Introduction
Hanwoo bones were boiled and resulting infusions are commonly ingested as soup in Korea. In general, the Hanwoo bones used for soup were the leg bone, foot, and tail. The foot refers to the bone below the kneecap and the leg bone refers to the two leg bones above the foot. The tail consists of several joints including the hip bone. The main components of Hanwoo bone infusions are collagen, chondroitin sulfate and inorganic materials, like calcium, sodium and magnesium (Park, 1986; Park and Lee, 1982; Seol and Jang, 1990). Among them, collagen and chondroitin sulfate are known to have effects on the bone and skin (Kim, 2003; Saito and Marumo, 2010; Salasznyk et al., 2004; Shizuka et al., 2013). Collagen has functions such as maintaining skin elasticity, strengthening joints and retaining moisture. However, due to the large size of the collagen molecule, ingested collagen is unable to absorb into the body. However, previous studies show that collagen was found in blood following the oral ingestion (Ichikawa et al., 2010; Koji et al., 2005; Sugihara et al., 2012), and the effects of the collagen on skin health have been published (Vivian et al., 2011; Zague, 2008). In another paper, the BioCellcollagen that is the gelatin made from the collagen of the chicken heart cartilage bone, improved blood circulation and reduced the aging of the skin (Schwartz and Park, 2012). In addition, Matsuda et al. reported the collagen peptides taken by pigs through oral administration increased the density of fibroblast and the diameter and density of collagen fibrils (Matsuda et al., 2006). Also, it was reported that mouse skin cell growth was promoted when treated with the collagen peptides (Yasutaka et al., 2009).
Normal epidermal cell differentiation continues as skin ages. However, the dermal matrix or connective tissue is reduced due to the reduction of keratinocyte division, causing skin become thin, dry and wrinkled (Kim et al., 2010). The connective tissue of the skin consists mostly of collagen and elastin. Collagen and elastin provide elasticity and strength to the skin. When elasticity and strength are weakened due to aging, the skin becomes prone to damages and aging (Makrantonaki and Zouboulis, 2007). Skin wrinkling is caused by an imbalance in collagen synthesis and decomposition. In young skin, the matrix metalloproteinase (MMP)-1 and 9 collagenases help to balance collagen synthesis and decomposition. As skin ages, the synthesis of the collagen decreases and the activity of MMP-1, 9 collagenase increases (Talwar et al., 1995). The increased MMPs within skin fibroblast due to repeated exposure to ultraviolet (UV) make skin collagen decomposition and causes skin-wrinkling (Imokawa, 2008). Skin aging can be identified by the growth rate of the skin fibroblast, suppression of collagenase and the amount of collagen synthesis. In order to determine the extent of skin-aging, activity level of senescence-associated β-galactosidase can be used for assessing senescence in mammalian cells. Through this study, we verified the effects of the Hanwoo bone infusions, which contain large amounts of collagen, on skin elasticity and senescence suppression.
Materials and Methods
The skin fibroblast used in the experiments was NHDF-c (adult human dermal fibroblast) obtained from Promo-Cell (Germany). Skin fibroblast was cultured in DMEM media (Welgene Inc., Korea) supplemented with 10% fetal bovine serum (Gibco BRL, USA) and 1% penicillin/streptomycin (Welgene Inc., Korea) at 37℃ in a 5% CO2 incubator (ASTEC, Japan). The used cell passage numbers is between three and ten. Skin fibroblast was cultured for 24 h until they reached 70% confluency, and then further cultured in the FBS-free media for 24 h. Each infusion of Hanwoo’s leg bone, foot and tail was added at the DMEM media without FBS, cells were cultured in 5% CO2 incubator (ASTEC, Japan) for 48 h. The control group was cultured in the media without the infusions.
The Bovine Collagen, that is the pure collagen was purchased from Biocolor Life Science (U.K.) and used for the control experiment.
The sample infusion that used in these experiments was prepared according to the optimization recipe presented by the Yoon et al. (2015). Four kg of each Hanwoo leg bone, foot, and tail was boiled in 20 L water for 12 h. Final volume is about 15 L. The Hanwoo leg bone infusion used the four Hanwoo leg bones was marked leg bone infusion or HLI. The Hanwoo foot infusion used the part of the Hanwoo’s foot under the kneecap was marked foot infusion or HFI. The Hanwoo tail infusion used the Hanwoo’s coccyx was marked tail infusion or HTI.
The cell proliferation rate was measured by using the Cell Proliferation Reagent WST-1 (Roche, Germany). The skin fibroblast was cultured in 96-well plates (4×103 /well) in DMEM media with10% FBS for 24 h and cultured additionally in the media without FBS for 24 h. Next, each infusion was added to the DMEM media without FBS and skin fibroblast was cultured in 5% CO2 incubator for 72 h. Skin fibroblast was cultured at 37℃ in a 5% CO2 incubator (ASTEC, Japan) for 4 h after adding 10 uL of Cell Proliferation Reagent WST-1 to each well. Absorbance was measured at 450 nm by using of ELISA Reader (Molecular Devices, UK).
The amount of the collagen synthesized by skin fibroblast was measured using the Sircol Collagen Assay Kit (Biocolor Life Science, U.K.), according to the manufacturer’s instructions. Skin fibroblast was cultured in 6-well plates (1×105 /well) in DMEM media with 10% FBS for 24 h. Next, each HLI, HFI and HTI was added to the separate DMEM medium with 1% FBS then incubated in a 5% CO2 incubator for 72 h. After collecting the culture media in microcentrifuge tube, the collagen in the media was extracted and concentrated for 18 h with collagen isolation and concentration reagent which contains polyethylene glycol in a TRIS-HCL buffer, pH7.6 (Biocolor Life Science). Then, microcentrifuge tube was centrifuged at 12,000 rpm for 10 min, without delay. The pellet of hydrated transparent collagen is invisible. The Sircol dye that selectively combines with collagen was added to the concentrated culture media, and ice-cold acid-salt wash reagent was added gently to the collagen-dye pellet to remove the unbound dye. The bound dye was released and dissolved by adding 0.5 M sodium hydroxide. The released Sirius Red Dye was measured calorimetrically at 550 nm by ELISA Reader (Molecular Devices).
Senescence-associated β-galactosidase (SA-β-Gal) activity, which is increased through aging, was measured by the Senescent Cells Histochemical Staining Kit (Sigma, USA) (Joseph et al., 2000; Ronald and Susan 2005). Skin fibroblast was cultured in 35-mm culture dishes (1×105 / well) with DMEM media with each HLI, HFI and HTI for 72 h. After fixing for 7 min with 0.2% glutaraldehyde and 2% formaldehyde, the staining solution (5mM potassium ferricyanide, 5mM potassium ferrocyanide, X-gal solution) was added to the media and incubated at 37℃ without CO2 for 24 h. The senescent skin fibroblast, dyed in blue, was examined under a microscope (×200) (Olympus, Japan)
Total protein was extracted from cells using lysis buffer. After SDS-polyacrylamide gel electrophoresis, proteins were transferred onto PVDF membranes using a semi-dry transfer unit-TE 70 (Amersham Biosciences, USA). The membranes were blocked with 4% skim milk and probed with the primary antibody at 4℃ for 12 h. Blots were washed and incubated with the second antibody for 1 h. Using the LAS-3000 (Fujifilm Co., Japan), the changes in protein expression were identified. MMP1, MMP9 (Santa Cruz, USA) and actin (Sigma-Aldrich Co., USA) were used as primary antibodies.
Results and Discussion
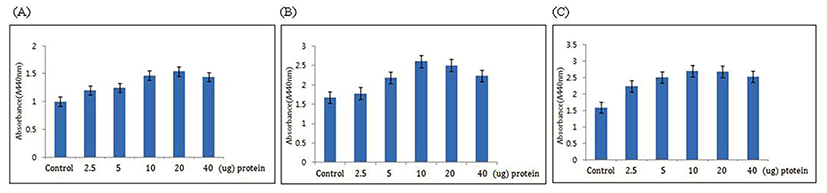
In order to confirm the effects of the HLI, HFI and HTI on the activation of the skin fibroblast metabolism, increments of the skin fibroblast division and cell growth rate were measured by treating skin fibroblast with the HLI, HFI and HTI. When treating skin fibroblast with the HLI, HFI and HTI, the protein concentration in each infusion was measured. When treating skin fibroblast with each infusion, skin fibroblast proliferation increased in comparison to the untreated control fibroblast. When comparing infusion concentrations, the concentration of infusion that displayed the highest growth rates was as follows: the 20 ug of Hanwoo leg bone protein showing 54% increased in growth rate, 10 ug Hanwoo foot protein at 60% and 10 ug Hanwoo tail protein at 65% (Fig. 1). Statistical analysis was carried out to configure the statistical significance of protein concentration differences (p<0.05). Consequently, the HLI, HFI and HTI increased the proliferation of skin fibroblast. Increase of the growth rate of skin fibroblast means increment in dermal tissue and connective tissue. Therefore, it was expected that the increase in the skin fibroblast growth rate may be effective in suppressing skin wrinkling.
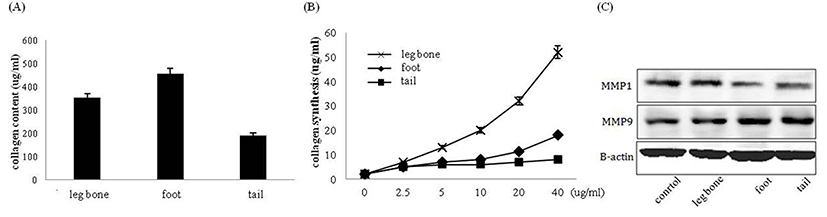
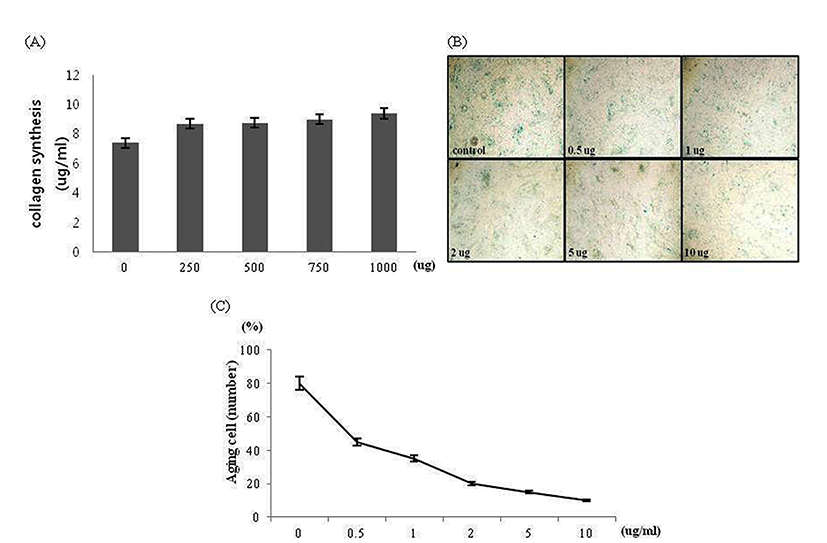
The amount of skin fibroblast collagen synthesis was examined as the next indicator regarding effects of the HLI, HFI and HTI on skin fibroblast metabolism. The collagen content in each infusion was measured to analyze the correlation between the amount of skin fibroblast collagen synthesis and the collagen content in each infusion (Fig. 2A). When comparing the collagen content in 1 mL of each infusion, the collagen content was highest in the HFI followed by the HLI and the HTI. This finding coincided with the results from the preceding studies by Yoon et al. (2015). Next, the amount of skin fibroblast collagen synthesis was measured after adding each infusion to the media of the skin fibroblast. Skin fibroblast treated with each infusion synthesized more collagen than untreated skin fibroblast. When cells were treated with 40 ug of protein from each infusion, the amount of the collagen synthesis was increased by approximately 50% with HLI, 20% with HFI, and 10% with HTI (Fig. 2B). The HLI, HFI and HTI increased the amount of skin fibroblast collagen synthesis, which showed its effectiveness in maintaining the skin elasticity. To this effect, the HLI showed the highest amount of the collagen synthesis, followed by the HFI and the HTI, respectively. In order to determine the exact amount of the collagen synthesis caused by infusion treatments, the amount of collagen content in each infusion was subtracted from the total amount of the collagen synthesis in media. In contrast to our expectations, direct correlation between the collagen content in infusion and the amount of the collagen synthesis of skin fibroblast treated with the HLI, HFI and HTI was not found. Thus, it was confirmed that the activation of the skin fibroblast metabolism was not directly related to the collagen content in the infusion. The results suggested that there is a synergetic effect from ingredients other than collagen. In order to confirm this expectation, the bovine collagen was only treated at skin fibroblast with different concentrations to examine the amount of the skin fibroblast collagen synthesis (Fig. 4A). The amount of collagen synthesis did not increase even when treated with the highest collagen concentration (1,000 ug/mL). Thus, the effect of each infusion on the synthesis of collagen may be the result of the synergetic effects of the collagen and the other ingredients in bone infusion. Next, it was necessary to confirm whether the increase in the amount of collagen synthesis was a result of the reduction of collagenase MMP-1 and MMP-9 amount or the result of increase in the amount of the collagen synthesis. By the western blot assay, the protein amount of MMP-1 and MMP-9 was analyzed following the treatment of skin fibroblast with the Hanwoo bone infusions. In case of the HLI, there was no significant changes in the protein amount of MMP-1 and MMP-9; small decrease in MMP-1 and MMP-9 protein amount was observed in the case of the HFI and the HTI (Fig. 2C). Thus, the increased amount of skin fibroblast collagen synthesis may be the effect of the increase in the collagen synthesis rather than the suppression of the collagen decomposition.
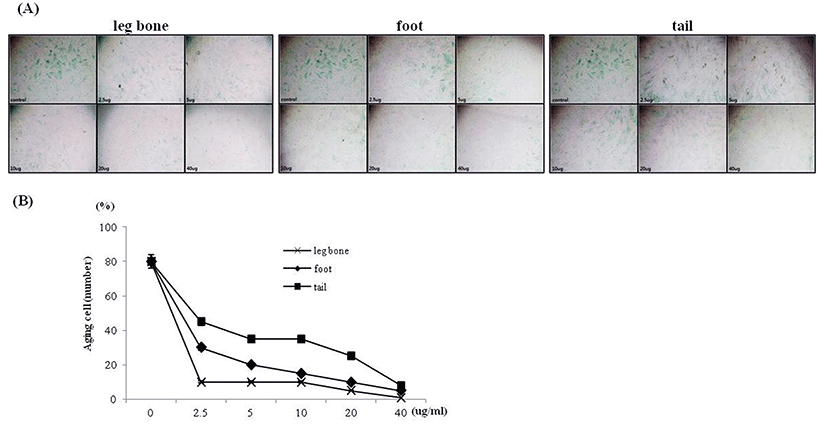
Since the treatment of each infusion increased the amount of skin fibroblast collagen synthesis, the infusions may affect skin elasticity. It was verified whether skin fibroblast senescence is effectively suppressed by infusion treatment, senescence-associated β-galactosidase was measured. Using the senescent cell staining kit, the aged cells were dyed so as to distinguish them from healthy cells (Fig. 3A). The number of aged cells in the fixed sector was counted (Fig. 3B). The results displayed high anti-aging efficacy of the Hanwoo bone infusions even in low concentration of each infusion. In the case of the 40 ug protein concentrate, only few senescent cells were observed. Statistical analysis was performed in order to identify whether there was a difference according to the concentrations. Analysis showed significant differences according to protein concentrations (p<0.001). The number of aged cells decreased remarkably in comparison to the fibroblast control group without the infusion treatment. The anti-aging effects of collagen was confirmed by culturing skin fibroblast with the bovine collagen in order to prove the effects of the collagen itself on the anti-aging of skin fibroblast (Fig. 4B, C). When skin fibroblast was treated with the highest concentration of bovine collagen, 10 ug/mL, the aging of the fibroblast was inhibited. However, the result was not as effective as when skin fibroblast was cultured with the Hanwoo bone infusions. From these results, it could be concluded that the anti-aging effects of each infusion on skin fibroblast was caused by collagen and other ingredients. This assumption would coincide with the result that bovine collagen was unable to increase the collagen synthesis in comparison to the Hanwoo bone infusions (Fig. 4A).
In this research, the infusions made from the Hanwoo parts such as the leg bone, foot and tail were added to skin fibroblast culture media in order to measure the effects of the Hanwoo bone infusions regarding the improvement of the elasticity of skin fibroblast and the inhibition of the skin aging. It was found that the aged cell number decreased as the proliferation of skin fibroblast and collagen synthesis increased due to culture treatment with the Hanwoo bone infusion in comparison to the control group. The skin fibroblast proliferation and the amount of the collagen synthesis increases depended on infusion concentrations. The highest levels of skin fibroblast collagen synthesis and anti-aging efficacy of skin fibroblast observed in the HLI, followed by the HFI and then the HTI. In accordance with the assumption that the main cause of this effect was the collagen from each infusion, skin fibroblast was treated only with bovine collagen and the skin fibroblast metabolism activation experiment was carried out. Unlike the assumption, treatment of the bovine collagen did not show skin fibroblast metabolic activation efficacy. This explains the result in which the collagen content of each infusion and activation of skin fibroblast metabolism did not correspond directly. When comparing collagen content per 1 mL of each infusion, the order of collagen content from the highest to lowest in each infusion was as follows: foot, leg bone, and tail (Fig. 2A). Research conducted by Zague (2008) confirmed that collagen is a major component in the anti-aging of the skin or skin elasticity. However, in the test confirming the collagen content of the Hanwoo bone infusion and each of their effects on skin fibroblast metabolic activation, it was observed that the collagen content of each infusion and their effects on the anti-aging of the skin and the skin elasticity does not correspond directly. The HFI contains a greater amount of collagen than the HLI, yet the effects on the activation of skin fibroblast metabolism are the greatest in the case of the HLI. Although he effect of the HFI was less than the effect of the HLI, there was no significant difference between the two. From such data, It may be speculated that collagen peptide type has an effect on the collagen function as well as the total collagen amount.
It is important to note that the effects of the Hanwoo bone infusion on skin fibroblast metabolism, rather than the effects of collagen itself, were observed. The other components of the Hanwoo bone infusion may have anti-aging effects on the skin and the skin elasticity in addition to collagen. In this study, the Hanwoo bone infusions prepared through the traditional method were added to skin fibroblast culture media and the activation of the skin fibroblast metabolism was confirmed through scientific experimental methods. The significance of this research is that it confirmed the effects of the traditionally prepared infusions on skin anti-aging through scientific methods. This research suggests that Hanwoo bone infusions may be used in place of other collagen peptides that are used to activate skin fibroblast metabolism. Furthermore, this conclusion highlights the Hanwoo bone infusion as natural food that activates skin fibroblast metabolism through oral ingestion.













