Introduction
One of most important components in meat products is fat, which improves product flavor and texture when added at the appropriate amount (Kumar et al., 2016). However, oxidation of lipid is one of the main factors deteriorating the quality and shelf-life of meat products (Dave and Ghaly, 2011). Synthetic antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have been used to inhibit the oxidation of various meat products (Chang et al., 1997). However, carcinogenicity and safety issues have been suggested for synthetic antioxidants (Ito et al., 1986); thus, several studies have examined replacing synthetic antioxidants with natural additives such as rosemary extract (Sebranek et al., 2005), golden thread (Coptis chinensis Franch), clove extract (Zahid et al., 2018), and sapota powder (Kumar et al., 2018).
Allium sativum, commonly known as garlic, exhibits antioxidant, antimicrobial, and anticancer effects and reduces cardiovascular diseases and blood cholesterol (Harris et al., 2001). However, the spicy and irritating flavor of garlic limits its use as a common food additive; this flavor is caused by allicin and its derivatives (Jang et al., 2018). Alliin, a major component of garlic, is converted to allicin by allinase released from vacuoles when the cells are damaged. As allicin is unstable, it is degraded to lipophilic organic sulfur compounds, such as diallyl sulfide, diallyl disulfide, diallyl trisulfide, and thiosulfinate, which cause a distinct aroma (Rose et al., 2005).
Several studies have examined reducing the irritant flavor of garlic by processing. Aged garlic (also known as black garlic or red garlic) is processed by aging fresh garlic for several weeks at temperatures of 40°C to 90°C (Jang et al., 2008). Aged garlic has a less irritating flavor and increased functionality as a result of browning reactions (You et al., 2011). Processed garlic has greater antioxidant ability than raw garlic (RG) owing to the higher total phenolic and flavonoid content during processing (Imai et al., 1994).
Garlic can also be processed by fermentation. For example, Bacillus subtilis, a Gram-positive, catalase-positive bacterium common in soil environment (also known as grass bacillus or hay bacillus), can be used for the production of fermented soybean foods such as cheonggukjang (Korea), natto (Japan), thua nao (Thailand), kinema (India) and douchi (China) (Steinkraus, 1997). It is known that soybean and sword bean fermented with B. subtilis have increased antioxidant ability due to increased phenolic contents and flavonoids (Han et al., 2015; Juan and Chou, 2010). Zhang et al. (2014) identified antioxidant peptides from peanut meal fermented with B. subtilis. Moreover, recent studies have demonstrated that extracts from garlic fermented with B. subtilis alleviate cardiovascular diseases and blood cholesterol (Park et al., 2016; Park et al., 2017).
Processed garlic studies have mainly focused on the antioxidative (You et al., 2011) and physicochemical properties (Choi et al., 2008) of aged garlic or steamed garlic, as well as products supplemented with processed garlic such as bread and sausage (Shin et al., 2011; Wang et al., 2012). However, little is known regarding garlic processed by fermentation. Thus, our aim was to compare the antioxidant ability of garlic fermented with B. subtilis and aged black garlic (AG) prepared by conventional heat treatment and their potential use as natural antioxidants when added to pork patties by analyzing lipid oxidation during vacuum packaged, refrigerated storage.
Materials and Methods
Extracts of RG, aged garlic, and garlic fermented by B. subtilis, as described by Yun (2017), were purchased from OZL DNF Co., Ltd. (Dam Yang, Korea). The pork loin and pork back fat used to produce the pork patties were purchased from GUMDON Corp. (Won Ju, Korea).
RG, AG, and FG extracts were filtered using Whatman filter paper (No. 1), freeze-dried, and stored at –80°C in a deep freezer. Samples were diluted with distilled water to concentrations of 0.2%, 0.6%, and 1.0% (w/v).
The Folin-Ciocalteu method was used to evaluate the total phenolic content of the three garlic extracts as described by Simoes et al. (2018). One milliliter of sample was mixed with 5 mL of 0.2 N Folin-Ciocalteu reagent for 10 min at 25°C. The mixture was supplemented with 4 mL of 7.5% (w/v) sodium carbonate and incubated at 25°C for 2 h. The absorbance was read at 765 nm using a spectrophotometer (Optizen 2120UV; Mecasys Co., Ltd., Daejeon, Korea). Distilled water was used as the blank control and flavonoid content was calculated with a standard curve by gallic acid.
The total flavonoid content was measured using Dowd’s method, as described by Adefegha et al. (2018). One milliliter of sample was mixed with the same amount of 2% (w/v) aluminum chloride then incubated for 10 min at 25°C. The absorbance of the mixture was read at 415 nm using an Optizen 2120UV spectrophotometer. Distilled water was used as the blank control and the contents were calculated based on a standard curve for quercetin.
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was measured using the methods of Blois (1958). One milliliter of sample was mixed with 5 mL of 100 μM DPPH/95% (v/v) ethanol and incubated for 30 min at 25°C in a dark room. The absorbance was read at 517 nm using an Optizen 2120UV spectrophotometer and 1 mL of distilled water was mixed with 5 mL of DPPH solution for blank controls. DPPH radical scavenging activity was determined as:
2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity was measured using the methods of Re et al. (1999). An ABTS stock solution containing 7 mM ABTS and 2.45 mM potassium persulfate was prepared and incubated in a dark room for 12 h to form the ABTS·+ radical. The ABTS stock solution was diluted with sodium phosphate buffer (pH 7.4) until the absorbance of the solution reaches 0.70±0.02 at 732 nm. Next, 2.9 mL of diluted ABTS solution were mixed with 0.1 mL of sample, incubated in a dark room for 10 min at 25°C, and the absorbance was read at 732 nm using an Optizen 2120UV spectrophotometer. Scavenging activity was calculated based on a standard curve for ascorbic acid.
The basic composition of pork patty batter mix was consisted of 75% lean pork, 24% pork back fat, and 1% salt; they were ground through a 3 mm plate, divided into five groups, and each group was pre-mixed prior to additional treatment. The five groups included the CON (control), patties with no additional treatment; the AA group, patties with 0.1% (w/w) ascorbic acid; the RG group, patties with 0.5% (w/w) freeze-dried garlic extract; the FG group, patties with 0.5% (w/w) freeze-dried FG extract; and the AG group, patties with 0.5% (w/w) freeze-dried aged garlic extract. All groups were mixed using a bowl mixer (SM246, Poking Industrial Co., Ltd., Hong Kong, China) for 7 min, the paste was weighed, and 80±2 g patties were formed using a rectangular burger press (Spikomat Ltd., Nottingham, UK), in order to ensure the size of the patties was exactly the same. Once the patties were produced, a total of three batches of patties from each of the five groups were vacuum packaged and stored at 4°C for 21 d. Samples were taken at 0, 3, 7, 14, and 21 d of storage.
The pH value of the pork patties was measured by homogenizing 2 g samples in 18 mL of distilled water by a homogenizer (AM-1, Nihon Seiki Kaisha Co., Ltd., Nagoya, Japan) for 1 min at 3,220×g. The pH of homogenate was then measured using a pH meter (LAQUA F-71, Horiba Co., Kyoto, Japan).
The 2-thiobarbituric acid reactive substances (TBARS) value was measured using the method of Buege and Aust (1978). Five grams of sample were mixed with 15 mL of distilled water plus 100 μL of 6% (w/v) BHT in ethanol solution and homogenized at 3,780×g for 1 min with an AM-1 homogenizer. Then, 2 mL of homogenate were mixed with 4 mL of TCA/TBA reagent (15% (w/v) trichloroacetic acid with 20 mM thiobarbituric acid solution) and heated in an 80°C water bath for 15 min. The samples were cooled in ice cold water, then vortexed and centrifuged for 10 min at 2,000×g, 25°C. After centrifugation, the supernatant of samples were filtered with Whatman filter paper No. 1, The absorbance of the filtrate was then measured at 531 nm using an Optizen 2120UV spectrophotometer. The malondialdehyde (MDA) concentration of the samples was determined using a standard curve for 1,1,3,3-tetraethoxypropane (TEP).
The volatile basic nitrogen (VBN) content of the samples was analyzed using Conway’s microdiffusion method. Five grams of sample were mixed with 15 mL of distilled water and homogenized at 2,240×g for 1 min; the volume was then adjusted to 50 mL with distilled water in a mass cylinder. The homogenate was filtered with Whatman filter paper No. 1 and 1 mL of the filtrate was added to the outer chamber of the Conway unit. Next, 1 mL of 0.01 N boric acid and 100 μL of Conway reagent (0.066% (w/v) methyl red and 0.066% (w/v) bromocresol in ethanol) were placed in the inner chamber of the Conway unit; 1 mL of 50% (w/v) potassium carbonate was added to the other side of the outer chamber of the Conway unit. The unit was then sealed, slowly agitated in a horizontal direction to mix the reagents in the outer chamber, and incubated at 37°C for 120 min. Following incubation, the inner chamber of the Conway unit was titrated with 0.02 N sulfuric acid. The VBN concentration was determined as follows:
Results and Discussion
As demonstrated in Fig. 1A, the total phenolic content of RG, AG, and FG significantly increased with increasing amounts (2, 6, and 10 mg/mL) of sample (p<0.05). Additionally, the total phenolic content of AG and FG was significantly higher than that of RG (p<0.05). The total phenolic content decreased in the following order: AG>FG>RG (p<0.05).
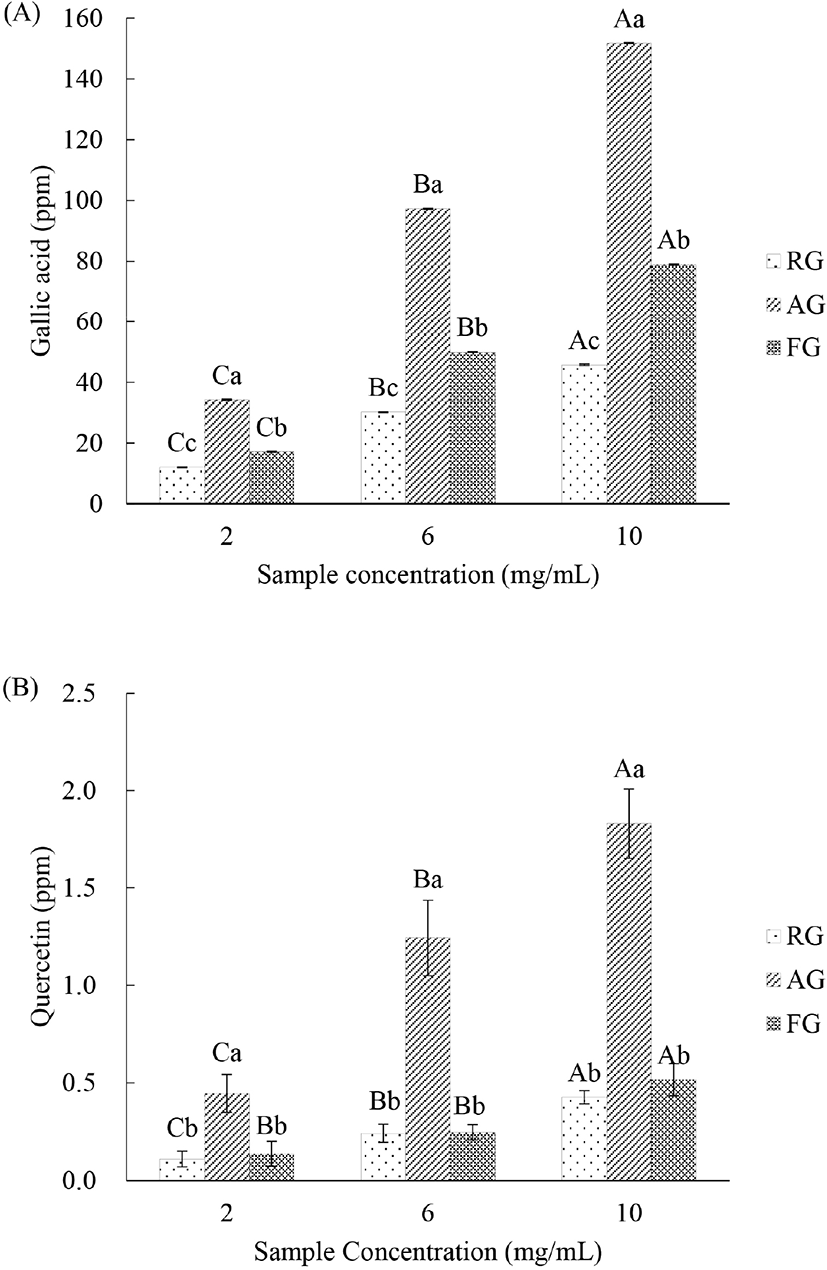
As demonstrated in Fig. 1B, the total flavonoid content of RG, AG, and FG significantly increased with increasing amounts (2, 6, and 10 mg/mL) of sample (p<0.05). Additionally, the total flavonoid content of AG was significantly higher than that of FG and RG (p<0.05).
Phenolic compounds and flavonoids, such as myricetin, caffeic acid, quercetin, chlorogenic acid, and ferulic acid are representative antioxidants in garlic (Beato et al., 2011) and have strong antioxidant ability owing to their hydroxyl groups (Harborne, 1986). Choi et al. (2008) found that the total phenolic and flavonoid content was increased in aged garlic because of the conversion of compounds to phenolic compounds during high-temperature processing. Fermented black soybean prepared with B. subtilis exhibits elevated phenol and flavonoid content (Juan and Chou, 2010). On the other hand, Yang et al. (2012) have reported that Allium cepa (onion) fermented with B. subtilis did not show a significant increase in flavonoids. Similar results were reported by Lee et al. (2016); garlic fermented with various Lactobacillus spp. exhibited only a slight increase in the total phenolic content and flavonoids.
The DPPH and ABTS radical scavenging activity of RG, AG, and FG significantly increased with increasing amounts (2, 6, and 10 mg/mL) of sample (p<0.05). The DPPH and ABTS radical activities were significantly higher in AG and FG than in RG (p<0.05; Fig. 2).
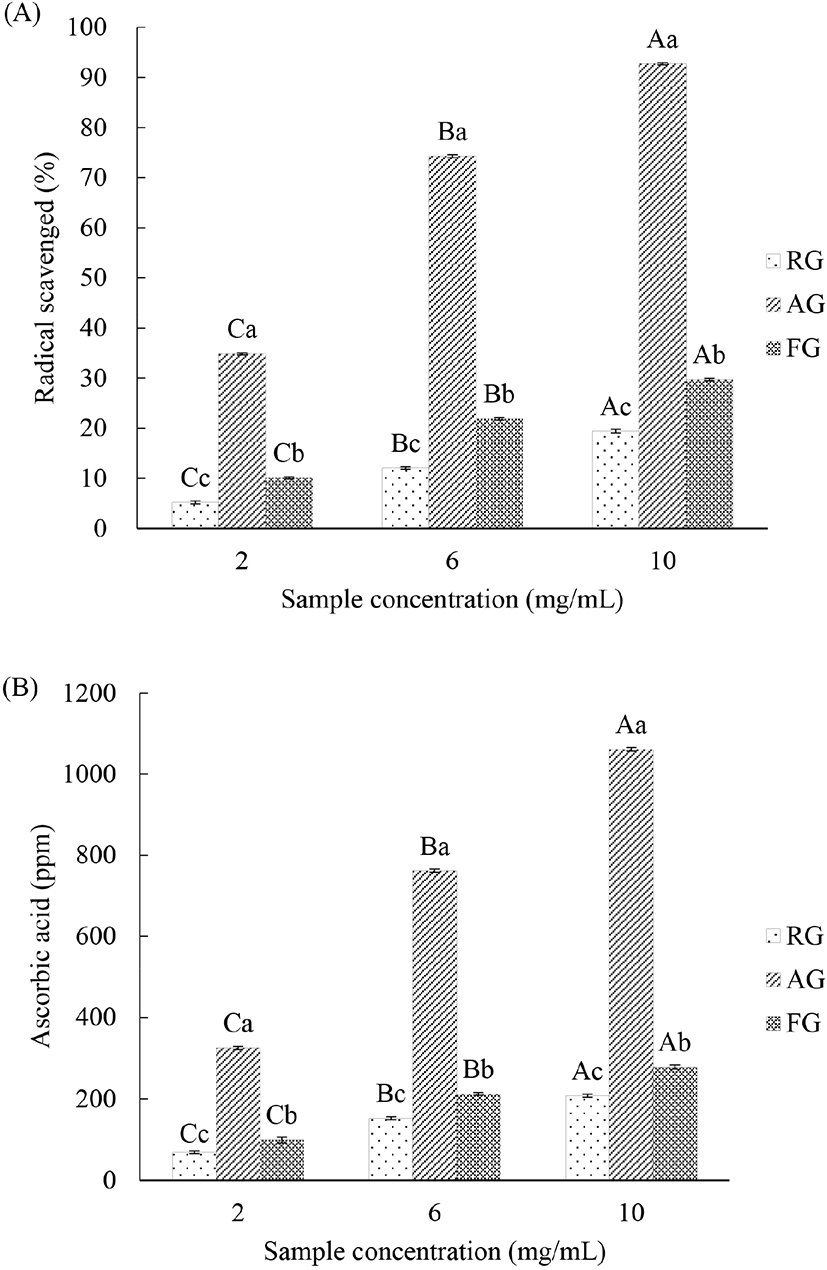
DPPH and ABTS radical scavenging activities are commonly calculated by measuring the reduction of free radicals by electrons transferred from antioxidants (Blois, 1958). Phenolic compounds and flavonoids are known to have antioxidant abilities such as regenerating α-tocopherol, scavenging free radicals, and chelating metal ions (Rice-Evans et al., 1996). This is due to aromatic features, conjugated structures with numerous different hydroxyl groups, which make phenolic compounds effective electron or hydrogen atom donors, scavenging free radicals and reactive oxygen species (Zhang and Tsao, 2016). The higher antioxidant power of AG and FG compared to RG might be related to the higher levels of total flavonoids and phenols (Fig. 1).
The change in pork patty pH values during storage are presented in Fig. 3. The initial pH value was 5.62–5.75 and AG and FG showed a significantly lower pH compared to CON at 0 d (p<0.05). The pH values in all groups significantly decreased with time until 7 d (p<0.05). After 7 d, the pH value of AG and FG continued to significantly decrease until 21 d (p<0.05).
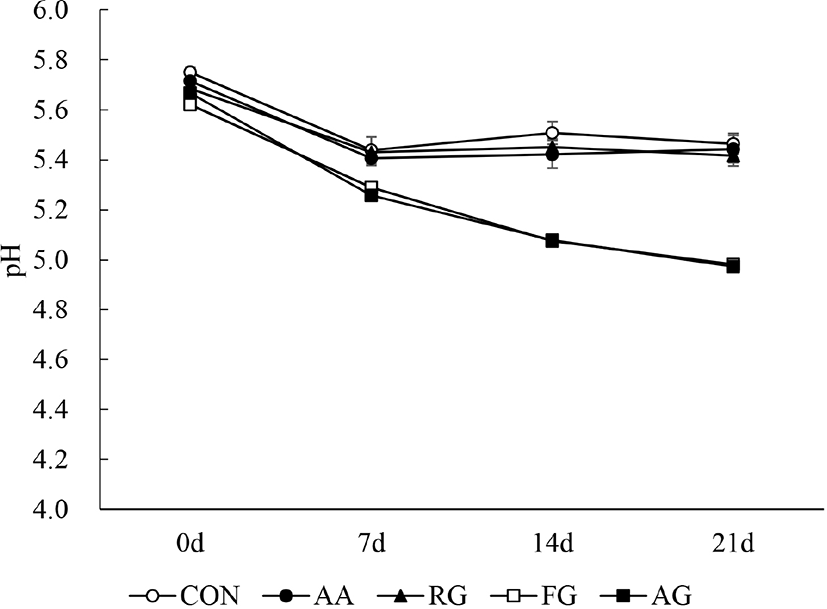
Decreased pH during the storage period has been associated with the activity of lactic acid bacteria (Shin et al., 2017). Garlic is known to contain lactic acid bacteria including Leuconostoc spp., Weissella spp., and Lactobacillus spp. (Jung et al., 2012). These lactic acid bacteria may have also remained in the aged garlic, decreasing pH of AG. In addition, the decrease in the FG pH value might be due to B. subtilis fermentation, which uses sugar as a growth substrate and produces acid via pyruvate (Sini et al., 2007). The increase in pH might be due to protein decomposition and amine production by microorganisms in the meat (Biswas et al., 2004). It is assumed that the high antioxidant ability of AG and FG (Fig. 2) effectively inhibited microorganism growth, thus preventing the increase in pH value.
Fig. 4 demonstrates the changes in the pork patty TBARS values during refrigerated storage. The TBARS values of all groups significantly increased with time (p<0.05). AG and FG showed significantly lower TBARS values compared to CON, AA, and RG (p<0.05). Additionally, the final TBARS values of the patties at 21 d were 0.25–0.77 and AG showed the significantly lowest TBARS value compared to the other groups. The TBARS values increased in the following order: AG<FG<AA<RG<CON (p<0.05).
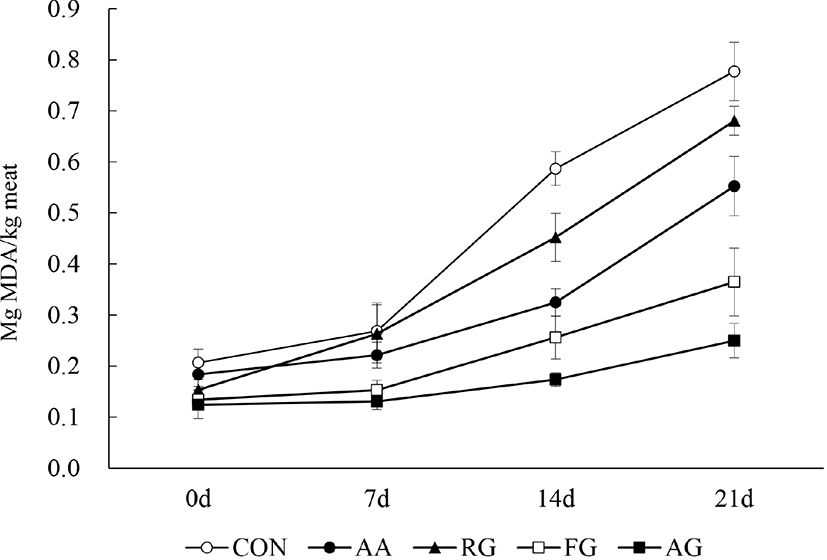
The TBARS assay quantifies the MDA content of meat, a secondary product of lipid oxidation shown to deteriorate meat quality (Choe et al., 2017). It has been reported that a rancid flavor appears in meat products when the TBARS value is more than 0.5 mg MDA/kg meat and that the meat is non-edible if the value reaches 1 mg MDA/kg meat (Park et al., 1988). Ibrahim et al. (2010) reported that various phenolic compounds contained in food additives are strongly correlated with inhibition of lipid oxidation, via free radical neutralization and metal ion chelation. This suggests that the phenolic compounds of AG and FG (Fig. 1A) contributed to the decrease in pork patty TBARS values.
The changes in pork patty VBN values during storage are shown in Fig. 5. The initial VBN value at 0 d did not show significant difference between the groups (p>0.05). The VBN value of all groups significantly increased with time (p<0.05). At 21 d, the VBN values of AG and FG were significantly lower than those of the CON and RG groups (p<0.05).
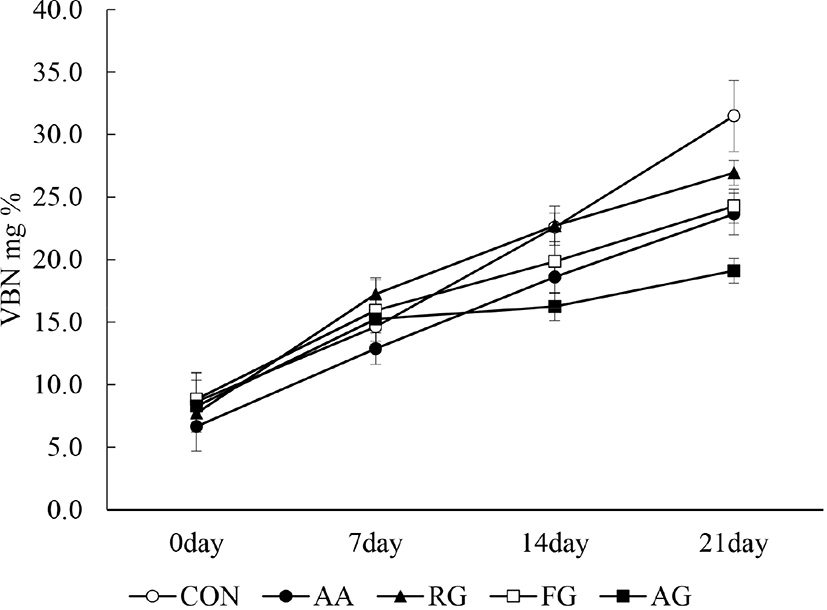
VBN is related to spoilage microorganisms and enzymes in meat products and is an indicator of meat quality (Cai et al., 2015). The main cause of increased VBN values is amino acid decomposition by proteolytic Gram-negative bacteria during storage (Lefebvre et al., 1994). Allium species, such as garlic, are known to have an antimicrobial effect (Harris et al., 2001) and phenolic compounds are known to have antimicrobial abilities owing to hydroxyl groups, which reacts with sulfhydryl groups or other protein elements of bacteria (Cowan, 1999). This suggests that the AG and FG groups had a greater antioxidative and antimicrobial capacity because of their phenolic compounds, compared to the CON and RG groups.
Conclusion
The effect of the antioxidant activity of RG, FG, and AG on pork patty lipid oxidation during refrigerated storage was investigated. The DPPH and ABTS radical scavenging ability of AG and FG was higher than that of RG. This is due to higher total phenolic content found in AG and FG, which is a representative antioxidant compound in garlic. Furthermore, the pH, TBARS, and VBN values of pork patties containing 0.5% (w/w) of AG or FG were lower than those of the CON and RG groups. As these assays are related to lipid oxidation and the growth of spoilage microorganisms in meat products, we hypothesize that the high antioxidant and antimicrobial power of AG and FG prevented pork patty spoilage, increasing shelf-life. Taken together, these results suggest that AG and FG extracts can be used as natural antioxidative additives for inhibiting pork patty lipid oxidation. As AG has already been used as an additives in food products, further studies on FG are needed to assess the effect of FG extracts on texture, color, and sensory evaluation including customer preference of the products, in order to determine the practicality of using FG as an antioxidant in meat products.













