Introduction
Proper supply of nutrients is critical for infant growth. Human milk is an ideal source of nutrition for infants; however, infant formula becomes an indispensable nutrient source if the nutritional components of human milk are inadequate or if infants are unable to ingest human milk. The effects of nutritional contents on the normal growth of infants have been extensively studied. (Neri-Almeida et al., 2009; Om et al., 2007; Shapiro AD et al., 1986; Vasquez-Garibay et al., 2005).
Nucleotides are involved in energy metabolism of organisms and are the basic units of DNA and RNA. They are also essential physiological constituents and affect the growth of premature babies. Therefore, nucleotides are important components of the human body (Neri-Almeida et al., 2009; Vasquez-Garibay et al., 2005).
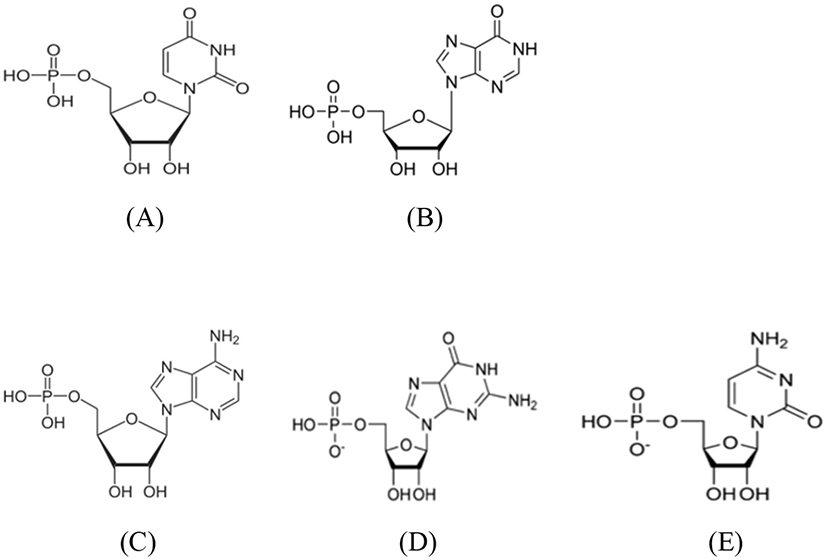
In particular, the European Union (EU), Australia, and New Zealand have specified the standard amounts of five nucleotides, namely, cytidine 5′-monophosphate (CMP), uridine 5′-monophosphate (UMP), adenosine 5′-monophosphate (AMP), guanosine 5′-monophosphate (GMP), and inosine 5′-monophosphate IMP) in food. Furthermore, research interests in infant products are continuously being promoted, and standard amounts of nucleotides are now being added to infant food even in Korea (Om et al., 2007).
Commercially distributed infant formula is mainly processed from the milk of cattle, sheep, and goat (Ministry of Food and Drug Safety, 2016b; Livestock product processing standards and component standards, 2016). It can also contain milk-processed products as the main ingredient, including nutrients such as, minerals, vitamins, and human milk ingredients necessary for the growth and development of infants added subsequently. Ever since the nutritional function of nucleotides was emphasized in 1965, nucleotides are being added to infant formulas available in today’s market (Alles et al., 2004). Nucleotides (Fig. 1) are used by most manufacturers of infant formula in Korea and abroad for the purpose of nutrition enhancement (Ministry of Food and Drug Safety, 2016b).
The use of nucleotides in food meant for infants and mothers is gradually increasing worldwide for improving the quality of nutrition, although domestic analytical methods for establishing the standard amounts are lacking. In contrast, the nutritional function and concentration of nucleotides in food are being actively studied across the globe using high-performance liquid chromatography-mass spectrometry (HPLC-MS), various capillary devices such as electrophoretic mass spectrometer (capillary electrophoresis coupled to mass spectrometry, CE-MS), hydrophilic liquid chromatography (hydrophilic interaction liquid chromatography, HILC), and HPLC (Garcia-Gomez et al., 2013; Laourdakis et al., 2014; Liao et al., 2011; Perrin et al., 2001; ShanShan et al., 2016).
The main aim of this study was to establish a rapid and simple analytical method for determining the concentration of five nucleotides in dairy products using solid phase extraction (SPE) clean-up and high-performance liquid chromatography with diode array detector (HPLC-DAD). The newly improved method proved to be appropriate for rapidly analyzing nucleotides in infant formulas and other dairy products compared to the conventional methods.
Materials and Methods
The five nucleotide standards—AMP, CMP, GMP, IMP, and UMP—and the internal standard, thymidine 5′-monophosphate (TMP), were purchased from Sigma (St. Louis, MO, USA). Water and methanol used for all experiments were purchased from Budick and Jackson (Ulsan, Korea), potassium bromide and ethylenediaminetetraacetic acid (EDTA) disodium salt dihydrate were purchased from Sigma, potassium dihydrogen phosphate was purchased from Kanto (Tokyo, Japan), phosphoric acid was purchased from Junsei (Tokyo, Japan), and potassium hydroxide and sodium chloride were purchased from Junsei.
Analytical samples for testing the current method included 20 samples of powdered milk (infant food) and 5 samples of cow milk. All infant powdered milk samples were purchased from the Daejeon local market and online market, and stored at room temperature. Cow milk samples were collected at Chungcheongnam-do dairy farm and subsequently stored at −18°C.
Five standard stock solutions were prepared by placing 100 mg of each standard in 100 mL volumetric flasks and adjusting the volume with distilled water to obtain 1,000 mg/L solution of each standard. Five working standard solutions for the calibration curves were prepared by placing 1 mL of each sample in a 10 mL volumetric flask, adjusting the volume with distilled water, and then shaking the mixture to obtain a 100 mg/L solution. Thereafter, the solution was diluted with distilled water to 0.5, 1, 5, 10, and 20 mg/L and was used as the final working standard solution. However, stock and working standard solutions were prepared prior to every experiment.
Laboratory instruments were used for general quantitative analysis. Whatman No.1 filter paper (Maidstone, UK) was used for filtration and Macherey-Nagel Chromabond SB 6 mL/1,000 mg column (Duren, Germany) was used for SPE. The vacuum manifold used was from Supelco (Maidstone, UK). To filter the final extracted solution, a 0.22-μm nylon filter (Adventec, Japan) was used. The Agilent 1100 Series HPLC Value System (Agilent, Santa Clara, CA, USA) and the Agilent 1200 Series DAD HPLC System (Santa Clara, CA, USA) were used. The analytical conditions of the instrument (HPLC-DAD) were optimized prior to initial use. To effectively detect nucleotides in milk powder and human milk, the sensitivity was analyzed using DAD, and the Capcellpak UG 120 C18 column (4.6×250 mm, 5 cm, Shiseido, Japan) was used for separation of nucleotides. Five optimum separation conditions were used. The mobile phase used was KH2PO4 (10 mM, pH 5.6), flow rate was 0.6 mL/min, column temperature was 40°C, total analysis time was 25 min, and injection volume was 10 μL.
Validation of the newly improved analytical method was based on the guidelines on standard procedures provided by the Food and Drug Testing Act developed by the Department of Evaluation of Food and Drug Safety Evaluation Institute. Guidelines for validating the chemical method (Ministry of Industry and Trade, 2012a; Ministry of Industry and Trade, 2012b; National Institute of Food and Drug Safety Evaluation, 2016) were notified by the Ministry of Industry and Trade Resources Department Technical Standards Institute. To verify the improved method, verification factors were selected according to the purpose of each method, accuracy, precision, linearity, selectivity, limit of detection, limit of quantitation, and recovery test performed (Table 1). All experiments related to verification were repeated thrice. Linearity of nucleotide concentration was evaluated using five standard solutions (0.5, 1, 5, 10, and 20 mg/L), and the intensity of each concentration was measured to plot the calibration curve. Linearity was expressed as correlation coefficient (r2). To evaluate the limit of detection and limit of quantitation, standard solutions of five nucleotides were diluted to the required concentration and the signal-to-noise ratio (S/N ratio) was calculated. To measure the inter-day and intra-day precision, the experiment was replicated three times per day and repeatedly conducted for three days. To determine the recovery rate, 2 mg/kg (the concentration of nucleotides in milk powder) of each of the five nucleotides were added and the recovery rates were determined before and after the addition.
Results and Discussion
The buffer (1 M NaCl, 5 mM EDTA) used as the extraction solvent in the AOAC method attempted to remedy the drawback that it did not dissolve completely during preparation of the reagent. EDTA used in the AOAC method is a colorless crystalline powder with a melting point of 240° and its solubility in water is 0.2 g/100 mL at 22°C. Hydrophilic EDTA is not soluble in ethanol/ether and chelates metal ions. In this study, these features were used in the extraction solvent, which contained the soluble disodium dihydrate salt of EDTA. The spiking test showed 84.69% to 102.72% (Table 1, intra-day and inter-day accuracy) when the reagent solvent was used.
HPLC-DAD, high-performance liquid chromatography with diode array detector; CV, SD of measured concentration/means of measured concentration; LOD, limit of detection, signal-to-noise (S/N) ratio=3; LOQ, limit of quantitation, S/N ratio=10; CMP, cytidine 5′-monophosphate; UMP, uridine 5′-monophosphate; AMP, adenosine 5′-monophosphate; GMP, guanosine 5′-monophosphate; IMP, inosine 5′-monophosphate.
The mobile phase and detection conditions of the AOAC method involve gradient conditions and three wavelengths for interpretation of the results. However, the disadvantage of this method is the longer time required for interpretation, which was circumvented by using an isocratic elution method and single wavelength in the new method. The optimization conditions of the improved nucleotide test method are shown in Fig. 2.
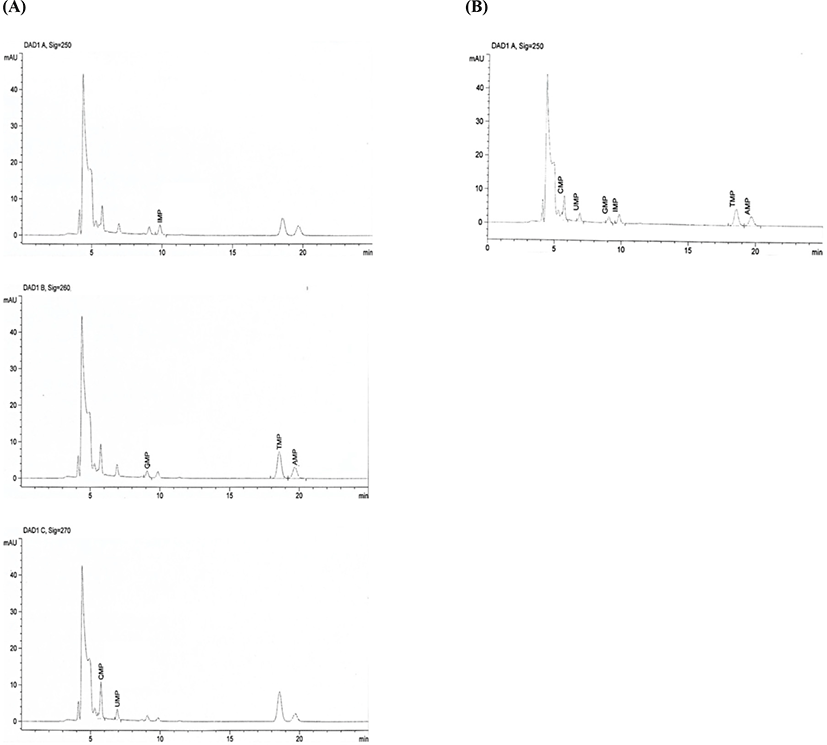
Fig. 3 shows a chromatogram analyzed at three wavelengths (250, 260, and 270 nm) using the conventional AOAC method, and at a single wavelength (250 nm), using the improved method. Fig. 3 (A) is a chromatogram of the analyte according to the wavelength, as shown in AOAC. Three wavelengths, 250 (IMP), 260 (AMP, GMP), and 270 nm (CMP, UMP), possess properties of good resolution and maximum absorption. Table 2 shows that the results obtained for the amounts of nucleotides were similar between the AOAC and improved methods (p<0.05). Because there are no unknown peaks (interference peaks), the wavelength range is not wide enough for the three wavelengths to affect the maximum value at 20 nm, and it is considered that TMP is calculated using the internal standard. Therefore, a single wavelength can be selected and analyzed quickly and accurately. We confirmed that the results obtained using the improved method were not significantly different from those obtained using the conventional method. The analysis time of the improved method was shorter than that of the conventional method, which is based on three wavelengths.
We identified differences in the nucleotide concentration (1.99–29.39 mg/100 g) of 20 infant formula samples, shown in Table 3. Our findings validated the concentrations displayed on the label in 16 out of the 20 products analyzed here. The nucleotide concentrations detected in recent studies [0–51.9 mg/100 g (Yiping et al., 2011) and 8.04–18.16 mg/100 g (YE et al., 2010)] are in agreement with the concentrations observed in present study (1.99 to 29.39 mg/100 g). Analysis of the cow’s milk revealed that the concentration of each of the five nucleotides present in the samples was in the range of 0.28–0.86 mg/100 g.
Nucleotides are widely used in commercial infant formulas and other dairy products due to the beneficial effect on the immune response of infants and enhancement of mineral absorption. However, currently there are no standardized test methods for evaluating the nucleotide content of infant formulas and dairy products, and therefore an universal protocol for determining the content of nucleotides is urgently required. A rapid and simple analytical method for determining the concentration of five types of nucleotides in dairy products was improved using HPLC-DAD.
Following optimization of the mobile phase and detection conditions the improved method shortened the analysis time from 40 min to 25 min. It is expected that the improved test method will be utilized by the nucleotide manufacturers such that they can rapidly and easily analyze the nutritional content in the final product. In addition, the results of this study can be used as basic data for analyzing the nucleotide content in the future.













