Introduction
Synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and propyl gallate (PG) are widely used to prevent oxidative degradation in the food industry. The oxidation of lipids and proteins is considered as a major cause of food spoilage, especially in meat and meat products. Thus, the use of synthetic antioxidants is the most effective method to control oxidation, which reduces the formation of undesirable oxidation products, enhances nutritional value and sensory quality and extends the shelf-life of muscle foods during storage (Jia et al., 2012; Shah et al., 2014). Since synthetic antioxidants may produce toxic compounds, consumers and meat technologists have become more interested in antioxidants from natural resources (Ibrahim et al., 2010; Jia et al., 2012). Recently, the utilization of natural antioxidants and antimicrobial agents extracted from various plants and fruits as food additives and preservatives has increased (Hinneburg et al., 2006).
Annatto (Bixa orellana L) seed has been used as a natural colorant in many traditional foods found in Asia. Annatto ranks second place in economic importance worldwide among all natural colorants and its extract shows antimicrobial and antioxidant properties (Yolmeh et al., 2014a). The color of the pigment from the outer layer of annatto seeds ranges from yellow to red and is affected by the concentration of the color compounds (Taham et al., 2015). The main color pigments of annatto seeds are bixin and nor-bixin, extracted from the outer coating of the seeds (Taham et al., 2015). Several previous studies have measured the antioxidant activities and color properties of different annatto extracts (Cardarelli et al., 2008). On the other hand, numerous studies were performed based on the extraction of bixin compounds from annatto seeds using different techniques (Rodrigues et al., 2014; Taham et al., 2015; Yolmeh et al., 2014a). Although most of the studies focused on the determination or optimization of the extraction methods for the compounds and their antioxidant activities, studies on the antioxidative and antimicrobial effects of annatto seed extracts on meat and meat products are limited. Therefore, the objectives of the present study were to evaluate the efficacy of different level of annatto seed powder in preventing lipid and protein oxidation, and to determine the physicochemical and antioxidative activities of pork patties during 14 d of refrigerated storage.
Materials and Methods
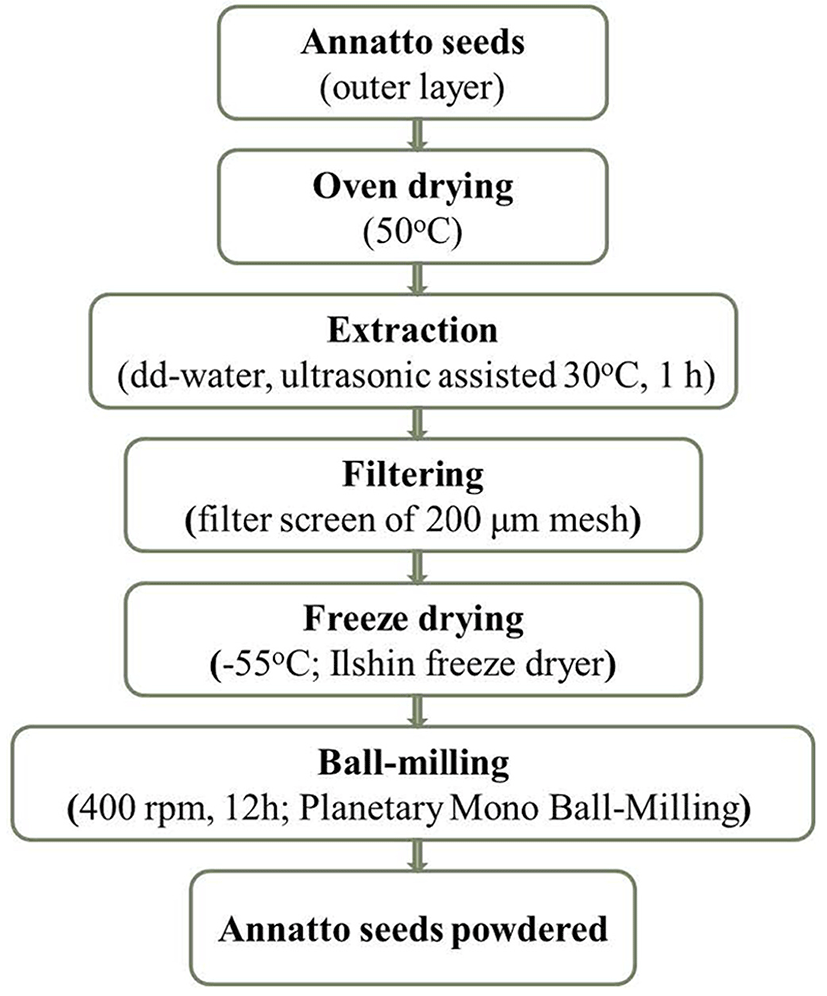
Annatto seeds were purchased from the local market in Central Highland, Vietnam. The fresh materials were oven-dried at 50℃ for about 72 h until the weight stopped changing. Then, they were extracted using double distilled (dd)-water in combination with ultrasonic assistance at a frequency of 40 kHz with 300 W of generation power (Ultrasonic JAC 2010, 330W, Korea) at 30℃ for 1 h. The resulting solution was filtered twice using a 200 μm mesh filter screen (Chung GyeSang Gong Sa, Korea), then was kept in the deep freezer at −70℃ prior to lyophilization at −55℃ (Ilshin freeze dryer, Korea) for about 5 d until it was completely dried. After drying, the product was ground with a ball-milling grinder (Planetary Mono Ball-Milling, Pulverisette, Fritsch, Germany) for 12 h at 400 rpm (Fig. 1). Finally, the annatto seed powder was used for further analysis to determine its phytochemical components and physicochemical characteristics, and was also used for the manufacture of pork patties to evaluate the antioxidative and antimicrobial activity during storage.
The amount of carotenoids in the powder was determined according to the methodology described by Scott (2001) with slight modification. The amount of carotenoids in the powder was determined as bixin (oil soluble) and nor-bixin (water soluble) equivalents. The absorbance was measured in a UV spectrophotometer, and the bixin concentration was calculated based on the absorption coefficient (E1%1cm) of 3090 at 487 nm while the nor-bixin concentration was calculated using the absorption coefficient (E1%1cm) of 2870 at 482 nm (Smith, 2006).
The total phenolic compounds in each extract were measured as gallic acid equivalents using the Folin-Ciocalteau’s phenol reagent (FC reagent) according to the method described by Lin and Tang (2007). The ascorbic content was determined by using the method described by Park et al. (2008). Total flavonoids from the powder were determined and quantified according the aluminum chloride colorimetric method as described by Lin and Tang (2007) with slight modification.
1,1-diphenyl-2-pycrylhydrazyl (DPPH) radical-scavenging activity was determined by the method described by Huang et al. (2006) with slight modification.
DPPH radical − scavenging activity (%) = [(ABSctl − ABSspl) /ABSctl] × 100
Where: ABSctl is the absorbance value of the control group, and ABSspl is the absorbance of the samples.
Ferrous ion-chelating activities of the leaf were evaluated by measuring the inhibition of the formation of a Fe2+ ferrozine complex using the method described by Le et al. (2007) with some slight modification. The absorbance was then measured at λ = 562 nm with a UV − vis spectrophotometer (UV 1601).
The chelating activity was calculated as a percentage via the following equation:
Chelating effect capacity (%) = [(1 − ABSspl/ABSctl] × 100
Where: ABSctl is the absorbance value of the control group, and ABSspl is the absorbance of the samples.
The reducing power was measured via the method described by Huang et al. (2006). The absorbance at λ = 700 nm was measured and high levels of absorbance were regarded as reflective of high reducing power. Ascorbic acid (0-10 mg/mL) was used as the positive reference with the annatto powder.
The ABTS (2,2’-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) activity of the annatto seed powder was measured with the ABTS cation decolorization assay using the modified method described by Re et al. (1999). The absorbance was measured at λ = 734 nm after 20 min of reaction against the phosphate buffer saline blank. The results were expressed as the percentage of decolorization and calculated as mean values ± standard deviation (with n=3). The percentage of inhibition was calculated with the equation:
Inhibition percentage (% IP) = [(ABSctl − ABSspl)/ABSctl] × 100
Where: ABSctl is the absorbance value of the control group, and ABSspl is the absorbance of the samples.
The total antioxidant capacity of the annatto seed powder was evaluated by the method described by Prieto et al. (1999). The total antioxidant activity of the samples was expressed as milligrams of ascorbic acid equivalents per gram of dried matter.
The pH of annatto powder was measured in a suspension resulting from blending one g sample with 10 mL of deionized water for 2 min, using a pH meter (Mettler-Toledo AG, 8603, Switzerland). The Hunter color values were measured at 5 different locations using a color reader (CR-10, Minolta Co. Ltd., Japan) and expressed as lightness (L), redness (a) and yellowness (b).
The water-holding capacity (WHC) and oil holding capacity (OHC) were determined according to Beuchat (1977) and the solubility (%) of the powder was analyzed according to Kha et al. (2010) with slight modifications.
Five types of pork patties were prepared and the formulations are listed in Table 1. They consisted of a control (CTL) group of samples (without addition), a reference group (AA0.1), and three different concentrations (0.1, 0.25 and 0.5%) of annatto seed powder (ANT0.1, ANT 0.25, ANT0.5, respectively). Pork patties (approximately 65 g/patty) were formed using a conventional patty-maker to give average dimensions of 8.5 cm diameter and 1 cm thickness and then were stored for 14 d at 4±1 ℃ in a refrigerator.
| Ingredients (%) | Treatment* |
||||
|---|---|---|---|---|---|
| CTL | AA0.1 | ANT0.1 | ANT0.25 | ANT0.5 | |
| Raw meat | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 |
| Fat | 20 | 20 | 20 | 20 | 20 |
| Salt | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Ascorbic acid | - | 0. 1 | - | - | - |
| Annatto seed | - | - | 0.1 | 0.25 | 0.5 |
*Treatments: CTL, patties without annatto seeds; AA=0.1% of ascorbic acid; ANT0.1, 0.25, 0.5=patties containing 0.1%, 0.25% and 0.5% of annatto seed powder, respectively.
The pH values were measured using a pH-meter (MP-120, Mettler-Toledo, Switzerland), while the color of the patties was assessed using a Hunter color reader (CR-10, Minolta Co. Ltd., Japan). Ten measurements were made at different positions on the surfaces of the patty samples, and the mean values were calculated. Hunter color values were reported as lightness (L), redness (a) and yellowness (b). The total color changes (ΔE) between patties at day 1 and day 14 of storage were calculated with the equation below:
ΔE1–14 = [(L14 − L1)2 + (a14−a1)2 + (b14 − b1)2)]1/2
The secondary products of lipid oxidation during the storage period were determined with the TBARS assay according to Shinnhuber and Yu (1977). The reactive substances were measured at 532 nm using a spectrophotometer (UV-1601, Shimadzu, Australia). TBARS values were calculated with the following equation:
TBARS value (mg malondialdehyde/kg) = optical density (O.D.) × 9.48/sample weight (g)
POV were determined using the spectrophotometric method as described by Shantha and Decker (1994) with some modification. The absorbance of the sample was measured at 500 nm against a blank that contained all the reagents except the samples using a spectrophotometer (UV-1601, Shimadzu, Australia). The results of POV were expressed in milliequivalents of oxygen per kilogram of patty (meq/kg).
Total VBN content was determined according to the micro-diffusion method introduced by Conway (1962) with slight modification. Total VBN values were expressed in mg/100g.
Total plate counts (TPC) and violet red bile (VRB) agar were prepared for the determination of total viable counts and Enterobacteriaceae, respectively (Park and Chin, 2010). Finally, relevant colonies on the petri dish were counted and the results for TPC and VRB were expressed as Log CFU/g.
Data were analyzed and statistical comparisons were performed by using one-way or two-way analysis of variance (ANOVA). The significant differences were assessed with the Duncan post hoc test at p value < 0.05 using the Statistical Package for the Social Sciences (IBM SPSS version 20.0) software for Windows. Results were presented as the mean±standard deviation (n=3).
Results and Discussion
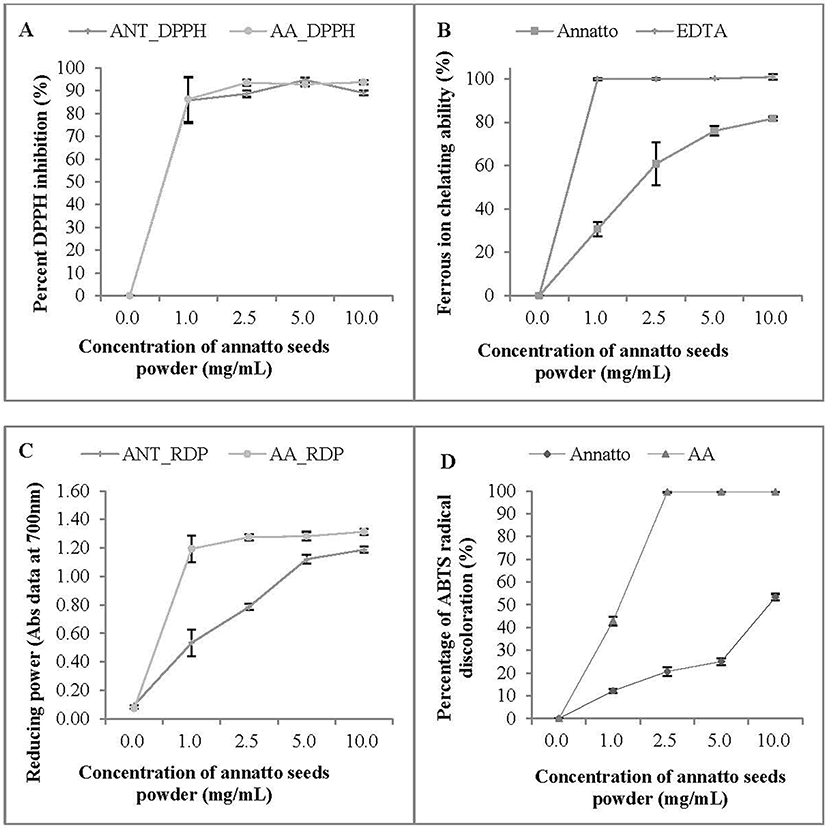
Phytochemical (Bixin, Nor-bixin, TPC, TFC and AA) compounds of annatto (Bixa orellana L.) seed powder are presented in Table 2. The antioxidant abilities are summarized in Figs. 2(A)-(D). The DPPH radical scavenging activities of ANT seed powder at different concentrations (1.0-10 mg/mL) ranged from 85.8 to 94.7%, and the concentration at 5.0 mg/mL showed the highest ability (Fig. 2A). It was observed that the DPPH radical scavenging activities of ANT seed powder were similar to those of AA. Ferrous ion chelating capability increased from 30.7% to 81.65%, with increasing annatto seed concentration from 1.0 to 10.0 mg/mL (Fig. 2B). Compared to EDTA, ANT seed powder had lower ferrous ion chelation. Reducing power (Abs at 700 nm) ranged from 0.53 to 1.19, showing an increase with increasing concentration. The reducing power of ANT seeds was lower compared to AA (1.0-10 mg/mL) (Fig. 2C). The ABTS ability also had a similar trend to the other in vitro antioxidant assays, and lower concentrations tended to have lower ABTS ability. The ABTS ability increased linearly with increasing concentration and the highest value was recorded at 10 mg/mL (Fig. 2D). Based upon these results, annatto seed powder was confirmed to show antioxidant activity. Many studies have stated that the presence of phenolic compounds in food, especially in fruit, could be important for consumers to prevent some diseases and cancers because of their beneficial health properties, which may be associated with their antioxidant capacities. Thus, annatto seed powder could be used as a natural antioxidant in various foods, especially meat products.
ATotal phenolic compounds was expressed as mg gallic acid equivalent /g d.m. of seed powder.
BTotal flavonoid contents are expressed as mg quercetin equivalent /g d.m. of seed powder.
CAscorbic acid content as mg /g d.m. of seed powder.
DTotal antioxidant capacity is expressed as mg ascorbic acid equivalent /g d.m. of seed powder.
The physicochemical properties of annatto seed powder are presented in Table 2. The mean pH value of annatto seed powder was 5.73. Viuda-Martos et al. (2012, 2015) reported that pomegranate juice arils bagasse had a pH of 4.4, pomegranate juice whole fruit bagasse had a pH of 4.5 and the pH of fig (Ficus carica L.) powder ranged from 5.10 to 5.49. In Hunter color values, the lightness value for annatto powder was 40.97, which is relatively dark. The lightness value was compared to those of other powders such as pomegranate, ranging from 62.2 to 62.8, and fig (Ficus carica L.) powder, ranging from 49.72 to 68.62 (Viuda-Martos et al., 2012; Viuda-Martos et al., 2015). The redness (a) value for annatto seed powder is 15.39, while the yellowness (b) value is 5.73.
The WHC is an important characteristic in the interaction between water and proteins that occurs in various food systems (Traynham et al., 2007). The WHC and OHC of annatto seed powder is 39.72% and 50.68%, respectively (Table 2). Moreover, WHC is an important processing parameter, which can enhance the cooking yield, especially in meat. The OHC is a techno-functional property that is influenced by the chemical structure of polysaccharides, which are present in plant resources and are highly related to their chemical and physical structures (Viuda-Martos et al., 2015). Based on the results for OHC and WHC, annatto seed powder may have stronger affinity for oil than water. Therefore, annatto seed powder, which has relatively higher OHC than WHC, may be favorable in food items. In addition, the solubility of annatto seed powder was 45.77%. Since solubility is directly associated with the dispersion and appearance of final products, the high solubility of annatto seed powder might be more favorable in emulsifying meat systems, such as sausages. Based on these results, annatto seed powder might be suitable for the improvement of cooking yield and retarding lipid oxidation in meat products during storage.
Changes in the pH values of pork patties during storage are shown in Table 3. The pH ranged from 5.61 to 5.86. No significant differences between ANT0.1 and ANT0.25 were observed, but ANT0.5 and AA0.1 were higher than the CTL. The present study showed that the pH values increased during refrigerated storage (p<0.05). Usually, the pH values of meat or muscle foods show significant increases with increasing storage length and this observation has been reported by several authors (Kim and Chin, 2015; Park and Chin, 2010). Moreover, changes in the pH values of meat during storage have an important effect on the quality, especially in sensorial characteristics such as odor, color, and texture (Li et al., 2014). Several factors could contribute to the changes of pH during storage in muscle foods such as the generation of lactic acid, the production of volatile basic components and alkaline substances by the decomposition of meat by microbial organisms and endogenous enzymes such as ammonia groups and amine groups (Cai et al., 2015; Li et al., 2014). In addition, Miao et al. (2015) reported that the increase of pH values in fresh pork during refrigerated storage was mainly caused by bacterial decomposition of meat components.
Means with different superscript letters (a-f) in the same column indicate significant differences at p<0.05. NS=not significant
*indicated significant at p<0.05, **: indicated significant at p<0.001.
1)Parameters: L=Lightness, a=Redness, b=Yellowness, TBARS=thiobarbituric acid reactive substances, POV=Peroxides values, VBN=Total volatile basic nitrogen. TPC: Total plate count, VRB: Violet red bile. 2) Treatment: CTL=patties without addition, AA0.1=0.1% Ascorbic acid; ANT0.1, 0.25, 0.5 = 0.1%, 0.25% and 0.5% of annatto seed powder, respectively.
The Hunter color values of pork patties during storage are presented in Tables 3-5. The lightness (L) increased and the redness (a) decreased (p<0.05), while no changes in yellowness were observed during refrigerated storage (p>0.05). As shown in Table 3, the lightness decreased while redness increased with the addition of annatto seed powder, as compared to the control (CTL) or reference (AA0.1). This clearly indicated that the Hunter color values of pork patties were affected by the addition of annatto seed powder, which has a strong color and changed the color to a slightly dark red as a result of the bixin and nor-bixin pigments. Therefore, a high increase of redness and a decrease of lightness were observed with increased levels of annatto seed powder, which might not be acceptable for the consumer. The present results are consistent with other findings of a positive correlation between plant pigments and the surface colors of meat products (Rodriguez-Corpena et al., 2011). Since the strong color pigments from plant extracts were transferred to the pork patties during processing, the final color of the meat products were changed. Although the redness decreased in all treatments throughout storage, the rate of decrease was quite different from those without treatment (p<0.05, Table 4). Since the interaction between treatment and storage time had a significant effect on the redness (Table 3, p<0.05), data were separated by treatment according to storage time or storage time according to the treatment. As shown in Table 4, the rapid decreases in the redness of the CTL and ANT0.1 samples might be partially due to rapid lipid oxidation, in which oxymyoglobin was changed to metmyoglobin in the control and at lower levels of annatto seed powder. Moreover, the results showed that the decrease in redness within the first 3 d was slow, but rapidly increased thereafter. When the changes in displayed color between day 1 and day 14 (ΔE1–14) are compared among treatments, CTL patties or those with 0.1% annatto seeds (ANT0.1) had more intense color deterioration than patties with higher levels (0.25 and 0.5%) of annatto seeds, or ascorbic acid (Table 5). This means that patties without annatto seeds and those with lower levels of annatto seeds (< 0.1%) had more rapid changes and less color stability during storage, whereas treatments with higher levels (> 0.25%) of annatto or ascorbic acid showed more resistance against discoloration than the control as shown in Table 6. The protective effect of annatto seed powder in preventing discoloration could be partially due to the polyphenol compounds or antioxidant agents in annatto seed powder. Several previous studies reported color changes in numerous raw meats as affected by various fruits during refrigerated storage. Rodriguez-Corpena et al. (2011) reported that avocado extracts preserved the color of raw pork during cold storage (4℃/15 d). Keokamnerd et al. (2008) concluded that rosemary extracts had an overall positive effect in maintaining the color stability and sensorial quality of chicken meat during storage. Ganhão et al. (2010) reported several types of natural resources extracts including strawberry, common hawthorn, dog rose, and blackberry that showed high capability to maintain the color of meat during storage, as compared to the control. On the other hand, several authors have reviewed the disadvantages of fruits or vegetables on the displayed color of meats, which can be unacceptable to consumers. Since the addition of annatto seeds led to marked changes in the displayed color of the pork patties as compared with the control, the final product might not be accepted by consumers. Thus, the differences in color values between the annatto treated patties and control should be minimized with the addition of other ingredients that can control the color.
Means with different lowercase superscript letters (a-f) in the same row indicate significant differences at p<0.05. Means with different uppercase superscript letters (A-E) in the same column indicate significant differences p<0.05.
1)Parameters: a=Redness, TBARS=thiobarbituric acid reactive substances, POV=Peroxides value, VBN=Total volatile basic nitrogen.
2)Treatments: see Table 1.
| Treatment* | Mean ΔE1–14 ± Standard deviation |
|---|---|
| CTL | 7.32 ± 0.25a |
| AA0.1 | 3.44 ± 0.79b |
| ANT0.1 | 8.15 ± 0.68a |
| ANT0.25 | 4.31 ± 0.83b |
| ANT0.5 | 4.76 ± 1.48b |
Means with different superscript letters (a,b) in the same column indicate significant differences at p<0.05.
*Treatment: see on the Table 1.
Means with different superscript letters (a-c) in the same column indicate significant differences at p<0.05.
*Treatment: see in Table 1.
Since there was an interaction between treatments and storage time with regard to TBARS values (p<0.001), the data were organized by treatment during storage or storage time according to the treatment (Table 4). The addition of annatto seed powder or ascorbic acid reduced the TBARS values. In fact, the TBARS values of CTL patties were highest at the beginning of storage, followed by patties containing 0.1% annatto. No differences in TBARS among patties treated with different amounts of annatto seed powder or with ascorbic acid 0.1% were observed during refrigerated storage. These results indicated that the TBARS values were affected by annatto seed powder during refrigerated storage, which might be partially due to the high level of total phenolic compounds in annatto seed powder, and could contribute to the retardation of lipid peroxidation. Numerous studies have reported that phenolic compounds from diverse plants play a major role in antioxidant activity (Kim et al., 2013; Maqsood et al., 2015). The donation of electrons and protons from phenolic and other bioactive compounds could prevent the initiation and propagation of lipid oxidation. Moreover, the inhibition of free radical reactions could be caused by the chelation of transition metal ions in addition to preventing oxygen attacks on radical chains (Jia et al., 2012; Shan et al., 2009). The spoilage value for chemical deterioration of meat and meat products is considered 1.0 mg MDA/kg meat and the TBARS values of the control patties indicated they began to spoil on storage day 3. However, the ANT0.1 samples reached the spoilage limit for TBARS value after 7 d of storage, while other treatments with higher amounts of ANT seeds did not even reach 1.0 during the entire storage period. These results were in agreement with the findings of Jia et al. (2012), who reported that the TBARS value in CTL pork patties was much higher than those of other samples containing black currant extract and exceeded 2.0 mg MDA/kg at storage day 6. The ANT0.25 and ANT 0.5 pork patties maintained low TBARS values and showed similar results as that of ascorbic acid (AA 0.1%) during storage up to 14 d. Lipids in meat and meat products are easily oxidized and form hydroperoxides, which decompose to secondary lipid oxidation products such as aldehydes and ketones, resulting in the production of rancid off-flavor, which might not be acceptable to consumers. Many types of fruit extracts from wild resources such as dog rose, blackberry, and hawthorn have shown very strong antioxidant activity, which can prevent the oxidation of lipids in raw pork patties during cold storage (Ganhão et al., 2010). Furthermore, Shan et al. (2009) reported that several natural extracts including cinnamon stick, oregano, clove or their by-products such as pomegranate peel and grape seeds added to raw sliced pork meat could act as inhibitors of lipid oxidation even up to 9 d of storage at room temperature (20℃). Therefore, the addition of ANT seed powder (from 0.25 to 0.5%) might be a good way to prevent lipid oxidation in meat products as shown in the lower TBARS slope of the ANT treatment rather than the control (Table 6).
POV is widely used as a chemical test for primary lipid oxidation in muscle food during storage. The POV of pork patties containing ANT seed powder are shown in Table 4. CTL samples were the highest (p<0.05), followed by pork patties with 0.1% ANT. No significant differences between AA0.1% and ANT0.25% were observed (p>0.05), whereas the pork patties containing ANT0.5% showed the lowest POV (p<0.05). Among the samples treated with annatto seed powder, pork patties with higher amounts of annatto had lower POV during storage. Furthermore, pork patties with annatto seeds combined with other products were more effective in lowering POV than those with annatto seeds alone. As shown in Table 4, POV tended to plateau at day 7 in all treatments (p>0.05), increasing rapidly thereafter up to day 14. The increases of POV after day 7 of storage could be partially due to the end point for the induction of lipid oxidation in pork patties. Peroxide value could not be qualified for the valuation of lipid oxidation due to the low stability of peroxides and the easy breakdown to non-peroxide compounds. However, a linear relationship has been observed between peroxide values and sensorial quality, especially in flavor scores during the initial stages of lipid oxidation (O’Brien, 2008). Therefore, high peroxide values usually mean poor flavor ratings in meat products, due to the presence of oxidized metabolites of fats.
VBN is one of the most important indicators of the freshness of meat, dairy or seafood products (Cai et al., 2015; Miao et al., 2015). It might be associated with the activity of several spoilage microorganisms and endogenous enzymes (Cai et al., 2015). The VBN values in all the samples showed a slight or no increase during initial storage (Table 4), but marked increase was observed after 7 d of storage. This might be due to the activity of spoilage bacterial and endogenous enzymes system in meat products. In the first 7 d of storage, the activity of microorganisms was still low, leading to a slight increase in the VBN value from 6.9 to 7.3 mg/100 g (p>0.05, Table 3). However, the VBN of all patties gradually increased (p<0.05) after 7 d and reached 9.5 on day 10 and 13.1 mg/100 g at by the end of storage (Table 3). At the end of storage, the VBN values of CTL samples AA0.1 and ANT0.5% did not show any difference and AA0.1 had higher VBN than samples treated with ANT01 and ANT0.25 (Table 4). Based on these results, it appears that treatment with very high amounts of ANT (0.5%) might causes protein degradation. Moreover, Miao et al. (2015) reported that the limit according to the national standard of China for hygienic standards for fresh meat from livestock was less than 15 mg/100 g of VBN in meat. Other countries have been considered VBN values less than 10 mg/100 g of meat. In this study, the control exceeded 10 mg/100 g of VBN after about 8 d of storage, however the other treatments took approximately 10 d or more to reach the same value, depending on the amount of annatto seed. Miao et al. (2015) showed that the VBN value of fresh pork had lower rates of increase and did not exceed the safety standard within the first 8 d of storage as compared to the control. Thus, pork patties containing ANT within the range of 0.1 to 0.25% could have reduced the VBN, resulting in the extension of shelf-life.
The results of microbial count are shown in Table 3. Although no differences in total microbial counts were observed among the treated patties, the addition of ANT seed powder to pork patties reduced the microbial count of Enterobacteriaceae, as compared to the control or reference (AA, 0.1%) (p<0.05) when the amount of ANT was higher than 0.25%. There have been several reports on the antimicrobial activity of annatto seeds in in vitro cultures. The present results might be supported by Venugopalan and Giridhar (2012), who stated that the ethanolic extracts of annatto seeds are capable against E. coli and B. cereus. Moreover, Yolmeh et al. (2014b) reported that the annatto dye was able to retard the microbial growth of E. coli and exhibited bactericidal effects on mayonnaise. The pooled mean of microbial counts (TPC and VRB) in all of the patties increased with increasing storage time (p<0.05). At 3 d of storage, the microbial counts for Enterobacteriaceae did not increase (p>0.05); however, they started to increase after 7 d of storage in all patties, indicating that the samples underwent spoilage. Therefore, ANT seed powder could have not only antioxidant activity, but also the potential for antimicrobial activity in pork patties.
Conclusions
Annatto seed powder had antioxidant abilities as well as water and fat holding capacity which might be suitable for incorporation with meat products. The redness values of pork patties containing annatto seed powder increased due to the presence of bixin and nor-bixin pigments (p<0.05). Moreover, pork meat patties containing annatto seed powder were found to have reduced lipid deterioration, which resulted in lower TBARS and POV than the control sample. The VBN values of the pork patties containing annatto seeds were lower than those of CTL at the end of storage (p<0.05). The addition of annatto seeds to meat products at a level of 0.25% appeared to be optimal for the manufacture of pork patties. Therefore, annatto seed powder could be a promising source for natural antioxidants and antimicrobial agents that could be used in the production of meat products to extend the shelf-life.