Introduction
Long-term exposure to stress exerts detrimental effects on an individual's overall well-being, serving as a predisposing factor for severe conditions, including anxiety and depressive disorders (Ataallahi et al., 2022). Chronic stress is characterized by symptoms such as impaired focus, cognitive decline, sleep disturbances, weight loss, profound exhaustion, and depression (Jianguo et al., 2019). From the perspective of the gut-brain axis, which has been actively studied in recent years, emerging evidence highlights a deep association between gut microbiota and depressive symptoms (Karl et al., 2018). The gut microbiota exerts substantial influence not only on nutrient metabolism but also on the modulation of the immune system, integrity of the intestinal barrier, and homeostasis of the central nervous system (Cryan and Dinan, 2012; Lee et al., 2022; Patra and Kar, 2021). Interestingly, recent studies underscore the association between dysbiosis, characterized by perturbed intestinal microbiota balance, and the onset of several metabolic diseases and psychological disorders (Cai et al., 2022; Jeong et al., 2021; Li et al., 2019). Hence, ongoing studies are actively investigating the potential of modulating the gut microbiome to improve anxiety and depressive disorders induced by chronic stress.
A growing body of research continues to report on specific probiotics, called psychobiotics, that have beneficial effects on the gut-brain axis and are suggested to alleviate psychological disorders by improving inflammatory responses, alleviating gut mucosal defects, and modulating neurotransmitters (Chen et al., 2021; Li et al., 2018b; Zareie et al., 2006). According to Li et al. (2018b), Lactobacillus helveticus, Lactiplantibacillus plantarum, and Bifidobacterium longum alleviated depressive behaviors and anxiety by improving interferon-γ, tumor necrosis factor-α, and indoleamine 2,3-dioxygenase-1 levels in the hippocampus of chronic mildly stressed mice (Li et al., 2018b). Additionally, it has been reported that treatment with Lacticaseibacillus rhamnosus and L. helveticus could improve chronic stress-induced gut barrier dysfunction and protect against bacterial translocation to the mesenteric lymph nodes (Zareie et al., 2006). However, there is still insufficient evidence regarding how chronic stress and psychobiotics affect the metabolism of the host and gut microbiota.
Fecal metabolomics is an analysis method that detects small molecules such as amino acids, sugars, fatty acids, and organic acids generated by the metabolic interactions between the microbiome and the host, providing insight into altered metabolic pathways (Vasquez et al., 2022; Zhang et al., 2023). Fecal metabolome analysis offers the possibility of identifying metabolites that can be used as indicators or biomarkers for the function of specific microbes, providing information to determine the role of the gut microbiota in health and disease (Zierer et al., 2018). Specifically, fecal metabolome analysis in stress models provides important evidence for understanding the physiological and biochemical changes in the gut in response to stress, which could give a perspective on how stress affects the various biological pathways. Furthermore, it could help us understand the impact of stress on the complex interactions in the gut-brain axis (Konjevod et al., 2021). Identifying altered metabolites in feces can be performed in two ways: targeted, analyzing only the specific metabolites of interest, and untargeted, comparing changes in the complete metabolomic profiles (Karnovsky and Li, 2020; Melnik et al., 2017). Most stress-related fecal metabolome analyses have focused on comparing fecal glucocorticoid or cortisol content as an indicator (Ataallahi et al., 2022; Josefson and Skibiel, 2021; Keay et al., 2006), and the effects of stress and psychobiotics using untargeted global metabolomic analyses of fecal metabolites have not been fully investigated. Therefore, this study was conducted to investigate the effects of probiotics on behavioral changes and the intestinal metabolome in mice subjected to unpredictable chronic mild stress (UCMS).
Materials and Methods
Male C57BL/6 mice were purchased from Central Lab. Animal (Seoul, Korea) at five weeks old. L. rhamnosus IDCC 3201 (L3201) was provided by Ildong Bioscience (Pyeongtaek, Korea). L3201 were suspended in saline before use for fresh administration. Mice were divided into three groups: control group (CTL group), stress control group (S. CTL group), and stress probiotics (L3201 group). All groups were orally administered saline or L3201 (1.0×109 CFU/mouse/day) from 6 weeks old. We determined the consumption of L3201 based on the results of previous studies (Lee et al., 2016). They were exposed to UCMS from 8 weeks old until sacrifice. Behavioral tests were started at 8 weeks old. Body weight was checked weekly. Mice had ad libitum access to food and water under standard conditions: 21°C–23°C, 45%–55% humidity, and 12 h light/dark cycle. All animal experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of Sejong University (SJ-20230110-01).
UCMS was slightly modified (Mineur et al., 2006; Oh et al., 2020). Stress groups were exposed to stressors such as tube restraint, tail tie-up, food deprivation, water deprivation, illumination, cold object, tilted cage, damp sawdust, sleep cycle change, foreign cage, and cold water bath. Two stressors were used on a randomized schedule in a day.
The EPM was used to measure anxiety-like behavior (Komada et al., 2008). The apparatus is 40 cm above the floor and has a center zone (5 cm×5 cm), two closed arms with walls (30 cm×5 cm×16 cm), and two open arms (30 cm×5 cm×0.5 cm). Mice were placed at the center facing the closed arm and freely explored for 5 min. The tests were recorded and analyzed by Any-maze software version 6.0 (Stoelting, Wood Dale, IL, USA).
The forced swim test was used to measure despair-like behaviors (Porsolt et al., 1977). The apparatus was filled with water (25±1°C) to a depth of 16 cm in a glass beaker (Ø 19×26 cm). Mice were placed in water and forced to swim for 6 min, and the last four minutes of the test were analyzed. All tests were recorded with a camera and analyzed by Any-maze software version 6.0 (Stoelting).
Fresh fecal samples were collected and stored at −80°C prior to metabolic analysis. Gas chromatography–mass spectrometry (GC–MS) analysis was conducted as reported (Thompson et al., 2020; Yoo et al., 2022) with slight modification. Briefly, 40 mg of fecal samples were homogenized in 1 mL of ice-cold methanol by vortexing and centrifuged at 15,000×g for 5 min at 4°C. The supernatant was filtered and thoroughly vacuum-dried. Methoxyamine hydrochloride in pyridine and N,O-bis(trimethylsilyl)trifluoroacetamide were used as derivatization agents. GC–MS analysis was conducted with a Trace 1310 Gas Chromatograph including the ISQ LT single quadrupole Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The detected metabolites were identified using AMDIS and the NIST Mass database (version 2.0, Gaithersburg, MD, USA). Statistical analysis was performed using Metaboanalyst 5.0 (Eom et al., 2022).
One-way analysis of variance (ANOVA) followed by Duncan’s post hoc test was conducted using SPSS statistics 23 (IBM, Armonk, NY, USA) to analyze the statistical significance between groups. Different superscript letters indicate significant differences. All data are presented as the mean±SEM.
Results and Discussion
A schematic diagram for the behavioral experiment is illustrated in Fig. 1A. The body weight of the S.CTL group decreased compared to the CTL group during the UCMS period, but the L3201 group did not differ from that of the CTL group (Fig. 1B). In addition, the S.CTL group showed a decrease in weight gain (%), but the L3201 group showed rescued body weight (Fig. 1C). The EPM was conducted to confirm the anti-anxiety effect of oral administration of the L3201 strain (Figs. 1D, E, F, G, H, and I). The EPM showed no difference in the total distance traveled in all groups (Fig. 1D). The S.CTL group spent significantly more time in the closed arms than the CTL group (Fig. 1E). On the other hand, the time spent in the closed arms of the L3201 group was similar to that of the CTL group (Fig. 1E). Additionally, the S.CTL group had a significantly decreased time spent in the open arms compared to the CTL group (Fig. 1F). However, there was no difference in the time spent in the open arms between the CTL and L3201 groups (Fig. 1F). The FST was performed to confirm the antidepressant effect of oral administration of the L3201 strain (Figs. 1H and I). The immobility time of the S.CTL group was significantly increased compared to that of the CTL group (Fig. 1I). The L3201 group tended to have an increased immobility time compared to the CTL group but a decreased immobility time compared to the S.CTL group (Fig. 1I). These results suggest that the administration of L3201 relieves stress-induced anxiety- and despair-like behaviors.
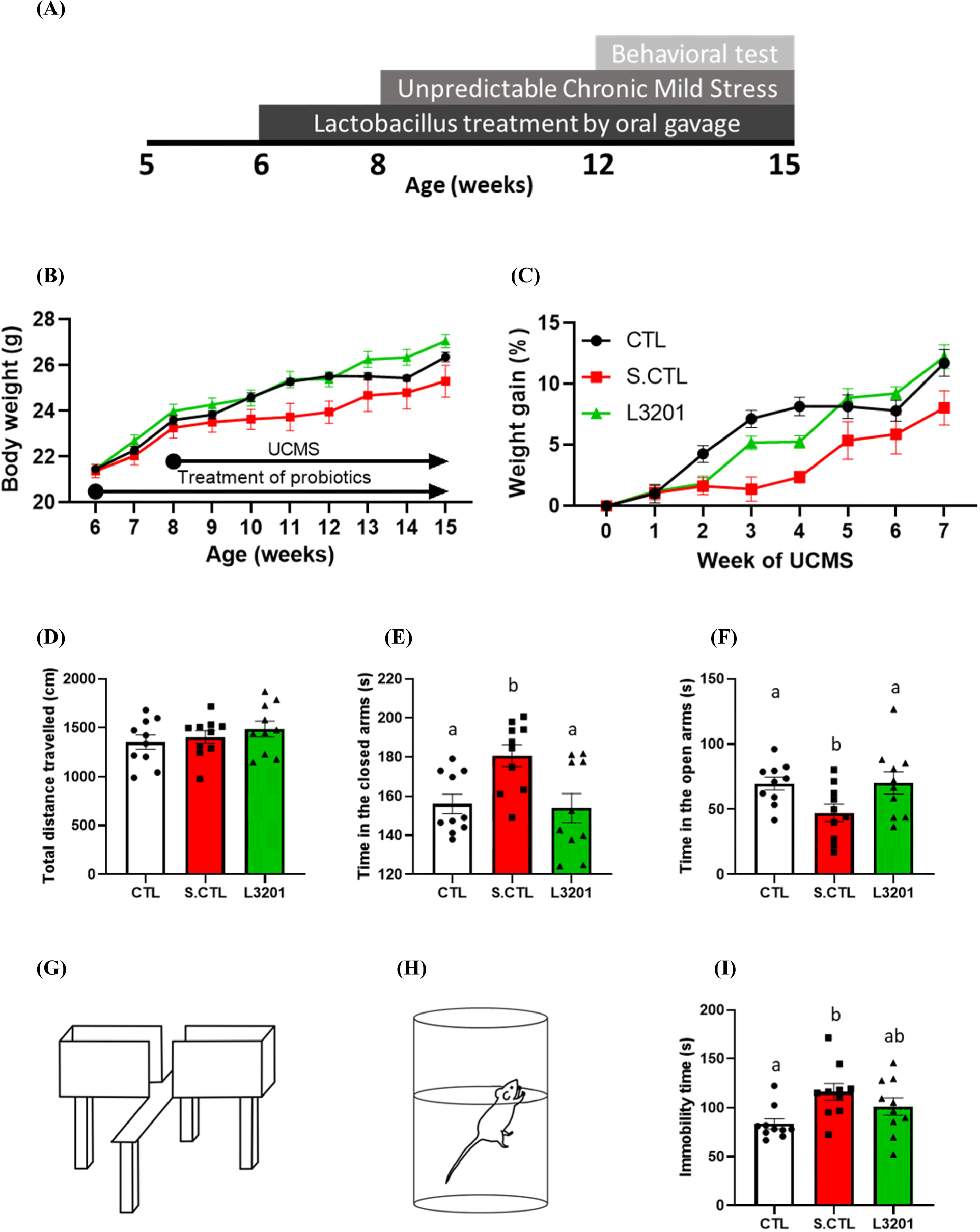
Chronic stress induces anxiety- and despair-like behavior in mice (Westfall et al., 2021). EPM is the most frequently performed test in rodents using open time as an anxiety index to identify anxiety-like behavior (Komada et al., 2008). Corticosterone- or UCMS-induced depressed mice spent less time in the open arms than normal mice in EPM (Peng et al., 2021; Zhu et al., 2020). The S.CTL group showed a decrease in open time, which is an anxiety-like behavior caused by stress. Stenman et al. showed the reduction of anxiety-like behaviors in EPM by administering probiotics in a depression model (Stenman et al., 2020). Consistent with previous anxiolytic results, the L3201 group showed a reversal in the reduced open time caused by UCMS, indicating a decrease in anxiety-like behavior.
Increased immobility time in the FST is used to measure a despair indicator (Porsolt et al., 1977). Previous research has shown that immobility is increased in mouse models of depression caused by corticosterone administration or UCMS (Badr et al., 2020; Mineur et al., 2006; Strekalova et al., 2005). Consistent with previous results, the S.CTL group showed increased immobility time, which indicated despair-like behavior caused by UCMS. Murray et al. (2019) and Stenman et al. (2020) showed the effect of reducing despair-like behaviors by reducing the immobility time of FST in a depression model through probiotics administration. Similar to previous studies, in the L3201 group, the increased immobility time induced by UCMS was alleviated, suggesting a stress-relieving effect of L3201 administration.
GC–MS analysis was performed to confirm the effect of oral administration of the L3201 strain (Figs. 2A, B, and C) on fecal metabolomics. As a result, a total of 53 metabolites, including amino acids, fatty acids, organooxygen compounds, and steroids, were detected; among them, 34 metabolites were identified. Multivariate analysis showed different metabolic profiles among the three groups (Fig. 2A). Within the identified metabolites, an upregulation was observed in the expression of 7 metabolites, while a decrease was noted in 26 metabolites, including L-alanine, L-isoleucine, and cholesterol, in the UCMS model compared to the CTL group (Fig. 2B). Conversely, administration of L3201 in the UCMS-induced model increased 13 metabolites, including L-alanine, L-isoleucine, and propanoic acid, while 19 metabolites exhibited a decrease in their levels. Furthermore, among the metabolites downregulated by UCMS induction, D-galactose, α-L-galactofuranoside, L-isoleucine, 4-methylmandelic acid, L-alanine, glycine, 2-ethylhexanoic acid, 3-oxaoct-4-en-2-imine, and propanoic acid were upregulated upon oral administration of L3201. In contrast, L-threose and α-tocopherol, which displayed upregulation in the S.CTL group compared to the CTL group, exhibited a downregulation in response to L3201 treatment.
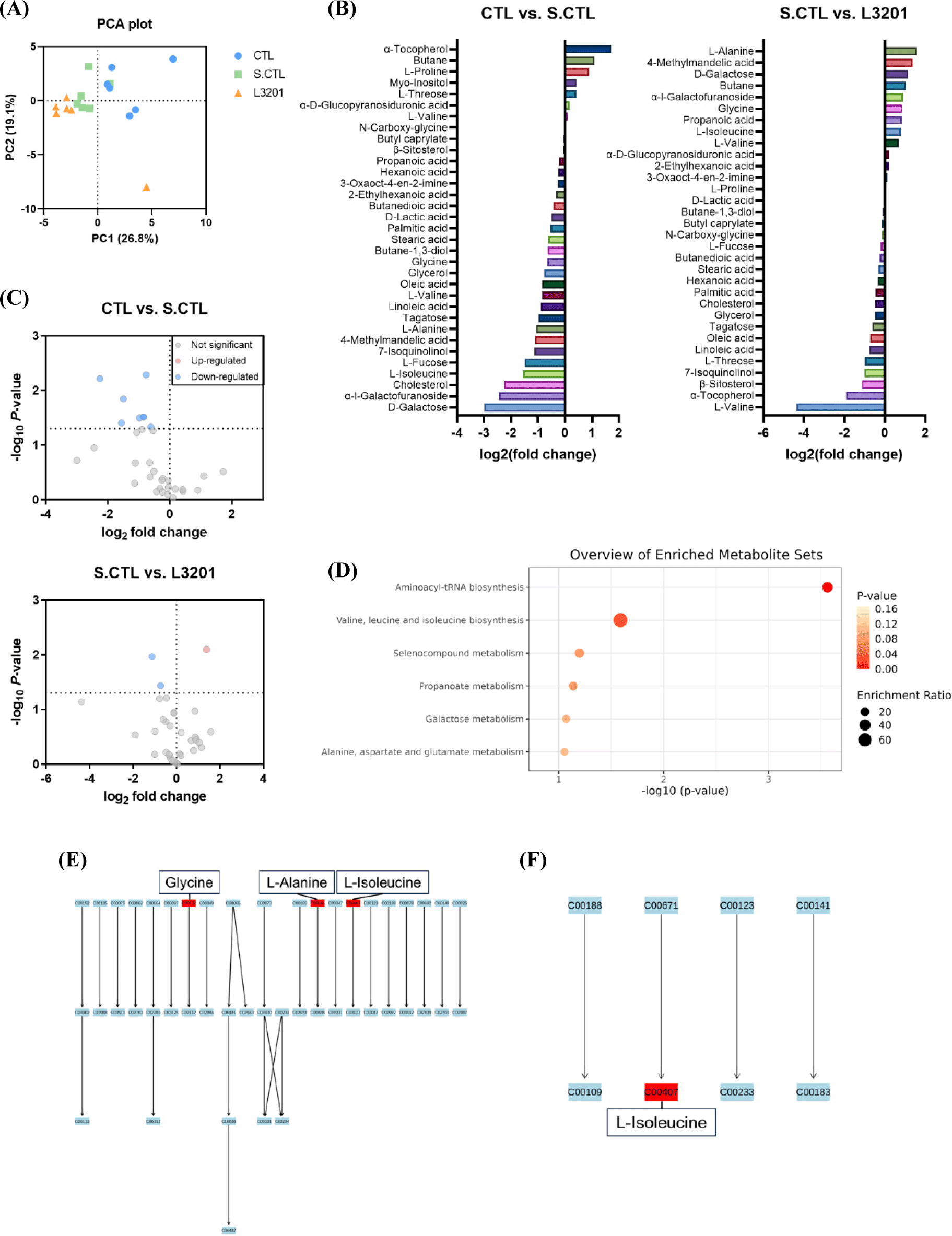
The volcano plot visualized the significantly altered metabolic compounds between the two groups (Fig. 2C). L-valine, glycerol, tagatose, oleic acid, stearic acid, cholesterol, L-isoleucine, and L-fucose were significantly reduced in the S.CTL group compared to the CTL group, and oleic acid and β-sitosterol were significantly reduced in the L3201 group compared to the S.CTL group. Interestingly, 4-methylmandelic acid was significantly increased in the L3201 group compared to the S.CTL group. KEGG pathway enrichment analysis showed that metabolites increased upon L3201 treatment under stress are related to aminoacyl-tRNA biosynthesis and valine, leucine, and isoleucine biosynthesis (Figs. 2D, E, and F). These results confirm that the induction of UCMS disrupts gut metabolism and that the administration of L3201 can ameliorate this imbalance.
Recent studies have shown that the gut metabolome is affected by psychological anxiety. A previous study showed that 11 specific metabolic pathways, including amino acid metabolism and lipid metabolism, were altered in the fecal metabolome of multiple depressed animal models under stress (Liu et al., 2022). It has been reported that glutamate, alanine, and L-serine in plasma could represent the extent of depression (Mitani et al., 2006). L-threonine, oxidized proline, serine, tyrosine, alanine, and isoleucine and related amino acid metabolism were found to be altered in the fecal metabolome of rats exposed to chronic stress (Jianguo et al., 2019). Furthermore, the hepatic metabolites of mice transplanted with feces from depressed patients showed significant changes in aminoacyl-tRNA biosynthesis (Li et al., 2018a). Consistent with previous findings, this study found that metabolites involved in aminoacyl-tRNA biosynthesis, such as L-alanine, L-isoleucine, and glycine, were reduced in the stress model compared to the control group.
Specific probiotics, called psychobiotics, have been reported to improve stress and depression. According to Ma et al. (2021), consumption of L. plantarum P-8 has been shown to reduce stress and anxiety in adults, and this has been attributed to an increase in gut microbial neuroactive metabolites upon probiotic intake. Treatment with L. rhamnosus HN001 (HN001) and Bifidobacterium animalis subsp. lactis HN019 (HN019), alone and in combination, improved anxiety-related behavioral indicators and significantly improved inflammatory markers in stress-induced mice (Huang et al., 2022). It was also found that consumption of L. rhamnosus Probio-M9 in stressed adults increased gut microbial diversity and altered intestinal metabolism, including arachidonic acid metabolism and amino acid metabolism (Zheng et al., 2021). Similar to previous studies, the current study showed that the metabolites related to aminoacyl-tRNA biosynthesis and valine, leucine and isoleucine biosynthesis, which were decreased by UCMS induction, were enhanced by the administration of L3201 (Figs. 2E and F). UCMS increases pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-12 like LPS (Reyes and Chandler, 2023; Zhao et al., 2019). In the previous study, IDCC3201 reduced TNF-a and IL-6 among the increased cytokines caused by LPS in cells (Chae et al., 2022). Although this study did not investigate a decrease in cytokine levels by L3201 administration, it is expected to reduce cytokines increased by stress. These results suggest that imbalances in amino acid metabolism play an important role in stress-related psychological disorders and that treatment with L3201 ameliorates these disruptions.
Conclusion
In this study, we induced UCMS in mice after oral administration of probiotics to reveal that probiotic administration was associated with improvements in depressive-like phenotypes and changes in the gut metabolome. Our results show that depressive-like behavioral patterns and perturbations in the gut metabolome of UCMS mice are ameliorated by supplementation with L3201. These findings suggest that probiotics play a key role in preventing stress-induced depression through the restoration of gut metabolic pathways.













