Introduction
Although beef is consumed after cooking in many countries, there are some countries, in which still consume raw beef dishes such as Yukhoe and beef sashimi in Korea and Japan, and beef tartare in western countries. However, the possibility for the existence of pathogenic bacteria in raw beef is very high, especially Listeria monocytogenes. L. monocytogenes is isolated from beef carcass and raw beef (Rhoades et al., 2009; Wieczorek et al., 2012). In addition, the pathogen remains a significant cause of foodborne illness characterized with high mortality rate up to 30%. It may cause sepsis, central nervous system infections, and maternal and fetal infections in pregnant women, the elderly, and immunocompromised people (CDC, 2013).
L. monocytogenes is widely spread in contaminated food and industrial environments (Lakicevic et al., 2015). Vitas et al. (2004) investigated the prevalence of L. monocytogenes in raw meat from open markets in Spain, and they reported that 34.9% of raw meat were L. monocytogenes contaminated before consumption. Indrawattana et al. (2011) investigated L. monocytogenes contamination on raw meats marketed in Bangkok, and reported that 15.4% of raw meat samples collected from supermarkets and open markets were contaminated with L. monocytogenes.Chiarini et al. (2009) investigated the prevalence of L. monocytogenes in poultry slaughterhouses and reported that 27.3% of non-food contact surfaces and 19.4% of products were contaminated with L. monocytogenes. In Brazil, 44.2% of L. monocytogenes isolates examined in poultry slaughterhouse during 1-year period were isolated from the thigh samples (Schäfer et al., 2017). L. monocytogenes strains attach to environmental surface and form biofilms, and the pathogen may be transmitted to food such as raw meat and dairy food (Pesavento et al., 2010). Although proper cleaning of processing plants is performed to control L. monocytogenes, the bacterium may exist in a slaughterhouse (Chiarini et al., 2009). L. monocytogenes can be cross-contaminated to raw meat in a slaughterhouse (Samelis and Metaxopoulos, 1999), and the pathogen can grow on raw meat at refrigeration temperature because L. monocytogenes is psychrotrophic (Carpentier and Cerf, 2011). The pathogen on raw beef can infect people through consuming raw beef dishes. Thus, non-thermal decontamination technology should be developed to improve the food safety of raw beef dishes.
Hydrogels are materials composed of a polymer backbone, water, and a crosslinking agent. The hydrophilic functional groups and three-dimensional polymeric structure of hydrogels may facilitate the absorption of water, biological fluids or antimicrobial substances (Bajpai et al., 2008). Hydrogels are applied to a variety of fields in medical, pharmaceutical and related fields, and are used as wound dressings, cosmetics, and drug delivery systems (Kamoun et al., 2017). However, no study has described the application of hydrogel patches in food industry. Thus, if the hydrogels formulated with antimicrobials are applied on food surface, it should be useful as a non-thermal decontamination technology for raw beef dishes. However, for the application on food, the hydrogel needs to be developed with edible polymers and antimicrobials.
In our previous studies, alginate and κ-carrageenan were used as appropriate materials as polymers for the preparation of hydrogels (Kim et al., 2017). The κ-carrageenan-based hydrogels showed improved physical properties, and Ca2+ ions in the gel improved the gel hardness (Campo et al., 2009; Kim et al., 2017). In addition, since alginate-based hydrogels showed high flexibility and non-toxic, alginate can be used as a polymer for hydrogels (Amsden and Turner, 1999). Chitosan is a natural polysaccharide that may inhibit the growth of several bacteria and fungi with low toxicity (Trad et al., 2018). The antimicrobials grapefruit seed extract (GSE) and citrus extract (CE) can be formulated in the hydrogels because they are natural and have high antimicrobial activities against L. monocytogenes even at low concentrations in our previous study (Kim et al., 2018). The main compounds in grapefruits and citrus seeds are polyphenols known as limonoids and naringenin. These compounds can inactivate more than 800 types of bacteria and viruses (Cvetnić et al., 2004).
Therefore, the objective of this study was to develop an antimicrobial hydrogel with edible polymers and antimicrobials to improve the food safety of Yukhoe (Korean beef tartare) by reducing L. monocytogenes cell counts.
Materials and Methods
Sodium alginate and κ-carrageenan were dissolved in distilled water, containing a co-polymer and/or crosslinkers. Hydrogel 1 was prepared as follows: 5% sodium alginate (Junsei chemical, Tokyo, Japan) and 1% chitosan (Carl Roth GmbH, Karlsruhe, Germany) were dissolved in 40 mL of glycerol (Daejung, Siheung, Korea). About 0.2% of CaCl2 (Samchum chemical, Pyeongtaek, Korea) was dissolved in 60 mL of water (H2O), and this solution was added to the glycerol mixture to provide Ca2+ ion. After stirring for 20 min, this mixture was poured into a flat square-shaped dish (SPL life science, Pocheon, Korea) and stored at 42℃ to dry the hydrogel for 2 days (Lee et al., 2003). Hydrogels 2, 3, and 4 were prepared as follows: 1% (hydrogel 2) or 2% of κ-carrageenan (hydrogels 3 and 4) in 100 mL of H2O were stirred on a heat block at 80℃ (Mohamadnia et al., 2008), followed by the addition of 1% chitosan (hydrogel 2), 1% CaCl2 (hydrogel 3), or 3% CaCl2 (hydrogel 4). The solutions were poured into the flat square-shaped plate, which were placed at room temperature (25℃) to form hydrogels.
Hydrogels were weighed and placed in distilled water (control), 0.1% grapefruit seed extract (GSE; ES Food, Gunpo, Korea) solution, and 0.1% citrus extract (CE; ES Food) solution for 30, 60, 120, and 240 min, followed by incubation at room temperature for 5 min to allow the surfaces to dry out. The hydrogels were weighed, and the swelling ratios of the hydrogels were calculated by the following equation:
Where Ws is the weight of the swollen hydrogel, and Wo is the weight of dried hydrogel.
Fifteen L. monocytogenes strains SMFM-SI-1, SMFM-SI-2, SMFM-SI-3, SMFM-SI-4, SMFM-SI-5, SMFM-SI-6, SMFM-SI-7, SMFM-SI-8, SMFM-SI-9, SMFM-SI-10, SMFM-SI-11, SMFM-SI-12, SMFM-SI-13, SMFM-SI-14, and SMFM-SI-15 isolated from carcass surface of cattle and swine in slaughterhouses (Oh, 2017) were cultured in 10 mL of tryptic soy broth with 0.6% yeast extract (TSBYE; Becton, Dickinson and Company, Sparks, MD, USA) at 30aC for 24 h. After incubation, 100 CL of cultures were transferred to 10 mL of TSBYE and incubated again at 30LC for 24 h. The cultures were centrifuged at 1,912×g and 4CC for 15 min, and the cell pellets were washed twice with phosphate-buffered saline (PBS, pH 7.4; 8.0 g of NaCl, 0.2 g of KH2PO4, 1.5 g of Na2HPO4, and 0.2 g of KCl in 1 L of distilled water). These suspensions were mixed, and their optical densities were adjusted to 0.1 at 600 nm with UV-spectrophotometer (OD600). The diluted suspension was spread onto the surface of tryptic soy agar with 0.6% yeast extract (TSAYE; Becton) with sterile swab. The prepared hydrogels (1×1 cm) were placed in 0.1% GSE or 0.1% CE solution for 30, 60, 120, and 240 min to make antimicrobial hydrogels, and they were then placed on the TSAYE surfaces. After incubation at 30yC for 24 h, the clear zones (mm) around the antimicrobial hydrogel were measured.
L. monocytogenes (OD600=0.1) was inoculated on the surfaces of raw beef (sliced round; 3×3 cm), and the samples were left at room temperature for 10 min to allow the bacterial cell attachment. The antimicrobial hydrogels were placed on the surface of the inoculated samples, and stored at 4℃ for 0 and 60 min. After storage, the antimicrobial hydrogels were removed, and the beef surfaces were swabbed by sterile swabs. The swabs were immediately placed in tubes containing 10 mL of 0.1% BPW (buffered peptone water; Becton, Dickinson and Company, Sparks, MD, USA), and vortexed for 1 min. Swab samples were serially diluted with 0.1% BPW, and the diluents were spread-plated on PALCAM agar (Becton, Dickinson and Company). The plates were incubated at 30℃ for 48 h, and colonies were counted manually.
Each experiment was performed twice in triplicates per trial. The data for the antimicrobial activity of hydrogels were analyzed with the GLM procedure of SAS® (version 9.2, SAS Institute, Cary, NC, USA). The differences between the LS means were determined by the pairwise t-test at α=0.05.
Results and Discussion
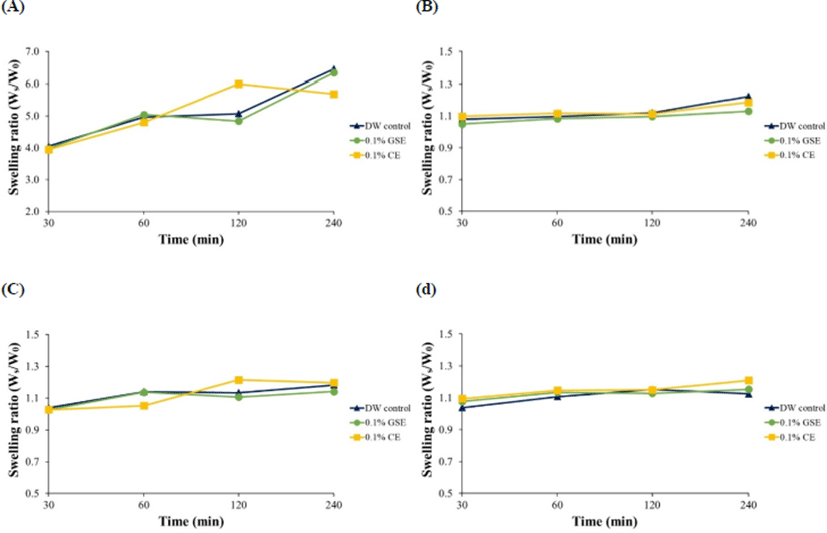
The hydrogels were prepared by two polymers such as alginate and κ-carrageenan with co-polymer, and cross-linkers to provide cation (Ca2+) which improves crosslinking between polymer molecules. When the hydrogels were placed in antimicrobial solutions to allow them absorb the antimicrobials, they showed different absorbing responses. In addition, the swelling of hydrogels is important in biomedical and antimicrobial applications. Thus, SRs of the hydrogels were determined after the absorption of the antimicrobials into the hydrogels, and only SR of hydrogel 1 increased as absorbing time increased, but the SRs of other hydrogels were unchanged (Fig. 1). This result indicates that hydrogel 1 (5% alginate+1% chitosan+0.2% CaCl2+40% glycerol) effectively absorbed antimicrobials. A study by Paşcalău et al. (2013) compared swelling of alginate and κ-carrageenan, and described that alginate had better swelling than κ-carrageenan because of cross-linking density with polymeric network. Thus, alginate hydrogel may show high SR in this study.
The hydrogels were allowed to absorb the antimicrobials for 30, 60, 120, or 240 min, and antimicrobial hydrogel 1 showed the biggest clear zone size (p<0.05), and its size was generally increased as the absorption time increased (Table 1). Although the other antimicrobial hydrogels also formed clear zones, their antilisterial activities were lower (p<0.05) than those of antimicrobial hydrogel 1 (Table 1). This result indicates that antimicrobial hydrogel 1 has obvious antilisterial activity. However, the hardness of the antimicrobial hydrogels decreased after 120 min, regardless of the hydrogel type (data not shown). This effect may be associated with the released antimicrobials because of an opposite elasticity force, created by balancing the stretching of the network at the equilibrium (Ganji et al., 2010; Kojima et al., 1998). Hence, the optimum antimicrobial absorption time for the preparation of antimicrobial hydrogels was determined to be 120 min.
To determine if antimicrobial hydrogel 1 can be applied on the raw beef, the antimicrobial hydrogel 1 absorbed antimicrobials for 120 min was placed on L. monocytogenes-inoculated raw beef. The antimicrobial hydrogel 1 formulated with 0.1% GSE or 0.1% CE reduced L. monocytogenes cell counts by 1.1 (93.6%)–1.4 Log CFU/cm2 (96.0%), but the hydrogel 1 formulated with no antimicrobials did not reduce L. monocytogenes cell counts (Table 2). Andritsos et al. (2013) showed that contamination level of L. monocytogenes in L. monocytogenes-positive samples was usually <100 CFU/g, and Khen et al. (2015) also showed that L. monocytogenes levels in L. monocytogenes-positive beef surfaces were 100–200 CFU/g. Thus, the reductions caused by the antimicrobial hydrogels should be meaningful. Several other studies have demonstrated that the 1% GSE was effective in inhibiting L. monocytogenes cell counts more than 2 Log CFU/g. In addition, the edible coating containing nisin with 1% GSE was greatly enhanced the antimicrobial effect (ca., 2.8 Log CFU/g reductions at 4℃ and 10℃) against L. monocytogenes on the meat sausage (Theivendran et al., 2006). Our research results showed that even less concentration of the antimicrobials than other studies as shown above reduced L. monocytogenes cell counts on the raw beef surface.
In conclusion, among the four hydrogels, antimicrobial hydrogel 1 (5% alginate+1% chitosan+0.2% CaCl2+40% glycerol) treated with 0.1% GSE or 0.1% CE for 120 min was the most efficient formulation to reduce L. monocytogenes cell counts on raw beef, which is a material for Yukhoe. Therefore, applying the antimicrobial hydrogel 1 should be useful in improving the food safety of Yukhoe.













