th, 7th and 14th d, and reached the highest values on the 28th d.
The lactic acid contents of treatment groups were higher than the control group in a statistically significant way (p<0.01). However no statistically significant difference was determined amongst them. Maltodextrin usage for the encapsulation of peptide increases the formation of lactic acid. Kovačević et al. (2014) showed that the maltodextrin additon to Kulenova seka formulation increases the formation of lactic acid and rapid pH decreases than the other sugar sources.
When the variance analysis results were examined, the effects of the ripening period (d), the treatment (peptide addition) and the ‘ripening time × treatment’ interaction on the water activity of the sucuk samples were determined to be statistically significant (p<0.01).
The Duncan multiple comparison test results for the average water activity values depending on the ripening period (d) in the sucuk samples produced with the addition of encapsulated collagen peptides are given in Fig. 3. and the Duncan multiple comparison test results for the average water activity values depending on the peptide addition (treatment) are given in Table 2.
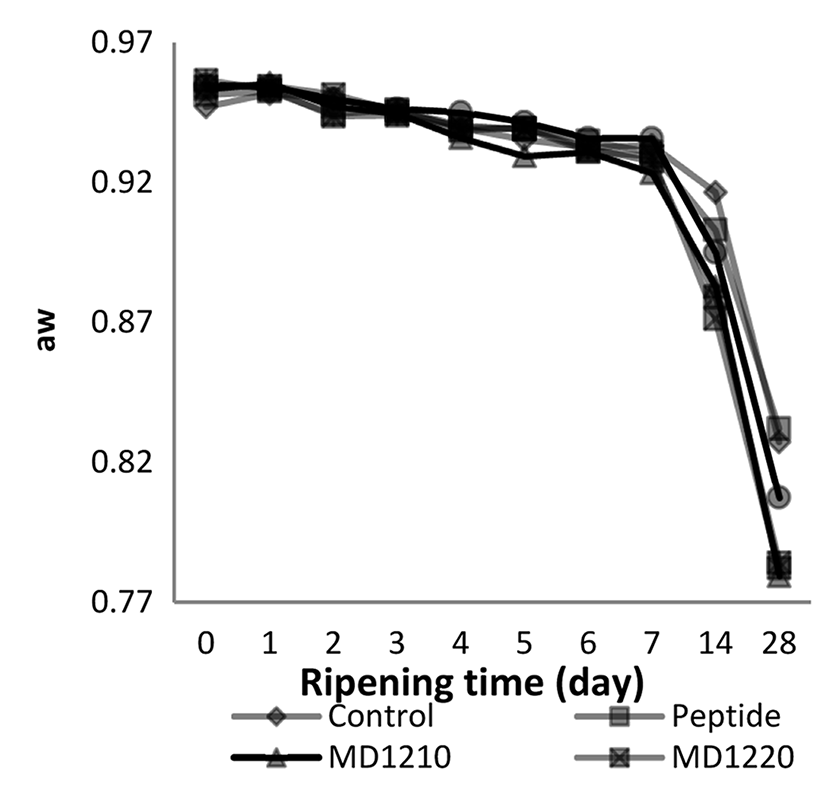
The effect of the ripening time × treatment interaction on the water activity levels of the sucuk samples is given in Fig. 3.
The aw values of the sucuk samples decreased in a statistically significant way with the onset of the ripening period. The aw values of the samples decreased below 0.90 from the 14th d.
Herranz et al. (2005) reported that the addition of free amino acids in dry fermented sausage production did not significantly change the aw values of the sausage samples; and the average value of all the groups was around 0.807.
It was determined that between the aw values of the sucuk samples in the control group and the groups produced with the addition of peptide and MD1920 (0.927-0.928) and the aw values of the samples produced with the addition of MD1210, MD1220 and MD 1910 (0.918-0.920) there was a statistically significant difference (p<0.01). However there was no statistically significant difference amongst them (p<0.05).
When the variance analysis results were examined, the effects of the ripening period (d), the treatment (peptide addition) and the ‘ripening time × treatment’ interaction on the moisture contents of the sucuk samples, were determined to be statistically significant (p<0.01).
The Duncan multiple comparison test results for the average moisture contents depending on the ripening period (day) in the sucuk samples produced with the addition of encapsulated collagen peptides are given in Table 2 and the Duncan multiple comparison test results for the average moisture content depending on the peptide addition (treatment) are given in Table 2. The effect of the ‘ripening time × treatment interaction on the moisture contents of the sucuk samples is given in Fig. 4.
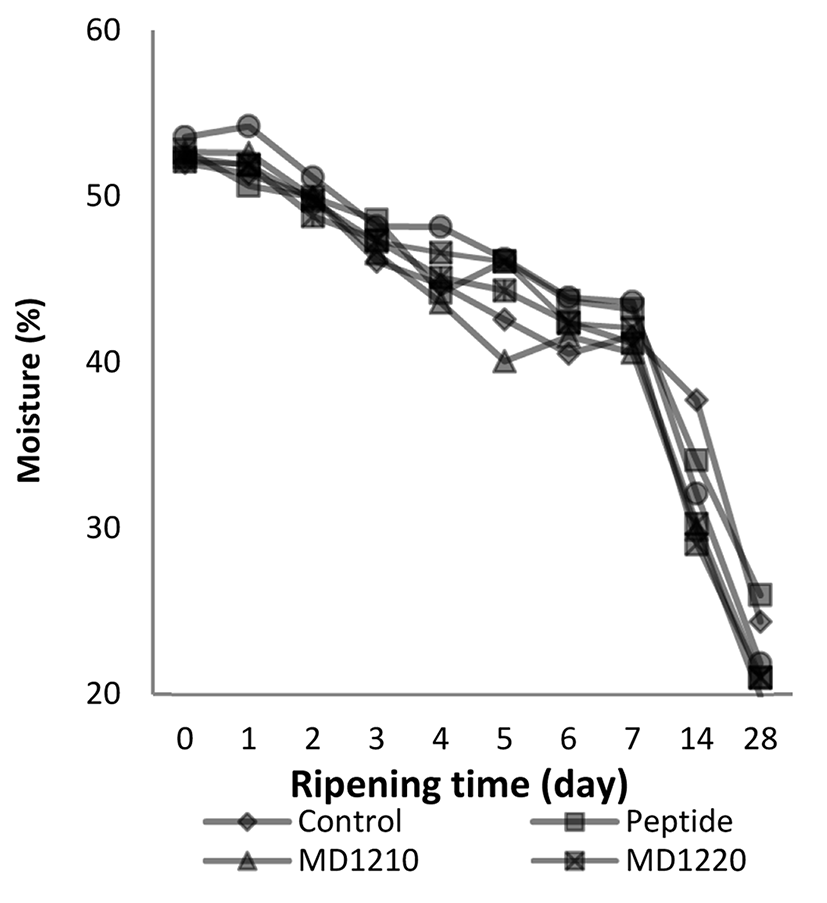
The moisture contents of the sucuk samples decreased during the ripening period. The moisture content that was at the level of 52.58% initially dropped to 22.34% on the 28th d. This decrease was due to the fact that a decrease was observed in the water contents of the fermented sucuks, depending on the ambient temperature and humidity.
Herranz et al. (2005) reported that when free amino acids were added to the dry fermented sausages, the amount of the dry substance in all sausage samples was around 71% after ripening. The moisture contents of the sucuk samples are consistent with the literature.
Coşkuner et al. (2010) conducted studies on the traditionally and heat-treated sucuk samples and they determined the moisture contents of the sucuks produced with the traditional method as a result of 7th d fermentation at 32.8±0.3%.
The highest level of moisture content was determined in the MD1920 group, and there were statistically significant differences between the other groups (p<0.01), with the exception of the peptide added group. There was no statistically significant difference between the moisture contents of the sucuks in the control group and the peptide added groups. The lowest moisture content (41.73%) was determined to be in the MD1210 group.
When the results of the variance analysis were examined, the effects of the ripening time (d), the treatment (peptide addition) and the ‘ripening time × treatment’ interaction on the TBA values of the sucuk samples were determined to be statistically insignificant (p<0.05).
The Duncan multiple comparison test results for the average TBA values, depending on the ripening time (d) in the sucuk samples produced with the addition of encapsulated collagen peptides and the test results for the average TBA values depending on the peptide addition (treatment) are given in Table 3.
a,bMean values followed by different superscripts within the same column indicate a statistically significant difference between the mean values (p<0.01), *;(p<0.05). Values represent the mean ± standard error. 28 d of ripening.
Peptide, Free collagen hydrolysate; MD1210, Collagen hydrolysate encapsulated with MD12 (%10 CH- %90 MD12); MD1220, Collagen hydrolysate encapsulated with MD12 (%20 CH- %80 MD12); MD1910, Collagen hydrolysate encapsulated with MD19 (%10 CH- %90 MD19); MD1920, Collagen hydrolysate encapsulated with MD19 (%20 CH- %80 MD19).
The effect of the ripening period on the TBA values of the sausage samples was analysed. It was determined that the TBA values reached the highest level on the 3rd d (0.673 mg MDA/kg sample) and dropped to the lowest level on the 28th d (0.445 mg MDA/kg sample). Between the average TBA values of the 3rd and 7th d, and those of the 0th and 14th d, no statistically significant difference was determined.
TBA values of the sucuk samples and difference between the groups were statistically not significant. The TBA values of the treatment groups were found between 0.530 and 0.559 mg MDA/kg.
Soyer and Ertaş (2007) showed that the oxidation degree in sucuk samples affected by storage time and fat content. Bozkurt and Erkmen (2007) concluded that the TBARS values affected by the fat content and ripening period. They said that the TBA formation was the highest at first 5 d and concluded that lipid oxidation was started from the 5 d and continues during ripening.
The variance analysis results for the antioxidant activity data of the water-soluble proteins extracted from the sucuk samples produced with the addition of encapsulated collagen peptides are given in Table 3.
When the variance analysis results were examined, the effects of the ripening period (d) and the treatment (peptide addition) on the antioxidant activity levels of the water-soluble proteins extracted from the sucuk samples, were determined to be statistically significant (p<0.01). The effect of the ‘ripening time × treatment’ interaction was determined to be statistically insignificant (p<0.05).
The Duncan multiple comparison test results for the average antioxidant activity levels of the water-soluble proteins extracted from the sucuks produced with the addition of encapsulated collagen peptides depending on the ripening period (d) and the average values depending on the treatment are given in Table 3.
The difference between the days when the antioxidant activities of the water-soluble proteins extracted from the sucuk samples increased (depending on the ripening period) was observed to be statistically significant (p<0.01). It was determined that the highest antioxidant activity value was reached on the 28th d (46.95%) and there was not a statistically significant difference between the averages of the 7th d (42.70%) and the 14th d (46.43%). It was also determined that there was not a statistically significant difference between the 3rd d antioxidant activity levels and the 7th d antioxidant activity levels.
Vaštag et al. (2010) reported that the radical scavenging activity of the water-soluble proteins extracted from Petrovská Kolbása was initially (0th d) 27.61±0.73%. They reported that the effect increased in a statistically significant way as with the ripening process went on, and it reached the level of 50.08±1.25% on the 90th d.
In this study there was no statistically significant difference between the control group and the peptide added and the MD1920 added sucuk samples among themselves, and the encapsulated peptide added sucuk samples among themselves in terms of the antioxidant activity values. The antioxidant activity was significantly high in the encapsulated peptide added samples. It was determined that the antioxidant activity values of the water-soluble proteins extracted from the sucuk samples with different peptide additions varied between 33.99 and 40.48%. It can be said that the fermentation goes fast than the control group and peptide added group because of the usage of maltodextrin to encapsulation wall material. Antioxidative peptides release by proteolytic hydrolysis of meat proteins due to the microbiota activity. According to Fernández et al. (2015) microbial activity in the fermented sausage significantly influences the low molecular weight nitrogen compounds and antioxidant activity. They reported that when the starter cultures adaptation are well and have high proteolytic activity increases the antioxidant activity of the nitrogen compounds extracts.
Tøstesen (2012) reported that proteins and peptides exhibit bioactive characteristics in fermented samples due to the chemical changes they undergo during fermentation, while peptides added to the samples may have been converted to amino acids and aromatic compounds by microorganisms.
The effect of the ripening period (d) on the ACE inhibitory activity results of the water-soluble proteins extracted from the sucuk samples was determined to be statistically significant (p<0.01). On the other hand the effects of the treatment and the ‘ripening time × treatment’ interaction, were determined to be statistically not significant (p<0.05).
The Duncan multiple comparison test results for the average ACE inhibitory activity results of the water-soluble proteins extracted from the sucuks produced with the addition of encapsulated collagen peptides depending on ripening the period (d) and the average values depending on the treatment (peptide addition) are given in Table 3.
The ACE inhibitory activities of the water-soluble proteins, extracted from the sucuk samples, began to increase from the 3rd d and the highest ACE inhibitory activity was reached on the 14th d (44.99%). When the Duncan results for the ACE inhibitory activities were examined, it was determined that the differences between the 3rd, 7th, 14th and 28th d were not significant while the difference between the 0th and 1st d was statistically significant.
Vaštag et al. (2010) reported that the initial (0th d) ACE inhibitory activity of the water-soluble proteins extracted from Petrovská Kolbása was 27.11±2.163%; and this activity level might be due to garlic components. They determined that the activity increased compared to the initial ACE inhibitory activity as the ripening process continued, it was 40% on the 15th d and 60% on the 30th d. While the results obtained on the 14th d of the study are consistent with the results obtained on the 15th d by Vaštag et al. (2010), no difference exists between the 28th d and 30th d values.
It can be said that bioactive components are formed during the fermentation process as a result of the breakdown of proteins. This is the reason why a significant difference does not occur in the ACE inhibitory activity values during the ripening period after fermentation might be due to the deceleration of fermentation and the peptides being fragmented into smaller molecules.
When the effects of the application groups on the ACE inhibitory activity values of the water-soluble proteins extracted from the sucuk samples were examined, the difference between the average values was determined to be statistically insignificant (p<0.05). It was determined that the ACE inhibitory activity values, varied between 39.71% and 42.45%.
Tøstesen (2012) reported that any ACE inhibitory activity was not observed in the control group samples of liver paste, meatball and Wiener sausages and reported that the IC50 value of the ACE inhibitory activity in the salami control group was 364 mg/mL and it was stronger than the hydrolysate added samples. Also observed that it could be stated that this situation might have arisen due to the chemical changes that the lactic acid bacteria caused in the proteins and the peptides during fermentation; while the proteins in the salami samples of the control group were disintegrated into peptides with the ACE inhibitory activity, the peptides in the hydrolysate added samples might be converted to amino acids and aromatic compounds by microorganisms. In our study it can be said that the addition of peptides and encapsulated peptides may be changed by lactic acid bacteria activity during fermentation but same bacteria fermented the meat proteins to peptides which have ACE inhibitory activity.
The texture profile characteristics of the sucuks produced with the addition of encapsulated peptide were determined on the 14th d of the ripening. The variance analysis results for the TPA data of the sucuk samples produced with the addition of encapsulated collagen peptides are given in Table 4.
a-cMean values followed by different superscripts within the same column indicate a statistically significant difference between the mean values (p<0.01). Values represent the mean ± standard error.
Peptide, Free collagen hydrolysate; MD1210, Collagen hydrolysate encapsulated with MD12 (%10 CH- %90 MD12); MD1220, Collagen hydrolysate encapsulated with MD12 (%20 CH- %80 MD12); MD1910, Collagen hydrolysate encapsulated with MD19 (%10 CH- %90 MD19); MD1920, Collagen hydrolysate encapsulated with MD19 (%20 CH- %80 MD19).
When the variance analysis results were examined, the effect of the treatment (peptide addition) on the hardness value of the sucuk samples was determined to be statistically significant (p<0.05), while its effect on the adhesiveness, springiness, cohesiveness, gumminess, chewiness and resilience values were not statistically significant (p<0.05).
The hardness values of the sucuk samples were examined, it was observed that the hardness values of the encapsulated peptide added groups were high and there was no statistically significant difference among them and between them and the peptide added sample. Compared to the control group, the hardness values of all the groups with additives were determined to be high in a statistically significant manner (p<0.05). The hardness and chewiness values of encapsulated peptide added sucuk samples are higher than the control group because of the pH decline near to isoelectric point of meat proteins so water holding capacity of proteins are minimum level. Samples are more dry so hardness values higher than the control group.
Şanes (2006) demonstrated that the inulin, maltodextrin and wheat fiber addition to sucuks with different fat levels. They showed that maltodextrin added sucuk samples texture profile analyse results are similar to control group of sucuks with high fat ratio. Maltodextrin addition to sucuk samples which have low and medium fat, decreases gumminess and chewiness values but increases adhesiveness values.
Herranz et al. (2005) determined the hardness and the cohesiveness values of free amino acid added dry-fermented sausages between 131.8±7.8 to 153±12.2 N, −1.32±0.34 to −1.33±0.37 Ns respectively. They reported that there was not a statistically significant difference between the values they obtained and the values of the control group 140.2±7.7 N, −1.35±0.27 Ns. They also determined that the springiness values of the free amino acid added dry fermented sausages varied between 0.50±0.03 to 0.68±0.06 mm. They reported that there was a statistically significant difference (p<0.05) between the values they obtained and the value of the control group (0.54±0.07).
Herranz et al. (2005) also reported that the adhesiveness, gumminess and chewiness values of the free amino acid added dry fermented sausages varied between 0.43±0.06 and 0.49±0.08, 75.01±0.08 and 82.1±8.3 N, 0.41±0.01 and 0.51±0.01 J respectively. They reported that there was not a statistically significant difference (p<0.05) for adhesiveness between the values they obtained and the value of the control group (0.38±0.06) but there was a statistically significant difference (p<0.05) for the gumminess and the chewiness values they obtained and the value of the control group (53.6±5.3 N, 0.29±0.04J). They said that these differences could be attiributed to the activity of microbial flora due to amino acid addition.
Ikonic et al. (2015) studied the ripening conditions effect on proteolysis and texture of dry-fermented sausage Petrovská klobása and concluded that hardness and chewiness of treatment groups increased during processing and ındustrial sausages pH drop is higher than the traditional ones because of the fater fermentation process. This situation affects the drying of sausages and rhelogical properties of fermented sausages.
Discussion
The pH values of the encapsulated peptides, on the other hand, were found to be lower than those of the control group. The titrable acidity values of the sucuk samples increased during ripening. The aw values and the moisture contents of the sucuk samples were reduced, depending on drying, which occurred during ripening. The TBA values of the sucuk samples increased up to the 7th d during the ripening period, and then they decreased. The antioxidant activities of the water-soluble proteins extracted from the sucuk samples increased during the fermentation process; it was determined that the values obtained after the 7th d until the end of fermentation did not exhibit a statistically significant difference. The ACE inhibitory activity of the water-soluble proteins extracted from the sucuk samples was determined to increase until the 3rd d of the fermentation process, and did not exhibit a statistically significant increase during the rest of the ripening period.
Although addition of peptide causes high levels of pH in sucuk samples, these can be as effective as peptides coated with maltodextrin in terms of formation of lactic acid, and coated peptides can be used in sucuk production to achieve rapid acidity development. Peptides and encapsulated peptides can be added to sucuks as they do not have a significant effect on the texture characteristics of sucuk samples, except for hardness.













