Introduction
Meat is an important source of major nutrients and constituent of a daily diet. Change for consumers’ demands and increased market competitions have induced a requirement for improving the quality of meat products as beef patty by developing their nutrient values and advantageous-health qualities (Lopez-Lopez et al., 2011). Nevertheless, fresh meat and meat products are highly responsive to quality deterioration because of the higher nutrient constitution (Shah et al., 2014). Frozen storage has been allowed as the most efficient procedure for preserving the attribute for meat products over an extended period of time. The meat quality can be deteriorated because of the physico-chemical process and, consequently, various researches have been presented that the oxidation of lipid is a prime cause for losing the qualities for several types of meat plus processed meats during frozen storage (Ozer and Saricoban, 2010). In addition, auto-oxidation is occurred broadly leading to a reduced functional quality of meats and meat-based products throughout frozen storage (Mielnik et al., 2003).
One of the important factors for meat quality deterioration is the oxidative process which affects lipid, protein, carbohydrate, vitamins, and pigments. Oxidative deterioration induces mainly lipid oxidation and results in loss of nutritional quality and sensorial attributes and reduction of shelf-life for meat-based products (Soriano et al., 2018). Lipid originated reactive oxygen species and oxidizing myoglobin by-products lead to meat oxidation and dramatically decrease quality properties such as color, flavor, and nutrient value of meat products (Seo et al., 2019). Moreover, protein oxidation results in a change for amino acid structure, tenderness, and water-retention capability for meats and processed meats causing a decrease in meat products quality (Turgut et al., 2016).
The uses of antioxidants supplements have been proven to be an efficient plan for delaying or preventing the oxidizing procedures. Therefore, ascorbic acid (AA) is usually employed as an antioxidant supplement during processing of meats. AA is soluble in water, employed as the additives based on the rule of ‘proper amount’ to hinder deterioration of meat product quality, and is regarded for having no toxic impacts for the consumer (Carballo et al., 2018; Ozer and Saricoban, 2010). Moreover, Butylated hydroxytoluene (BHT) is a widely utilized artificial antioxidant which is efficient to purify peroxyl radical and restrain the origination of free radical. This is allowed to incorporate into meat and meat product to slow or inhibit the oxidation and expand the storage stability (Kumar et al., 2015). Nonetheless, consumer interests and requirements on naturally originated antioxidant substances have been increasing owing to the antinutritional and toxicological impacts for synthetically antioxidant substances like BHT, butylated hydroxyanisole (BHA) or propyl gallate (Carballo et al., 2018; Shah et al., 2014).
Clove (Syzigium aromaticum L.), is belonged to the Myrtaceae family, is a dried-up flower bud and is largely utilized in the food products as it possesses a specific aroma and effective health attributes. Clove contains phenolic constituents like tannins, sesquiterpenes, and triterpenoids which can show antioxidant activities (Ramadan et al., 2013; Zhang et al., 2017). Clove extract (CE) acquired from the entire clove bud has been widely examined to show high antioxidant actions in meat products (Shi et al., 2014). In that context, CEs as natural antioxidant have been employed for meats and meat-based products to increase lipid and protein stability against oxidization, enhance color stabilization and sensorial properties, and lengthen the shelf-life at the storage time (Shi et al., 2014; Zhang et al., 2017).
The uses of natural antioxidant have been expanded for improving the oxidizing stability for meats and meat-based products over the past years (Armenteros et al., 2016). Zhang et al. (2016) has recorded that CE as a natural antioxidant has been used potentially in meat products for improving the oxidizing stability. However, it has been utilized BHT and AA as antioxidants in the meat industries (Carballo et al., 2018; Cunha et al., 2018). Considering this, fresh beef patties were formulated with BHT, AA, and CE, and it was examined the comparison for the impacts of abovementioned antioxidants on oxidizing stability, color stableness, and heme iron content for fresh beef patties. Nonetheless, no comparative analysis has been conducted for the antioxidative effect of CE with AA and BHT on the stability of fresh beef patties, especially at frozen storage.
The purpose for the existing study was to determine the antioxidative effect of CE compared to AA and BHT on the stability of fresh beef patties. Cooking loss, pH, lipid oxidization, protein oxidization, heme iron level, and color values of fresh beef patties were evaluated during 6 months of frozen storage. Fresh beef patties with added no antioxidants were employed as the control.
Materials and Methods
Clove was purchased from the domestic market. The clove powder extract was got utilizing the method of reflux extraction. After grinding of clove, the powder was mixed into the distilled water (w/v, 1:5 ratios) and carried for extraction at 85°C for 7 h. Likewise, the extraction for residue was conducted using distilled water (1:5 ratios) for 14 h at 85°C. The extracted two solutions were filtrated with filter paper of Whatman No. 1. The eventual CE was collected after condensing the solution through a void rotating evaporator at 85°C. The CE was preserved at −60°C to continue analysis.
BHT, AA, 2-thiobarbituric acid (TBA), perchloric acid (PCA), sodium dodecyl sulfate (SDS), tris (hydroxymethyl) amino methane (TRIS) buffer, and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) were obtained from Sigma Aldrich (St. Louis, MO, USA). The whole chemicals utilized for the current research was of the highest purities of analyzed properties.
Fresh beef loin and beef back fat were acquired from the domestic market and ground independently utilizing a meat grinder (GG-22, German Knife, CA, USA) consisting of a plate set in 8-mm diameter holes. The study included 4 treatments, and three batches for each treatment were conducted 3 times for each storage month (mon). The preparation of all fresh beef patties was performed employing the same formulations. The beef loin, back fat, and all fresh constituents were combined absolutely in the correct proportion employing a patty mixer (5K5SS, KitchenAid, Michigan, MI, USA). The primary formulations contained 90.8% beef loin, 8.0% back fat, and 1.2% sodium chloride. The fresh beef patties (4) were prepared as control (without BHT, AA, and CE), added with 0.02% BHT (BHT), 0.05% AA (AA), and 0.1% CE (CE). The patties (45 g) were structured through a hand held patties maker. After packaging in the polyethylene packets, the fresh patties were kept at frozen (−21°C) storage for 0, 2, 4, and 6 mon to conduct the whole experiments in the laboratory of Meat Processing.
The pH value of beef patties was assessed employing an electronic pH meter (MP230, Mettler Toledo, Greifensee, Switzerland). The distilled water (30 mL) mixed patties (3 g) were homogenized utilizing an electronic homogenizer (T25 Ultra-Turrax, IKA, Germany) for 25 s. For evaluating the pH of the patties, the pH meter was calibrated through standard buffers of pH 4.01, 7.00, and 9.21 at 21°C.
The cooking loss (%) for fresh beef patties was measured by the calculation of weight difference between uncooked and cooked patties as followed:
Thiobarbituric acid reactive substances (TBARS) of beef patties were assessed to measure the lipid oxidation employing the minor modified method adopting from Cherian et al. (2007). Fresh beef patties (3 g) were homogenized through 3.86% perchloric acid (27 mL) and stored for 1 h at 5°C. The homogenate was centrifuged (1736R, Labogene, Seoul, Korea) at 2,100 g for 10 min. After filtrating the supernatant, filtrate (2 mL) was admixed into 20 mM TBA (2 mL) and allowed to stay at room-temperature for 14 h. The absorbance was evaluated at 531 nm spectrophotometrically (Cary 60 UV-Vis, Agilent Technologies, Santa Clara, CA, USA). The TBARS values were stated as mg malondialdehyde (MDA)/kg patties.
Thiol content for different patties was determined for measuring the protein oxidization using the method described by Vossen and De Smet (2015) with some modifications. The patties (2 g) were homogenized through 27 mL of 5% SDS in 0.10 M Tris buffer and transferred to the water-bath at 80°C for 30 min. The coolness formed homogenate was centrifuged at 6,000 g for 20 min. The filtrated supernatant (0.5 mL) was added to 2 mL of 0.1 M TRIS buffer (pH 8.0) and 0.5 mL of 10 mM DTNB (5,5′-dithiobis (2-nitrobenzoic acid) in 0.1 M TRIS buffer to evaluate the thiol content. Filtrated supernatant (0.5 mL) was incorporated into 0.1 M TRIS buffer (2.5 mL) to evaluate the protein content. Moreover, the reagent blank was formed by mixing 5% SDS in TRIS buffer (0.5 mL), 10 mM DTNB (0.5 mL), and 0.1 M TRIS buffer (2.0 mL). All solutions were allowed for reacting in the dark place at 5°C for 30 min. Absorbance for thiol content was then read at 412 nm. The calculation for thiol content was done employing the Lambert-Beer equation of ε412 = 14,000 M−1 cm−1, and the result was indicated in nmol of thiol/mg of protein. Protein content was determined at 280 nm utilizing a BSA standard curve.
Heme iron content of beef patties was measured employing the method explained by Ozer and Saricoban (2010) with minor modification. Beef patties (1 g) were added to 5 mL of acidified acetone solution (acetone:distilled water:HCl=90:8:2) in polypropylene tube. The tube was closed with a cap and permitted for standing in darkness condition at room-temperature for 1 h. The tube content was filtrated using Whatman GFA as glass filter paper, and the absorbance was evaluated at 640 nm.
The calculation for heme-iron content was performed by calculating the whole pigment as hematin employing the following formulas:
Color values like lightness (CIE L*), redness (CIE a*), and yellowness (CIE b*) for several patties were determined utilizing a colorimeter (Konica Minolta CR-400, Tokyo, Japan). The standard white plate (Y=81.2; x=0.3191; y=0.3263) was employed for calibrating the colorimeter, and each patty was measured twice. The measurement for chroma (C*) value and hue angle (h°) value was carried out utilizing two equations of {(a* + b*)1/2} and {tan−1(b*/a*)}, respectively.
The experiments contained a sum of 48 observations (four treatments×three batches×four storage periods) to conduct statistical analysis. All data were exhibited as mean values of 3 replications with the standard error of means. The data were examined utilizing a statistical software of Statistical Analysis System (SAS) containing 9.3 version. One-way analysis of variance (ANOVA) accompanied by Duncan’s multiple range tests (p<0.05) was utilized to assess significant differences among different categories for fresh beef patties and to assess the effect of storage period.
Results and Discussion
The measurement of pH and cooking loss was conducted for 6 mon of frozen storage and is displayed in Table 1. Non-significant change for pH value was noticed amongst all fresh beef patties throughout frozen storage times (p>0.05). Nonetheless, the pH value in all beef patty samples was significantly increased (p<0.05) from the first to last time of frozen storage. Ozer and Saricoban (2010) recorded that pH value for meat products increased significantly during frozen storage. This increasing for pH value is due to the production of ammonia arising from amino acids deterioration as protein denaturation in meat products. The results are in accordance with Mokhtar and Youssef (2014), who noticed that the beef burgers treated with BHT/BHA and CE showed no significant changes for pH values compared with the control during storage.
The cooking loss considers the loss of moisture and fat after cooking of meat products. The insignificant difference (p>0.05) for cooking loss was seen among all patty samples at all storage periods. Nevertheless, the cooking loss in all patties showed no significant changes among all storage times (p>0.05). It is reported that all antioxidants and storages times had no negative effects in cooking loss. No cooking loss in beef patties formulated with BHT, AA, and CE can be related to no fat and moisture loss. Non-significant change for cooking loss in chicken nugget was seen after formulation with antioxidants of sage, rosemary, and tea catechin (O’Sullivan et al., 2004). Basanta et al. (2018) also reported that patties sample with added plum pulp showed non-significant change for cooking loss in comparison to the control sample.
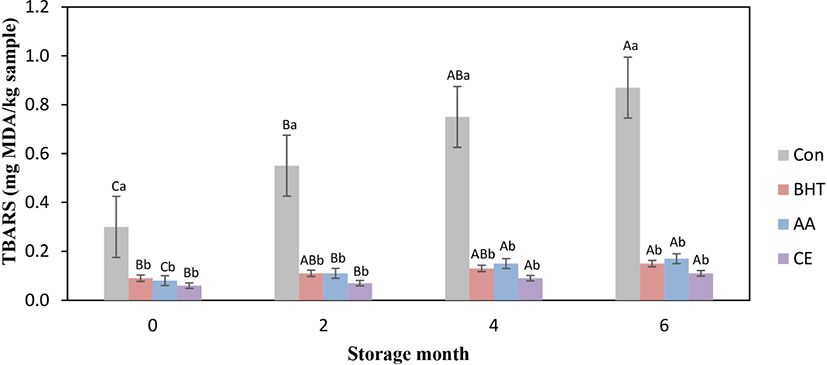
Lipid oxidization for fresh beef patties was assessed by measuring the TBARS value at frozen storage, and the findings are exhibited in Fig. 1. Incorporated antioxidants and storage months presented substantial (p<0.05) effect on TBARS values. Maximal TBARS was shown in control beef patties throughout the storage months; nonetheless, antioxidants formulated patties exhibited obvious reduction for TBARS values. TBARS value for all kinds of beef patties was significantly (p<0.05) increased from mon 0 to mon 6 of storage. The antioxidants like BHT, AA, and CE formulated fresh beef patties revealed significantly lower TBARS values during whole frozen storage periods when compared with the control (p<0.05). The findings noted that added antioxidants presented a positive effect on the oxidative stability for beef patties. The increase for TBARS value for the control could be due to the origination of increased MDA that has been considered as secondary products for lipid oxidization (Zhang et al., 2016). However, non-significant change for TBARS value was observed amongst BHT, AA, and CE contained beef patties for all storage periods (p>0.05). Ozer and Saricoban (2010) reported that chicken patties with added AA had significantly reduced TBARS value compared to the control patties. The substantial decrease for TBARS values was found in CE treated pork patties (Kong et al., 2010), chicken meat sample (Zhang et al., 2016), buffalo patties (Tajik et al., 2014), beef burgers (Mokhtar and Youssef, 2014), and pork sausages (Zhang et al., 2017). The current study is in agreement with such results and indicates that the natural antioxidant like CE can have been employed for enhancing the shelf-life for any meat product. This antioxidant effectiveness of CE has been performed owing to the phenolic constituents and the capacity for the hydrogen molecule donation to deactivate free radical (Baghshahi et al., 2014).
Protein oxidation of fresh beef patties was measured by the evaluation of thiol contents, and the findings are presented in Table 2. The significant decline for thiol content in whole beef patty samples were observed from mon 0 and 2 to mon 4 and 6 of frozen storage (p<0.05), indicated the proteins oxidization. A similar result has been found by Feng et al. (2016), who revealed that proteins oxidization caused diminished thiol content for pork sausage at storage time. From first to last time of storage, the reducing rate for thiol content in CE treated beef patties were lower compared to AA supplemented beef patties and the control. The decline percentages for thiol content were shown by ascending: control>AA>CE>BHT (12.31%> 11.20%>9.51%>8.72%, respectively). At the end of the storage, thiol content reduction of 12.31% was seen for the control patties; however, thiol content reduction for BHT, AA, and CE treated beef patties (8.72%, 11.20%, and 9.51%, respectively) was lower than the control patties. After mon 4 and 6 of storage, BHT and AA contained beef patties presented significantly higher thiol content by comparing with CE contained patties and the control patties (p<0.05), nevertheless, no significant change (p>0.05) for thiol content was found between BHT and AA contained beef patties. The CE formulated fresh beef patties presented significantly lower thiol content than all other patties for all storage months (p<0.05). The outcomes are similar to the research analyzed by Zhang et al. (2017), who observed that the significant reduction for thiol content was seen for CE formulated pork sausages compared with the control, and this occurrence might be done because of the balancing of the antioxidants and pro-oxidants effect for phenolic components in CE. Jongberg et al. (2011) stated that white grapes extracts led to a reduction in thiol contents for beef patties, and it might be occurred owing to the presence of ortho-phenolic substances in extracts. This thiol content reduction in beef patties by the addition of CE was presented because of the ortho-phenolic component (eugenol), which could react with thiol contents and produce thiol-quinone admixture; as a result, thiol content was decreased (Zhang et al., 2017). Nonetheless, silver carp fillet formulated with CE was seen to hinder the decline of thiol contents (Shi et al., 2014).
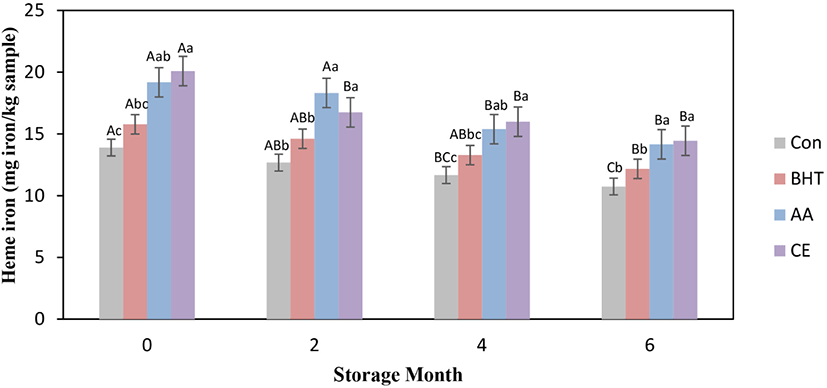
Meats and meat-based products are important sources for heme iron connected to protein. Heme iron content for fresh beef patties at frozen storage is presented in Fig. 2. The significant decrease (p<0.05) of heme-iron content was seen for all types of beef patties at frozen storage time from mon 0 to mon 6. The heme iron content in CE treated patties was significantly increased (p<0.05) when compared to the control and BHT treated beef patties at all storage times, whereas non-significant changes for heme iron contents were found between AA and CE treated beef patties (p>0.05). On mon 6, the beef patties formulated with AA showed significantly increased heme iron content in comparison to BHT formulated patties and the control (p<0.05). The results specified that AA and CE prevented the free of iron from heme-iron. The increase in heme-iron content for antioxidant contained patties could have been due to the increased level of soluble heme pigments and the contribution for increased extractability of heme pigment (Ozer and Saricoban, 2010). The reduction of heme-iron content occurred because of the releasing of iron caused by disruption of heme and the increase of frozen storage times (Benjakul and Bauer, 2001; Ozer and Saricoban, 2010). Purchas et al. (2003) stated that the drips freed from meat throughout storage comprised a substantial quantity of iron, especially soluble heme-iron. Moreover, Buzala et al. (2016) reported that heme-iron is mostly located in meat protein, is involved in the contribution of the bright red color in meat and meat products.
Color is a precious quality mostly of meat products and makes influencing the consumer for instant purchasing or refusing the meat products through observation (Soriano et al., 2018). The color values of fresh beef patties were evaluated for all storage months and are shown in Table 3. From mon 0 to mon 6, CIE a* and C* values were significantly reduced for all kinds of beef patties (p<0.05), nevertheless, non-significant change (p>0.05) in CIE L*, CIE b*, and h° values was seen for all beef patties. The CIE L* value for all patties presented non-significant variation (p>0.05) throughout the storage. The patties formulated with CE showed significantly increased (p<0.05) CIE b* value compared to the control at the final storage, while the non-significant change (p>0.05) for CIE b* value was observed in BHT and AA processed patties compared with the control. This result is in accordance with Radha Krishnan et al. (2014), who reported that the CIE b* values of the spice extract incorporated chicken meat samples were substantially higher in comparison with the control. After 4th and 6th mon for frozen storage, BHT, AA, and CE supplemented patties showed significantly increased (p<0.05) CIE a* and C* value by comparing with the control patties. Moreover, a significant decline (p<0.05) for h° value was seen for BHT, AA, and CE incorporated beef patties as compared to the control on mon 4 and 6. The lowered h° value has been linked to a lowered decline for red color (Yousuf and Srivastava, 2017), indicated that BHT, AA, and CE processed beef patties revealed lowered color decline compared to the control. The result indicated that added antioxidants (BHT, AA, and CE) showed preventative effects on discoloring for beef patties during frozen storage moment. This might have been related to a reduction for lipid oxidization by the addition of BHT, AA, and CE, since reduced lipid oxidization can cause reduced discoloration. The previous studies showed that lipid oxidization for meat products caused redness degradation (Hayes et al., 2011; Jung et al., 2012). The significant increase for CIE a* value was also observed in CE incorporated pork patties (Kong et al., 2010), chicken meat sample (Zhang et al., 2016), and pork sausages (Zhang et al., 2017). Moreover, Falowo et al. (2014) stated that a preventative impact for natural plant extract on discolorization for meats and meat-based products was found because of the antioxidative action of phenolic substances.
Conclusion
The incorporated BHT, AA, and CE in fresh beef patties prompted a significant decline of TBARS and h° values and increase of CIE a* and C* values at frozen storage for 6 mon as compared with the control (p<0.05). Inclusion of AA and CE led to significantly increased (p<0.05) heme iron content in beef patties when compared to BHT treated patties and the control. Moreover, BHT and AA added patties and the control showed significantly increased (p<0.05) thiol content compared to CE treated patties. Nevertheless, the percentage in a decrease for thiol content of CE treated patties was lower than the control and AA-treated patties from first to last time of storage. It is definitely seen that BHT, AA, and CE showed the antioxidant effect on fresh beef patties. The antioxidant impacts for three antioxidants were more pronounced for lipid oxidizing than protein oxidizing. In Sum, the inclusion for CE could have been employed as a safe and substitution of artificial antioxidants in beef patties preparation to efficiently prevent lipid oxidation and increase heme iron content and color stability. Therefore, the results can be concluded that CE can replace the application of AA and BHT when the formulation of fresh beef patties at frozen storage.













